Ch 19 Electrochemistry Dr Namphol Sinkaset Chem 201





























- Slides: 63

Ch. 19: Electrochemistry Dr. Namphol Sinkaset Chem 201: General Chemistry II

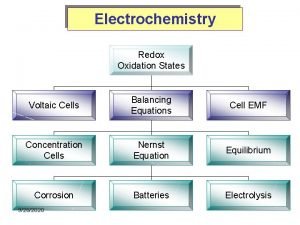
I. Chapter Outline I. III. IV. V. VII. Introduction Balancing Redox Reactions Galvanic Cells Standard Reduction Potentials E°cell, ΔG°, and K Batteries Electrolysis

I. Introduction • Reduction-oxidation (redox) reactions are a huge branch of chemistry. • Basically, they are reactions in which electrons are in motion. • Electrons in motion = electricity. • Concepts in this chapter can be used to explain fuel cells, batteries, electroplating, etc.

I. Redox Reactions • Recall that reduction and oxidation are coupled processes. § Reduction is the gain of electrons. § Oxidation is the loss of electrons. • We identify what is oxidized and what is reduced by examining oxidation numbers.

I. Assigning Oxidation Numbers 1) 2) 3) 4) 5) 6) 7) 8) 9) Atoms in elemental form have O. N. = 0. Charge on a monatomic ion equals its O. N. The sum of all O. N. must equal the total charge. For Group 1, O. N. = +1. For Group 2, O. N. = +2. For H, O. N. = +1 w/ nonmetals, -1 w/ metals and B. For F, O. N. = -1. For O, O. N. = -1 in peroxides and -2 in all others. For Group 17, typically O. N. = -1.

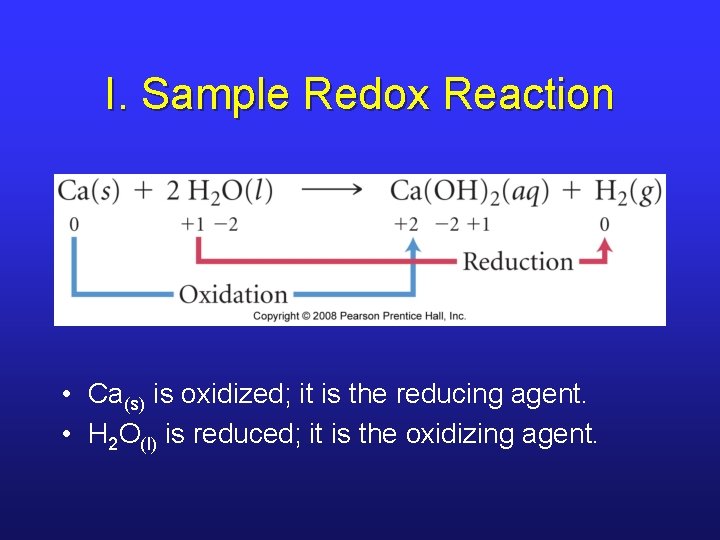
I. Sample Redox Reaction • Ca(s) is oxidized; it is the reducing agent. • H 2 O(l) is reduced; it is the oxidizing agent.

II. Balancing Redox Reactions • Balancing a redox reaction is more involved because mass and charge must both be balanced. • A common way to balance redox reactions is the “half-cell method. ” § In this method, we split the reaction into 2 half reactions: oxidation and reduction. § Each half is balanced separately, and then the two are recombined.

II. The Half-Cell Method 1) Identify what is being oxidized and reduced. 2) Write each half-cell reaction. 3) Balance each half-cell w/ respect to mass: § Balance elements other than O and H. § Balance O by adding H 2 O. § Balance H by adding H+. 4) Balance each half-cell w/ respect to charge by adding e-’s. 5) Make the # of e-’s in each half-cell equal by multiplying by an appropriate factor. 6) Add the two half-cells together. 7) Add required # of OH- to each side and simplify if redox reaction takes place in basic solution.

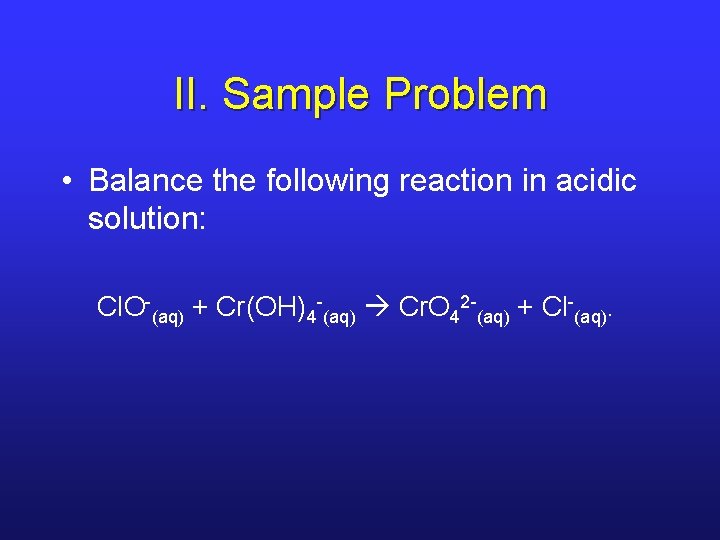
II. Sample Problem • Balance the following reaction in acidic solution: Cl. O-(aq) + Cr(OH)4 -(aq) Cr. O 42 -(aq) + Cl-(aq).

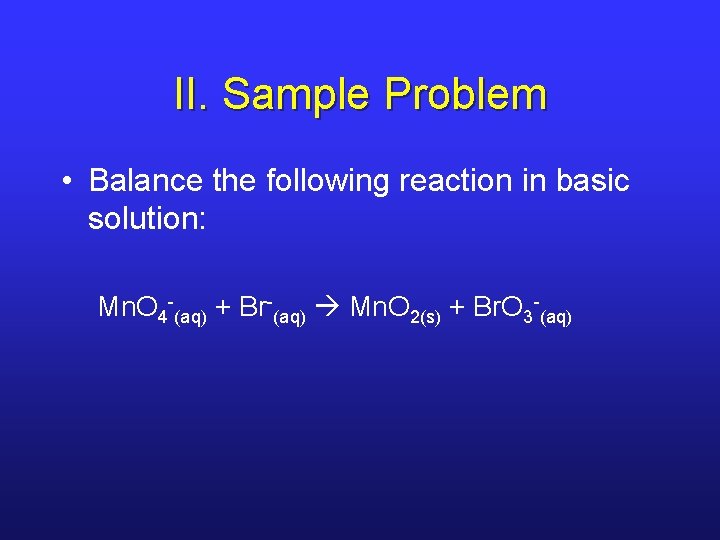
II. Sample Problem • Balance the following reaction in basic solution: Mn. O 4 -(aq) + Br-(aq) Mn. O 2(s) + Br. O 3 -(aq)

II. Inspection Method • A different way to balance redox reactions when you are given all reactants and products already. • e. g. Balance the following reaction. H 2 SO 4 + HCl H 2 S + Cl 2 + H 2 O

II. The Inspection Method 1) Assign oxidation states to all atoms. 2) Identify species in which oxidation states change and write them as half reactions. 3) Balance the atoms that are changing oxidation state in each half reaction. 4) Identify the number of e-’s involved in each half reaction. 5) Multiply the half reaction with the fewer number of e-’s so that the e-’s in each are equal. 6) Without changing these coefficients, balance the rest of the reaction by inspection.

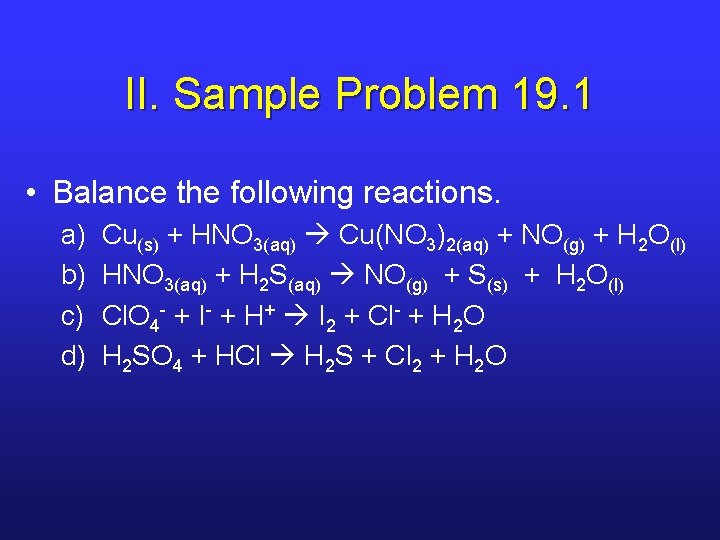
II. Sample Problem 19. 1 • Balance the following reactions. a) b) c) d) Cu(s) + HNO 3(aq) Cu(NO 3)2(aq) + NO(g) + H 2 O(l) HNO 3(aq) + H 2 S(aq) NO(g) + S(s) + H 2 O(l) Cl. O 4 - + I- + H+ I 2 + Cl- + H 2 O H 2 SO 4 + HCl H 2 S + Cl 2 + H 2 O

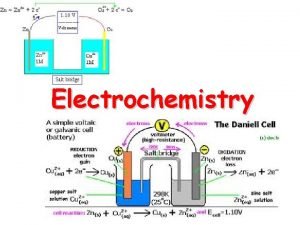
III. Generating Electricity • As stated earlier, electrical current is simply charge in motion. • Since an e- is charged, movement of electrons generates electrical current. • Some redox reaction occur spontaneously. § e. g. Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s)

III. Zn/Cu 2+ Redox • Zn(s) is oxidized to Zn 2+ which goes into solution. • Cu 2+ is reduced to Cu(s) which “plates out. ” • 2 e-’s are transferred from Zn to Cu 2+.

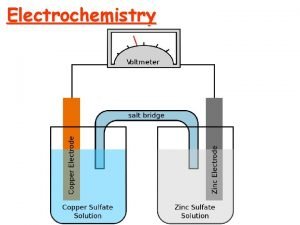
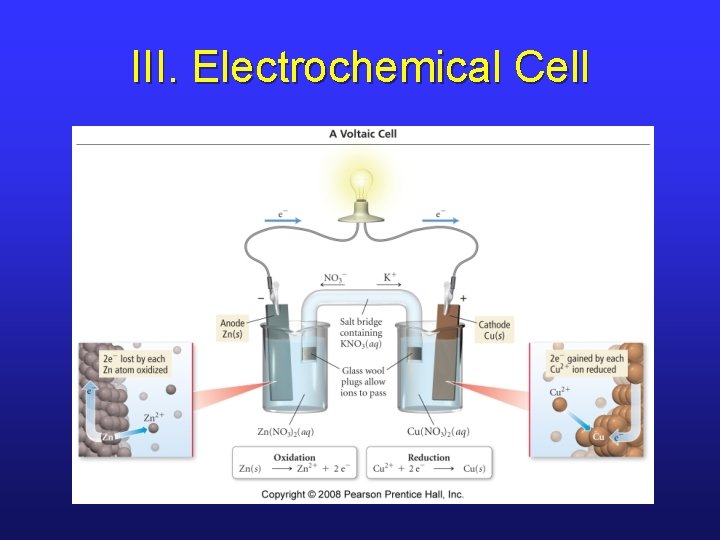
III. Making e-’s Travel • In this arrangement, we don’t really get electrons flowing, more of a transfer. • If we separate the components, we can force electrons to travel externally, and even do work for us. • This kind of setup is called an electrochemical cell.

III. Electrochemical Cell

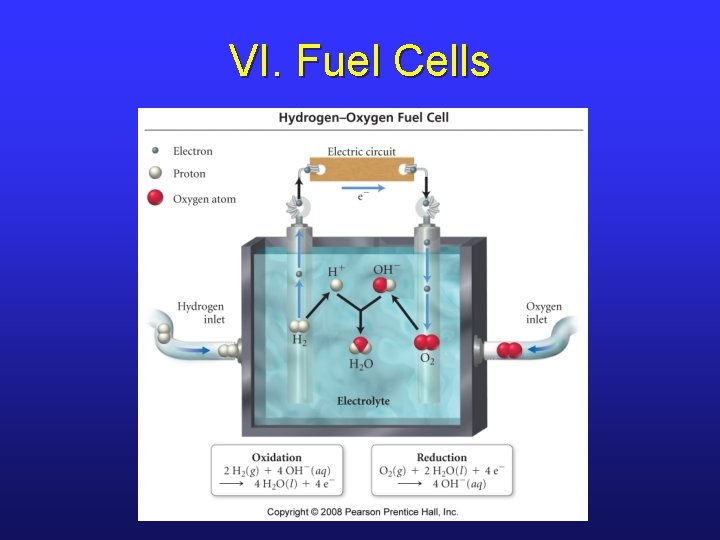
III. Electrochemical Cells • Voltaic (or galvanic) cells produce electrical current from a spontaneous redox reaction. • Important aspects: § Each part is called a half-cell. § Oxidation occurs at the anode. Electrons flow away from the anode. § Reduction occurs at the cathode. Electrons come to the cathode. § A salt bridge is necessary to prevent charge build up and complete the circuit.

III. Current and Potential • Two important aspects about electrons in motion are current and potential. • Electrical current is measured in amperes (A), which has units of coulombs (measure of charge) per second, C/s. 1 A = 1 C/s. • The current is driven by a potential energy difference called the potential difference. § Potential difference is a measure of the difference in PE per unit of charge. § The SI unit is the volt (V). 1 V = 1 J/C.

III. The River Analogy

III. Cell Potentials • The potential difference is the force that drives the movement of e-’s, so it’s also called the electromotive force (emf). • In a voltaic cell, the potential difference between the cathode and anode is called the cell potential (Ecell) or cell emf.

III. Standard Cell Potentials • If the cell is under standard conditions, the cell potential is the standard cell potential (E°cell) or standard emf. • For the Zn(s)/Cu 2+(aq) cell, E°cell = 1. 10 V. • The cell potential is a measure of the tendency of the redox reaction to occur spontaneously.

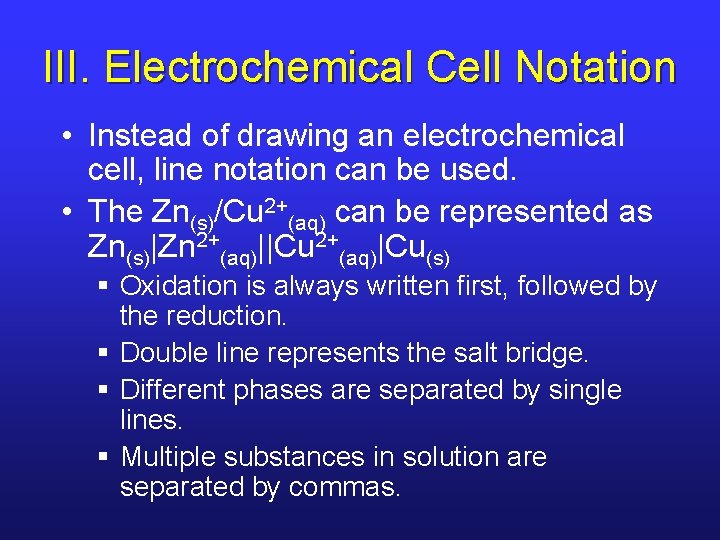
III. Electrochemical Cell Notation • Instead of drawing an electrochemical cell, line notation can be used. • The Zn(s)/Cu 2+(aq) can be represented as Zn(s)|Zn 2+(aq)||Cu 2+(aq)|Cu(s) § Oxidation is always written first, followed by the reduction. § Double line represents the salt bridge. § Different phases are separated by single lines. § Multiple substances in solution are separated by commas.

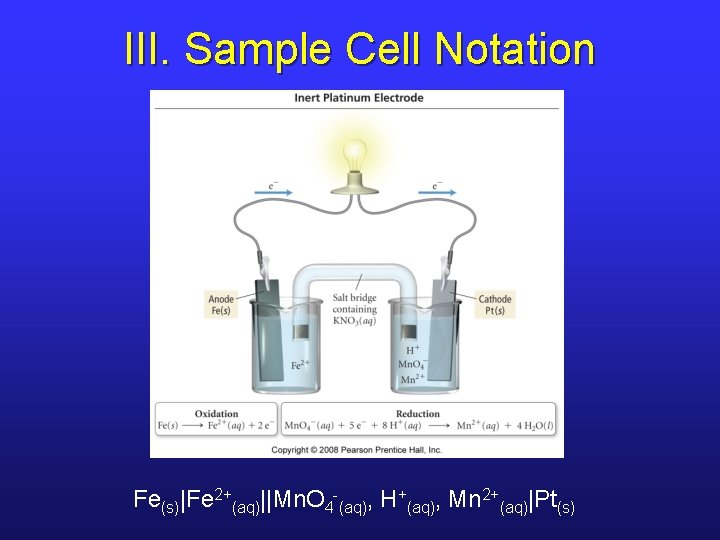
III. Sample Cell Notation Fe(s)|Fe 2+(aq)||Mn. O 4 -(aq), H+(aq), Mn 2+(aq)|Pt(s)

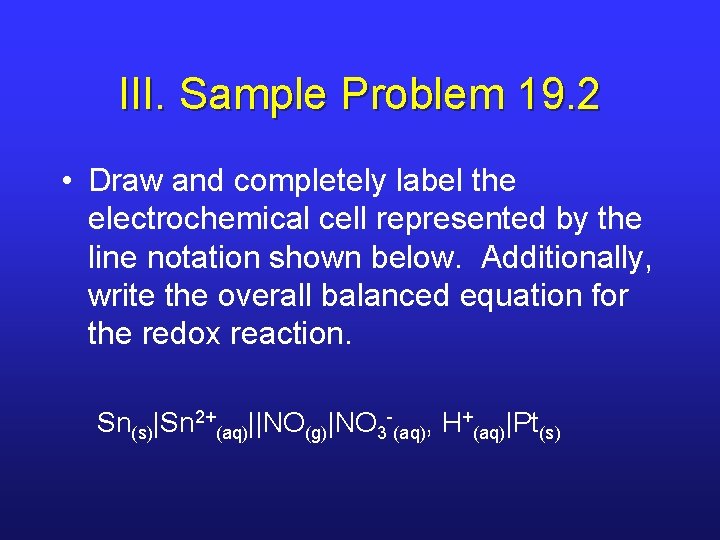
III. Sample Problem 19. 2 • Draw and completely label the electrochemical cell represented by the line notation shown below. Additionally, write the overall balanced equation for the redox reaction. Sn(s)|Sn 2+(aq)||NO(g)|NO 3 -(aq), H+(aq)|Pt(s)

IV. Calculating Cell Potentials • In an electrochemical cell, we can think of each half-cell as having its own potential. • Thus, E°cell is a sum of both half-cell potentials. • The half-cell with the higher potential will occur in the forward direction, forcing the other half-cell to occur in the reverse direction.

IV. Half-Cell Potentials • We can’t create a cell that has only reduction or only oxidation; thus, we can’t measure an absolute value of a half-cell. • We have to assign a particular half-cell a value of 0. 00 V and measure everything else relative to that.

IV. Standard Hydrogen Electrode • The standard hydrogen electrode (SHE) is normally assigned a potential of 0. 00 V. • We attach different half-cells to SHE, watch what happens (red or ox), and whatever potential comes out is assigned to the half-cell.

IV. Measuring a Standard Reduction Potential

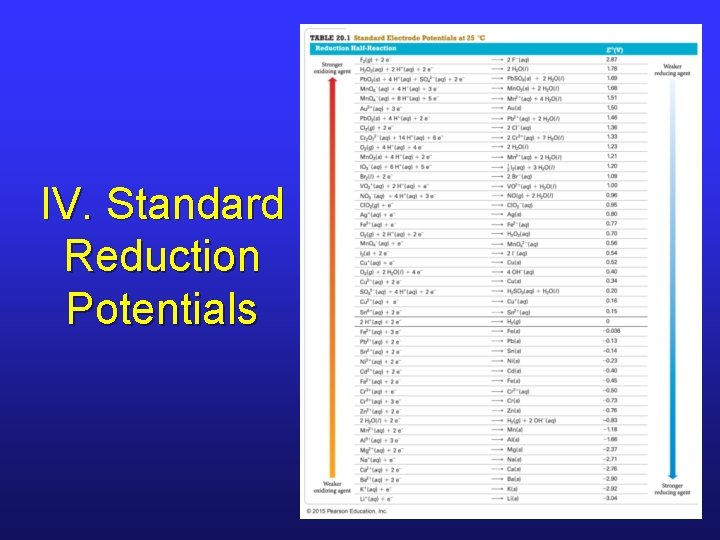
IV. Standard Reduction Potentials

IV. Important Points • The reduction potential of SHE is 0. 00 V. • Half-cells that undergo reduction when attached to SHE have a positive reduction potential. • Half-cells that undergo oxidation when attached to SHE have a negative reduction potential. • Substances at the top have strong tendency to be reduced; they are strong oxidizing agents.

IV. Important Points • Substances at the bottom have strong tendency to be oxidized; they are strong reducing agents. • E°ox = -E°red • For any cell, E°cell = E°ox + E°red. • Never multiply half-cell potentials by coefficients used to balance redox reactions.

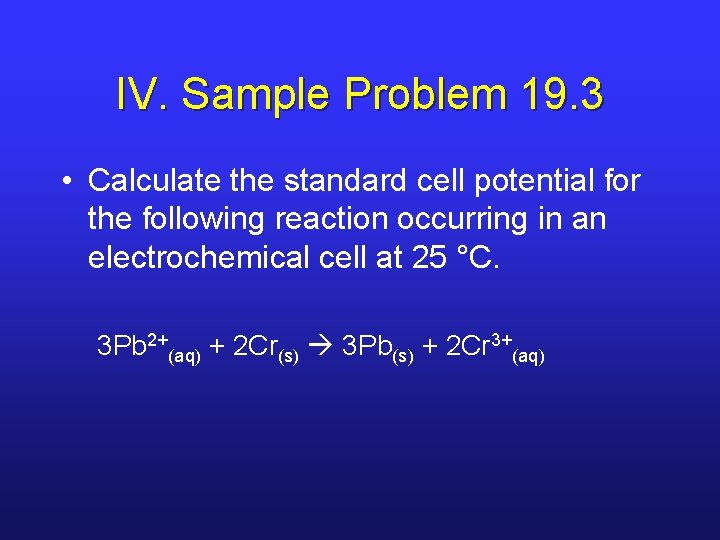
IV. Sample Problem 19. 3 • Calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25 °C. 3 Pb 2+(aq) + 2 Cr(s) 3 Pb(s) + 2 Cr 3+(aq)

IV. Predicting Spontaneous Redox Reactions • Positive cell potentials are spontaneous. • We can identify the half-cells in a reaction, look up their potentials, and sum to find overall cell potentials. • Thus spontaneity can be determined for any redox reaction.

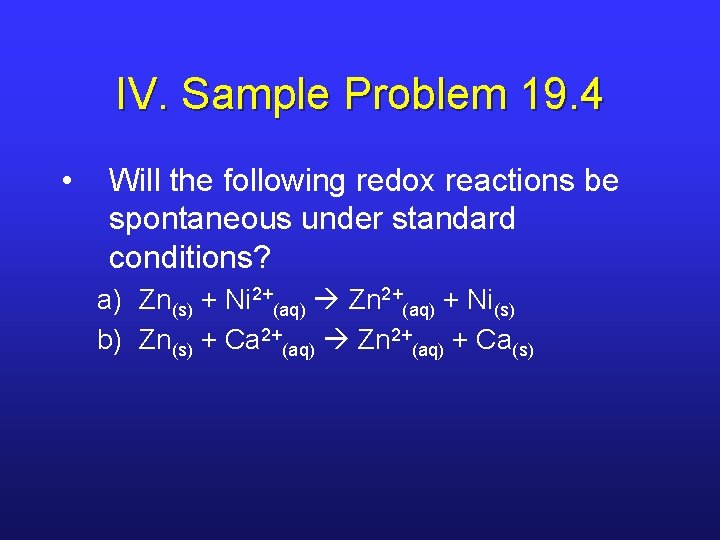
IV. Sample Problem 19. 4 • Will the following redox reactions be spontaneous under standard conditions? a) Zn(s) + Ni 2+(aq) Zn 2+(aq) + Ni(s) b) Zn(s) + Ca 2+(aq) Zn 2+(aq) + Ca(s)

IV. Sample Problem 19. 5 • Determine whether the following metals dissolve in HCl(aq), HNO 3(aq), both, or neither. a) Fe(s) b) Au(s) c) Ag(s)

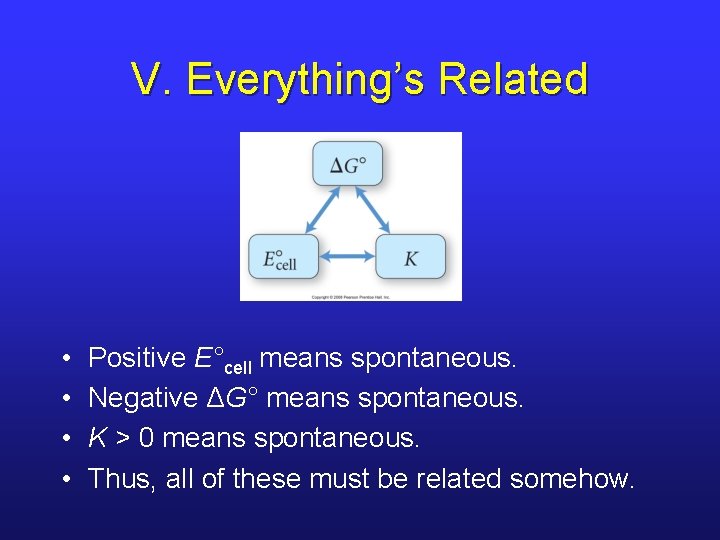
V. Everything’s Related • • Positive E°cell means spontaneous. Negative ΔG° means spontaneous. K > 0 means spontaneous. Thus, all of these must be related somehow.

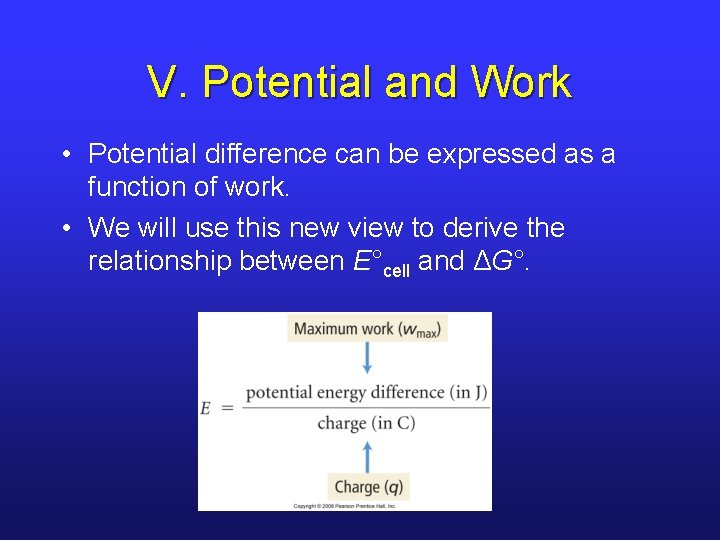
V. Potential and Work • Potential difference can be expressed as a function of work. • We will use this new view to derive the relationship between E°cell and ΔG°.

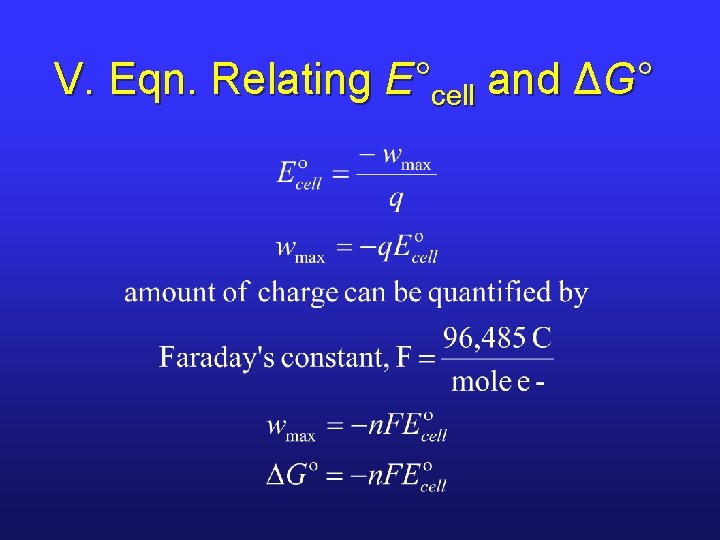
V. Eqn. Relating E°cell and ΔG°

V. Sample Problem 19. 6 • Using tabulated half-cell potentials, calculate ΔG° for the reaction 2 Na(s) + 2 H 2 O(l) H 2(g) + 2 OH-(aq) + 2 Na+(aq).

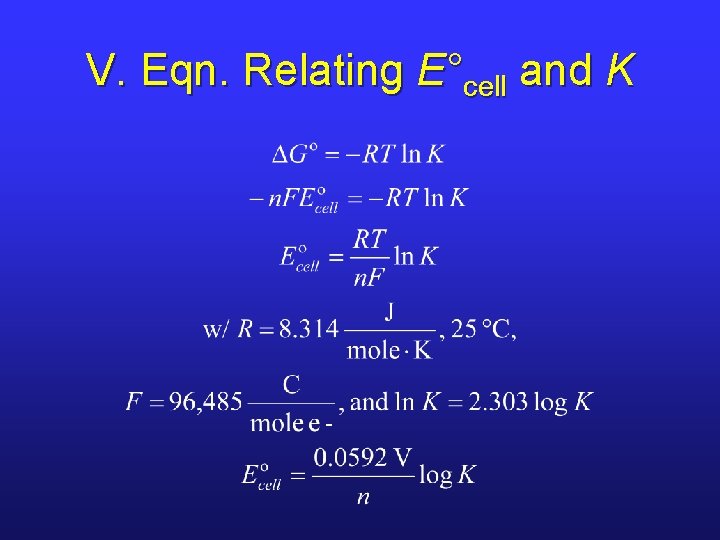
V. Potential and Equilibrium Constants • Using our new equation that relates standard cell potential to standard free energy, we can derive an equation between E°cell and K. • We start with the equation that relates ΔG° to K.

V. Eqn. Relating E°cell and K

V. Sample Problem 19. 7 • Use tabulated half-cell potentials to calculate the equilibrium constant for the two electron oxidation of copper metal by H+.

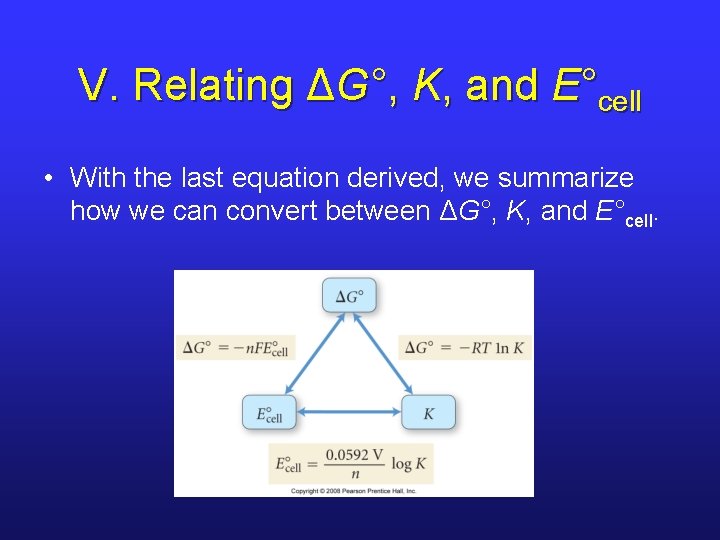
V. Relating ΔG°, K, and E°cell • With the last equation derived, we summarize how we can convert between ΔG°, K, and E°cell.

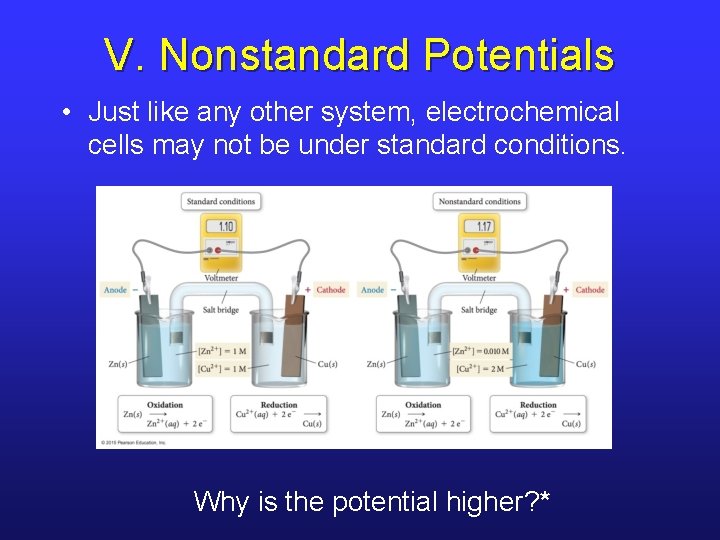
V. Nonstandard Potentials • Just like any other system, electrochemical cells may not be under standard conditions. Why is the potential higher? *

V. Calculating Nonstandard Cell Potentials • Although we can qualitatively predict whether Ecell is higher or lower than E°cell, we’d like to calculate an exact value. • We can derive an equation starting with the nonstandard ΔG equation.

V. Deriving the Nernst Equation

V. Nernst Eqn. Generalizations • Under standard conditions, log Q = log 1 = 0, so Ecell will equal E°cell. • If Q < 1, there are more reactants than products, so redox reaction shifts right; Ecell will be > than E°cell. • If Q > 1, there are more products than reactants, so redox reaction shifts left; Ecell will be < than E°cell. • If Q = K, then Ecell = 0.

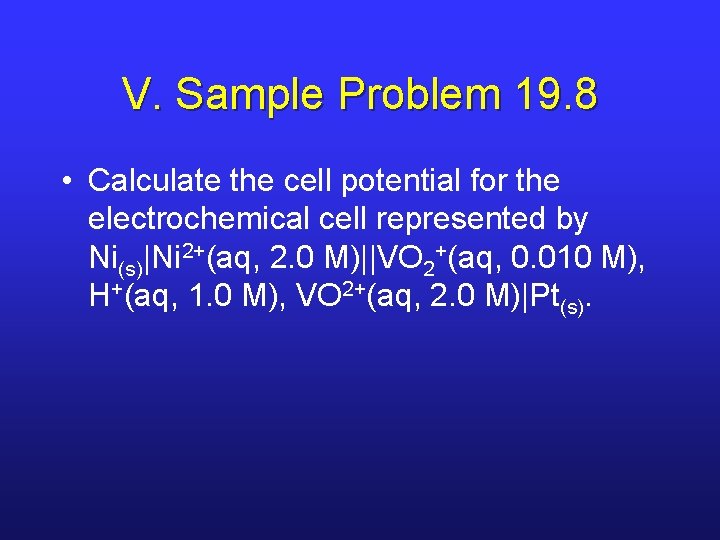
V. Sample Problem 19. 8 • Calculate the cell potential for the electrochemical cell represented by Ni(s)|Ni 2+(aq, 2. 0 M)||VO 2+(aq, 0. 010 M), H+(aq, 1. 0 M), VO 2+(aq, 2. 0 M)|Pt(s).

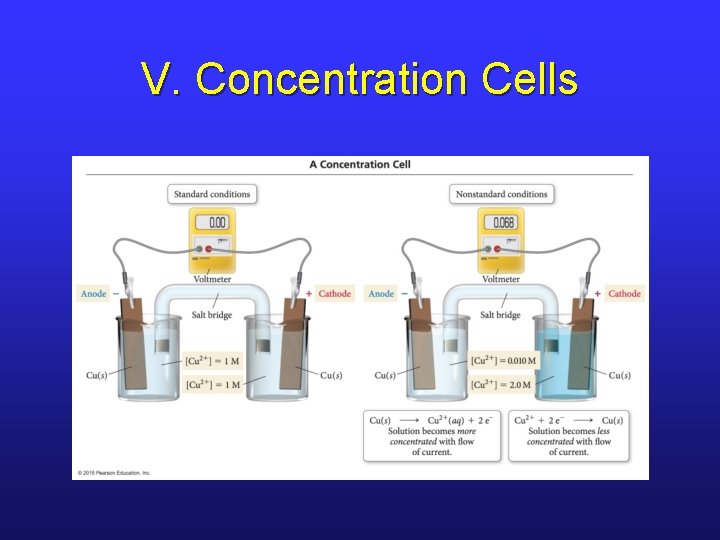
V. Concentration Cells

V. Concentration Cells • If the two half-cells are the same, there is no reason to reduce something on one side and oxidize the same thing on the other side. • However, if there are [ ] differences, there is a push to get to equilibrium. • Electrons flow in order to increase the [ ] of the dilute cell and decrease the [ ] of the concentrated cell.

VI. Using Electrochemistry • If constructed correctly, electrochemical cells can be used to store and deliver electricity. • We briefly look at dry-cell batteries, lithium ion batteries, and fuel cells.

VI. Dry-cell Batteries • Called dry-cell because there’s very little water. • Voltage derived from the oxidation of Zn(s) and the reduction of Mn. O 2(s). • In acidic battery, Mn. O 2(s) reduced to Mn 2 O 3(s). • In alkaline battery, Mn. O 2(s) reduced to Mn. O(OH)(s).

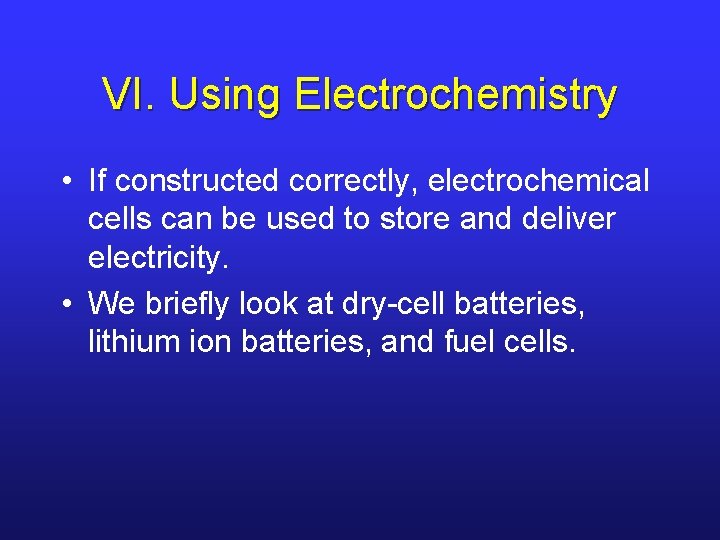
VI. Lithium Ion Batteries • This rechargeable battery works a bit differently. • Motion of Li+ from anode to cathode causes e-’s to flow externally and reduce the transition metal. • Battery is recharged by oxidizing the transition metal in the cathode.

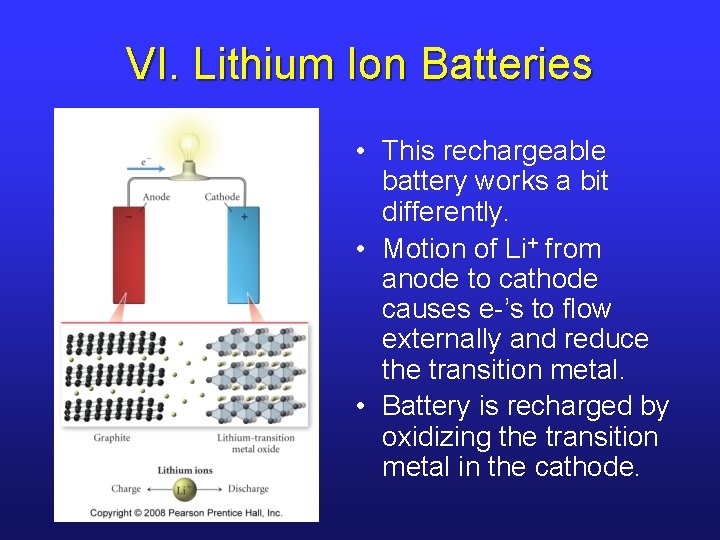
VI. Fuel Cells

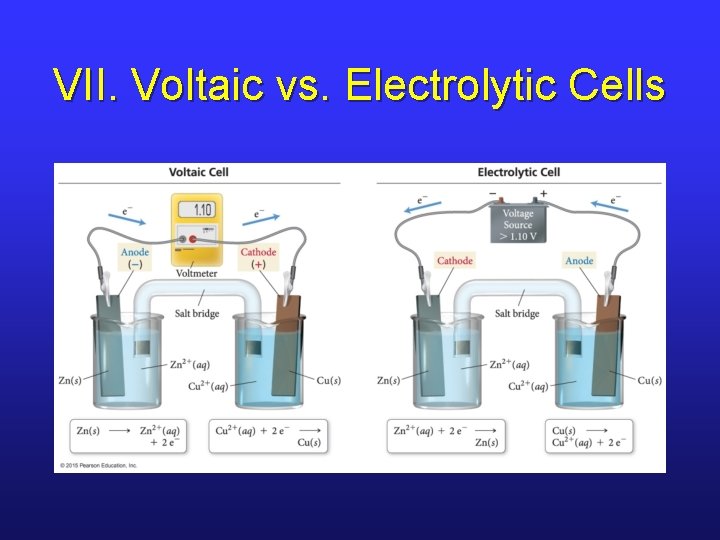
VII. Nonspontaneous Redox Reactions • Nonspontaneous reactions can be forced to occur by inputting energy. • Nonspontaneous redox reactions can be forced to occur by inputting energy in the form of electrical current. • This type of electrochemical cell is called an electrolytic cell.

VII. Voltaic vs. Electrolytic Cells

VII. Predicting Products • In an aqueous electrolytic cell, it’s possible that H 2 O can be reduced or oxidized. • To predict products, must consider potentials of all processes that could occur. • Processes that are easiest (least negative or most positive half-cell potential) will occur.

VII. Overvoltage • There is one problem in predicting electrolysis products. • Some half-cell reactions don’t occur at their expected voltage potentials! • e. g. 2 H 2 O(l) O 2(g) + 4 H+(aq) + 4 e- has Eox = -0. 82 V when [H+] = 1 x 10 -7 M. § However, kinetic factors require a voltage of 1. 40 V for this to occur.

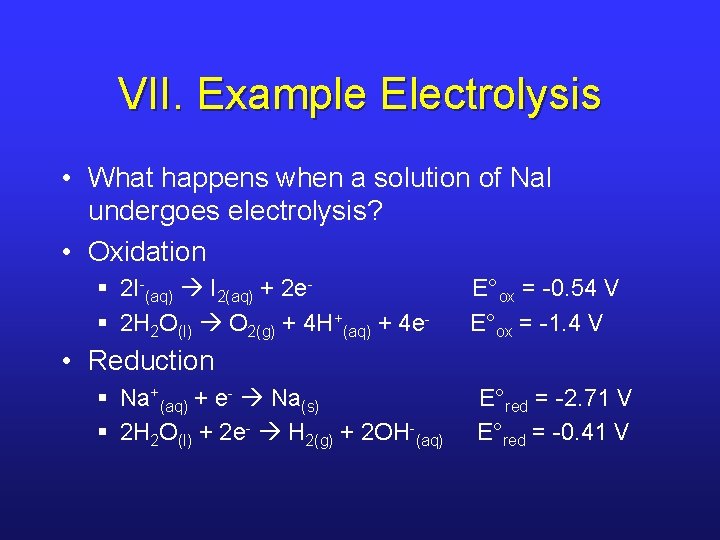
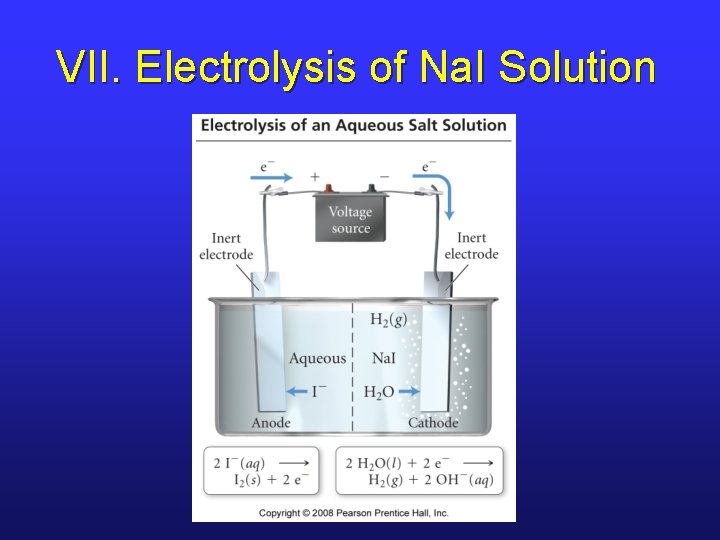
VII. Example Electrolysis • What happens when a solution of Na. I undergoes electrolysis? • Oxidation § 2 I-(aq) I 2(aq) + 2 e§ 2 H 2 O(l) O 2(g) + 4 H+(aq) + 4 e- E°ox = -0. 54 V E°ox = -1. 4 V • Reduction § Na+(aq) + e- Na(s) § 2 H 2 O(l) + 2 e- H 2(g) + 2 OH-(aq) E°red = -2. 71 V E°red = -0. 41 V

VII. Electrolysis of Na. I Solution

VII. Electrolysis Stoichiometry • The # of electrons in any redox reaction can be used as a stoichiometric ratio. • If we know how long an electrolysis takes place, and the magnitude of current that flowed, we can do stoichiometric calculations. • Important relationships: § 1 A = 1 C/s § F = 96, 485 C/mole e-

VII. Sample Problem 19. 9 • Copper can be plated out of a solution containing Cu 2+ according to the halfreaction: Cu 2+(aq) + 2 e- Cu(s). How long (in minutes) will it take to plate 10. 0 g of copper using a current of 2. 0 A?
 Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Ap chem electrochemistry review
Ap chem electrochemistry review Danielle cell
Danielle cell Electrochemistry balancing equations
Electrochemistry balancing equations Electrochemistry stoichiometry
Electrochemistry stoichiometry Ap chemistry electrochemistry
Ap chemistry electrochemistry What is electrochemistry in chemistry
What is electrochemistry in chemistry Electrochemistry tutorial
Electrochemistry tutorial Balancing redox reactions
Balancing redox reactions Electrochemistry balancing equations calculator
Electrochemistry balancing equations calculator Chapter 20 electrochemistry
Chapter 20 electrochemistry Electrochemistry
Electrochemistry What is e in nernst equation
What is e in nernst equation Electrochemistry lesson
Electrochemistry lesson Electrolysis table
Electrolysis table Khan academy electrolysis
Khan academy electrolysis Whats electrochemistry
Whats electrochemistry Intro to electrochemistry
Intro to electrochemistry Mass transport electrochemistry
Mass transport electrochemistry Cell notation
Cell notation Transport number chemistry
Transport number chemistry Cell chapter 21
Cell chapter 21 Electrochemistry
Electrochemistry Diagonal rule electrochemistry
Diagonal rule electrochemistry Is electrochemistry
Is electrochemistry Fundamentals of electrochemistry
Fundamentals of electrochemistry Ir drop
Ir drop Red cat electrochemistry
Red cat electrochemistry Introduction of electrochemistry
Introduction of electrochemistry Diagonal rule electrochemistry
Diagonal rule electrochemistry Junction potential
Junction potential Equilibrium constant formula electrochemistry
Equilibrium constant formula electrochemistry Applications of electrolytic cell
Applications of electrolytic cell Ir drop in electrochemistry
Ir drop in electrochemistry Electrochemistry balancing equations
Electrochemistry balancing equations Slidesdgo
Slidesdgo What is electrochemistry
What is electrochemistry Complex redox reactions
Complex redox reactions Spontaneity electrochemistry
Spontaneity electrochemistry Fundamentals of electrochemistry
Fundamentals of electrochemistry Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test What is polarization in electrochemistry
What is polarization in electrochemistry Interfacial method in electrochemistry
Interfacial method in electrochemistry Chem
Chem Chem pharma impex
Chem pharma impex Sig fogs
Sig fogs Photoelectron spectroscopy pogil
Photoelectron spectroscopy pogil Chem
Chem Chem 30 equilibrium
Chem 30 equilibrium Environics chempro x
Environics chempro x Chem
Chem Chem feed
Chem feed Astex viewer
Astex viewer Hkale chemistry
Hkale chemistry January 2012 chemistry regents answers
January 2012 chemistry regents answers Ap chem equilibrium
Ap chem equilibrium Chem lab
Chem lab Www.chem.purdue/gchelp/atoms/elements.html
Www.chem.purdue/gchelp/atoms/elements.html Topic 5 chemistry ib
Topic 5 chemistry ib Ario acronym chemistry
Ario acronym chemistry Chem 494
Chem 494