Ch 10 Liquids and Solids Dr Namphol Sinkaset















- Slides: 43

Ch. 10: Liquids and Solids Dr. Namphol Sinkaset Chem 200: General Chemistry I

I. Chapter Outline I. III. IV. Introduction Intermolecular Forces Phase Transitions Phase Diagrams

I. Condensed States • Liquids and solids are the condensed states because of the close proximity of atoms/molecules to one another. • This proximity leads to much more frequent interactions than in gases. • Interactions depend on chemical identity of the substance and determine many physical properties.

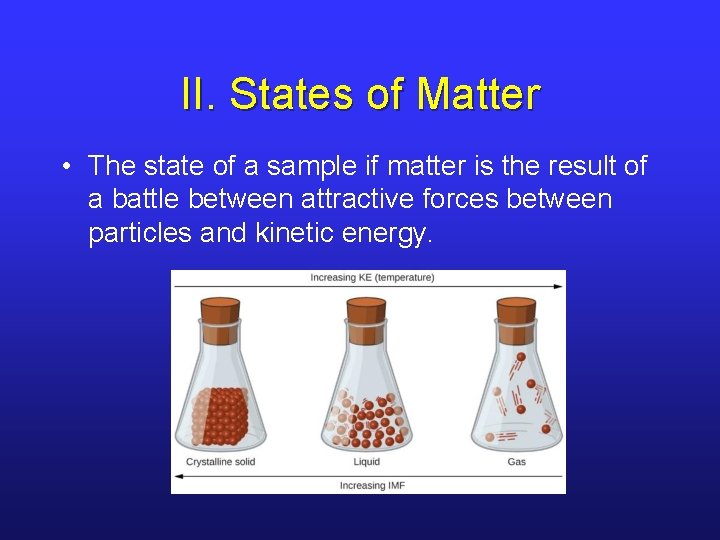
II. States of Matter • The state of a sample if matter is the result of a battle between attractive forces between particles and kinetic energy.

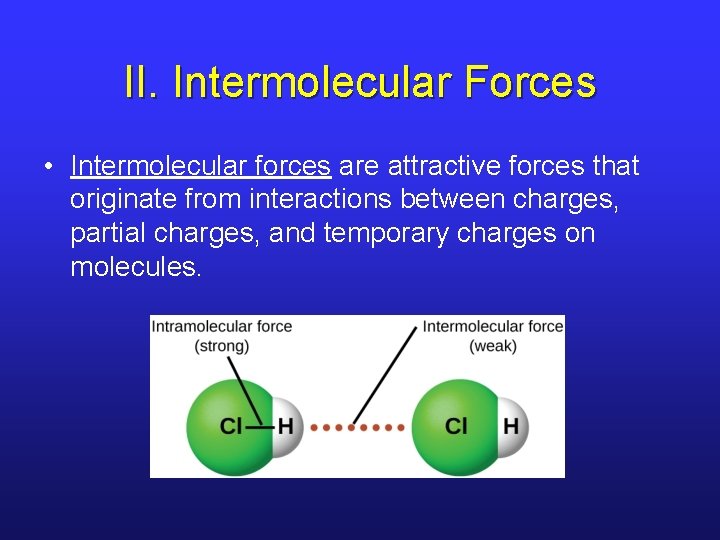
II. Electrostatic Forces • Every molecule in a sample of matter experiences two types of electrostatic forces. § Intramolecular forces: the forces that exist within the molecule (bonding). These forces determine chemical reactivity. § Intermolecular forces: the forces that exist between molecules. These forces determine physical properties.

II. Intermolecular Forces • Intermolecular forces are attractive forces that originate from interactions between charges, partial charges, and temporary charges on molecules.

II. Types of IM Forces • There are different kinds of IM forces, each with a different level of strength. § Dispersion forces § Dipole-dipole attractions § *Hydrogen “bonding”

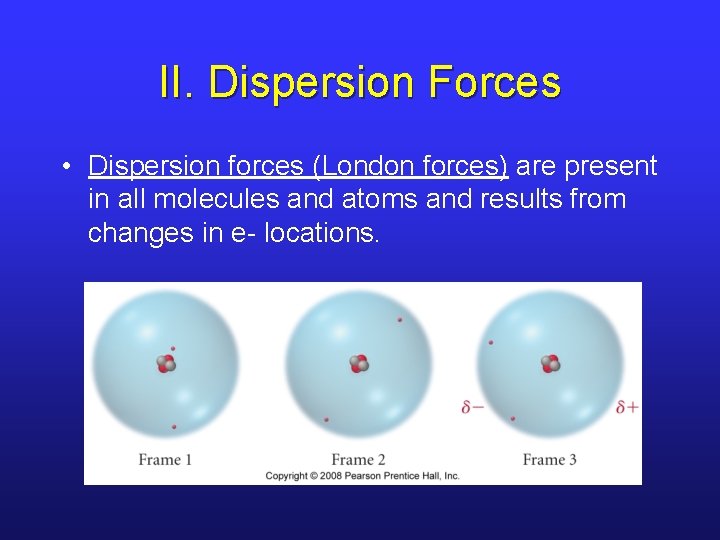
II. Dispersion Forces • Dispersion forces (London forces) are present in all molecules and atoms and results from changes in e- locations.

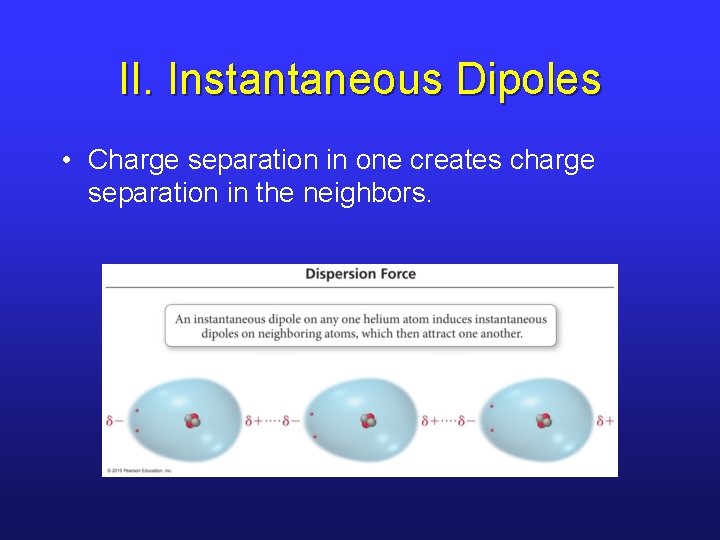
II. Instantaneous Dipoles • Charge separation in one creates charge separation in the neighbors.

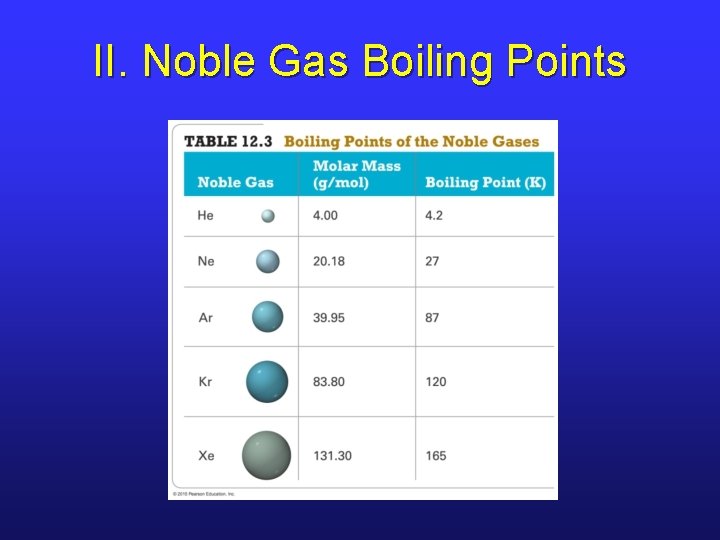
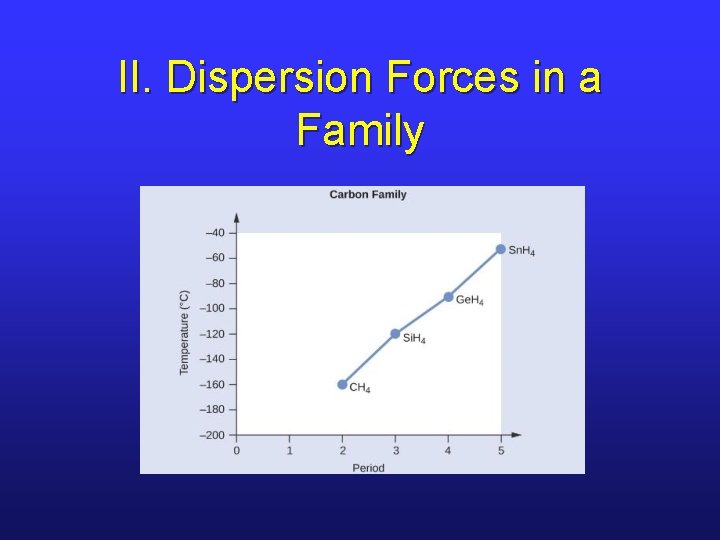
II. Dispersion Force Strength • The ease with which e-’s can move in response to an external charge is known as polarizability. • Large atoms with large electron clouds tend to have stronger dispersion forces. • Larger molecules tend to have stronger dispersion forces.

II. Noble Gas Boiling Points

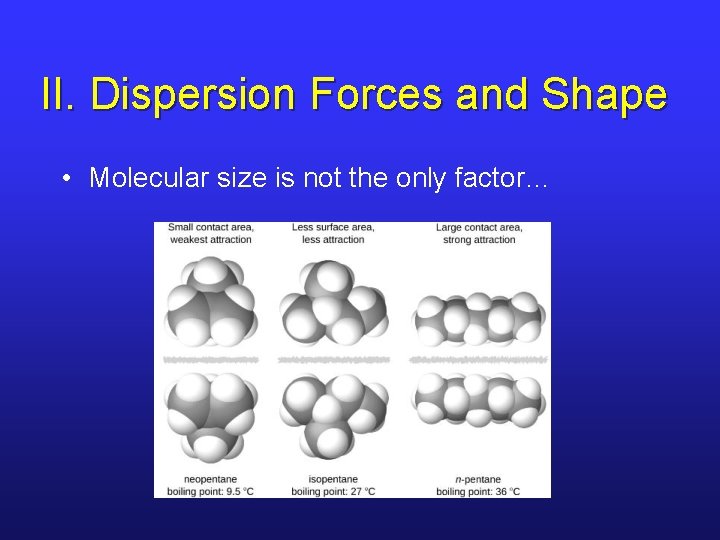
II. Dispersion Forces and Shape • Molecular size is not the only factor…

II. Dispersion Forces in a Family

II. Dispersion Forces in Action

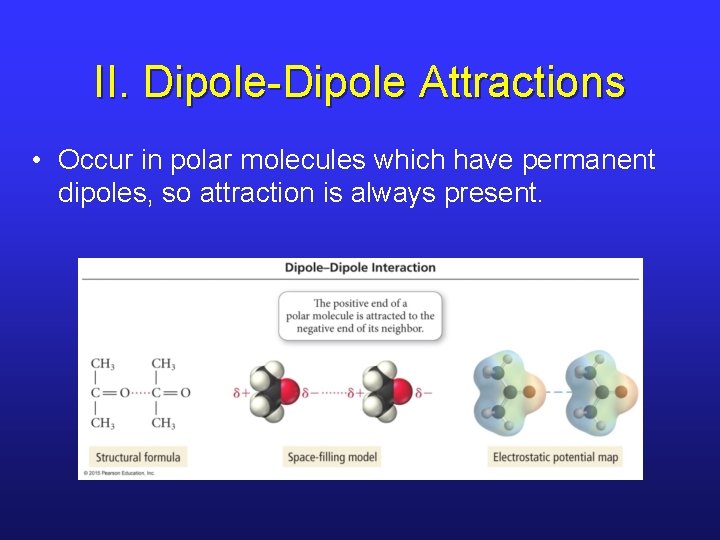
II. Dipole-Dipole Attractions • Occur in polar molecules which have permanent dipoles, so attraction is always present.

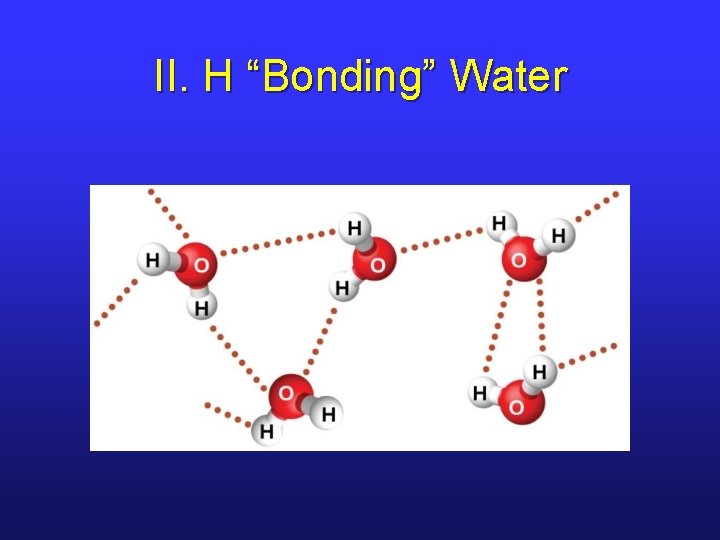
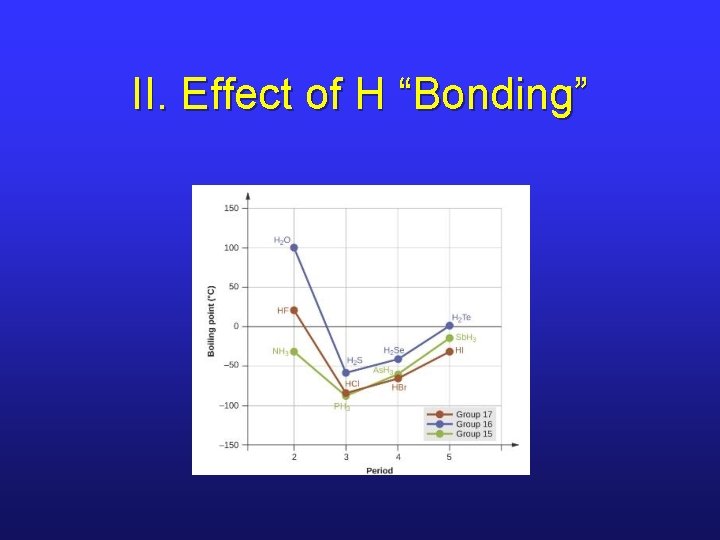
II. Hydrogen “Bonding” • This IM force is a misnomer since it’s not an actual bond. • Occurs between molecules in which H is bonded to a highly electronegative element (N, O, F), leading to high partial positive and partial negative charges. • It’s a “super” dipole-dipole attraction.

II. H “Bonding” Water

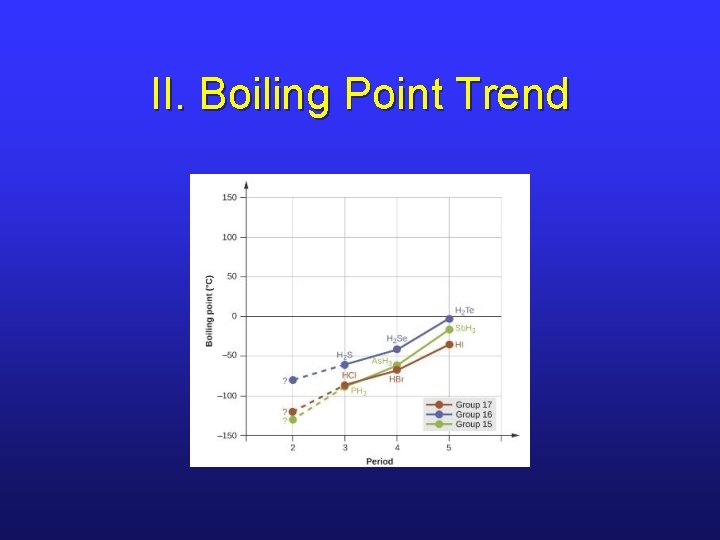
II. Boiling Point Trend

II. Effect of H “Bonding”

II. Sample Problem • Which substance has the highest boiling point and why? a) CH 3 OH b) CO c) N 2

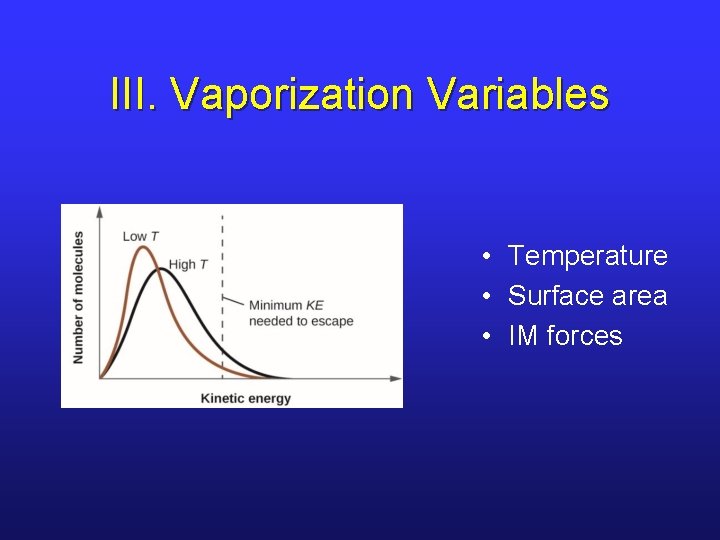
III. Vaporization and IM Forces • From experience, we know that water evaporates in an open container. • What factors influence rate of vaporization?

III. Vaporization Variables • Temperature • Surface area • IM forces

III. Energetics of Vaporization • As molecules evaporate, what happens to the temperature of the samples left in the beaker? • Vaporization is an endothermic process – it’s the reason why we sweat when we get too hot. • Condensation is an exothermic process.

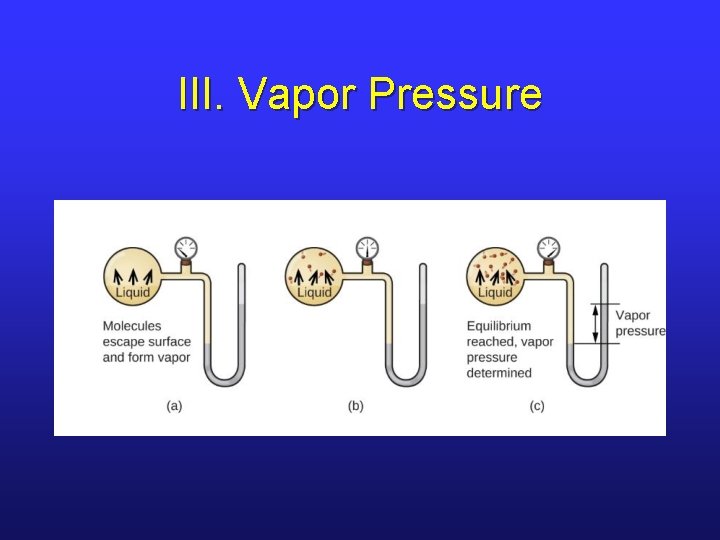
III. Dynamic Equilibrium • In an open flask, a liquid will eventually evaporate away. • What about a closed flask?

III. Vapor Pressure

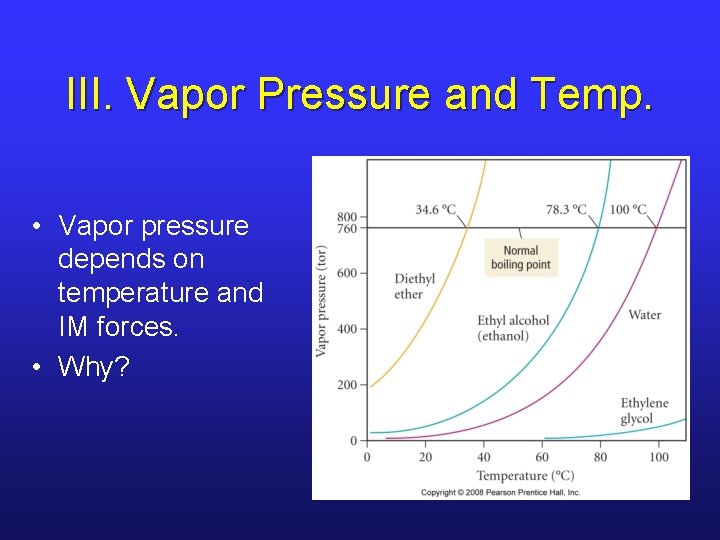
III. Vapor Pressure and Temp. • Vapor pressure depends on temperature and IM forces. • Why?

III. Boiling Point • When T is increased, the vapor pressure increases due to the higher # of molecules that can break away and enter gas phase. • What if all molecules have necessary thermal energy? • At this point, vapor pressure = external pressure, and boiling point is reached. • The temperature at which vapor pressure equals 1 atm is the normal boiling point.

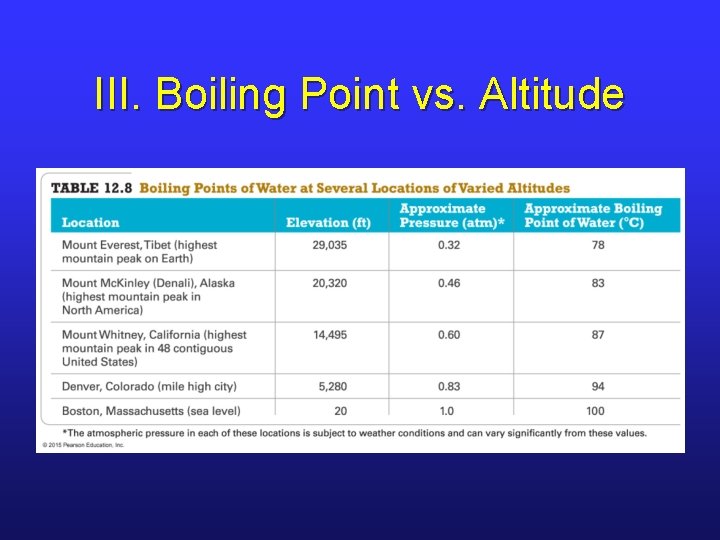
III. Boiling Point vs. Altitude

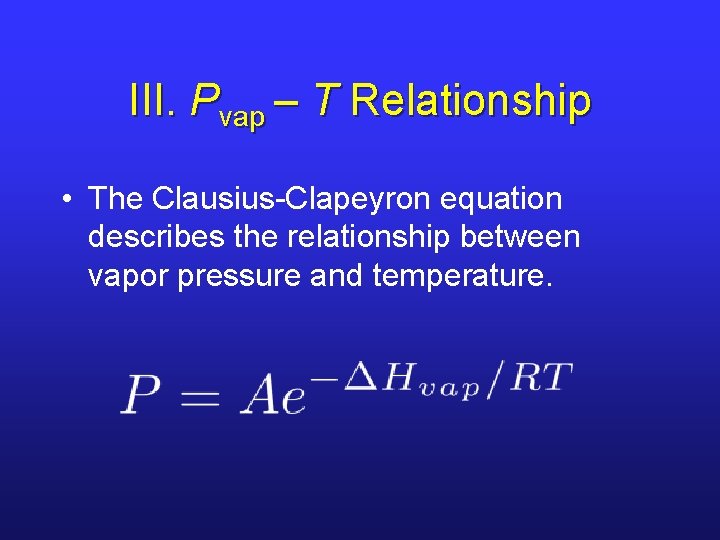
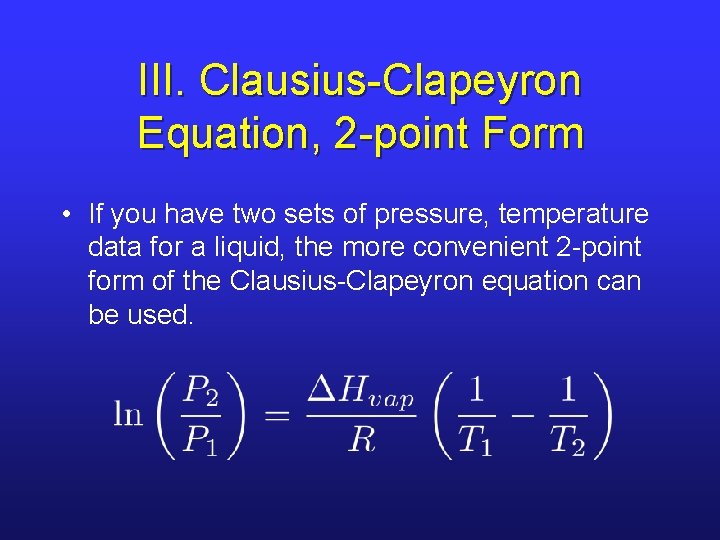
III. Pvap – T Relationship • The Clausius-Clapeyron equation describes the relationship between vapor pressure and temperature.

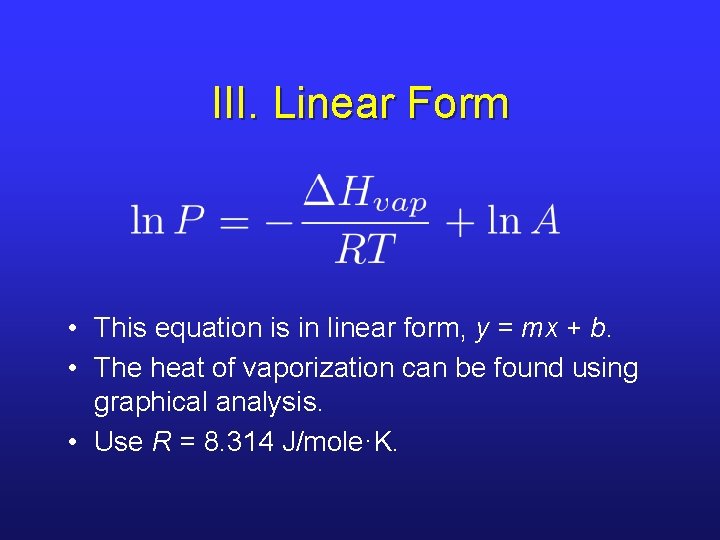
III. Linear Form • This equation is in linear form, y = mx + b. • The heat of vaporization can be found using graphical analysis. • Use R = 8. 314 J/mole·K.

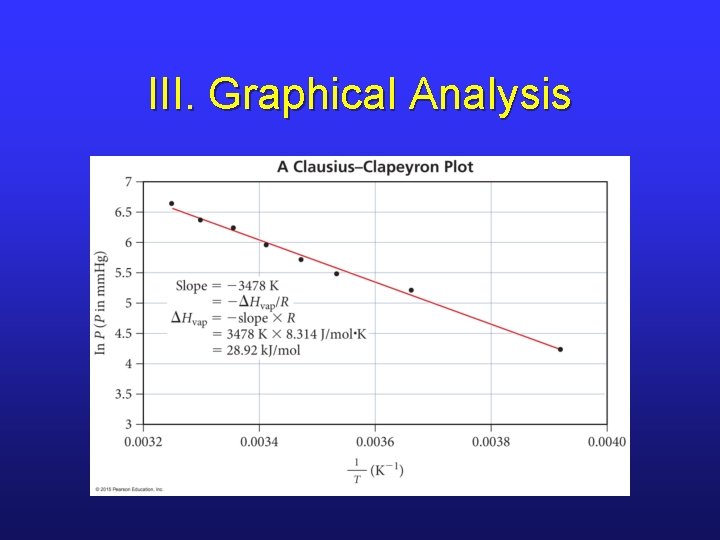
III. Graphical Analysis

III. Clausius-Clapeyron Equation, 2 -point Form • If you have two sets of pressure, temperature data for a liquid, the more convenient 2 -point form of the Clausius-Clapeyron equation can be used.

III. Sample Problem • Propane has a normal boiling point of -4. 20 °C and a heat of vaporization of 19. 04 k. J/mole. What is the vapor pressure of propane at 25. 0 °C?

III. Other Phase Changes • Sublimation is the direct conversion of particles from the solid phase to the gas phase. § Average KE is low, but always some that have enough KE to break away. • Fusion is the conversion of solid to liquid. • Also have deposition and freezing.

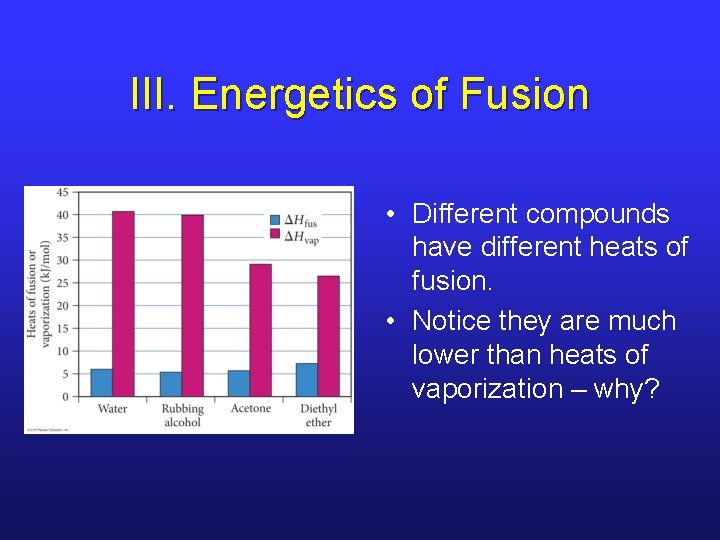
III. Energetics of Fusion • Different compounds have different heats of fusion. • Notice they are much lower than heats of vaporization – why?

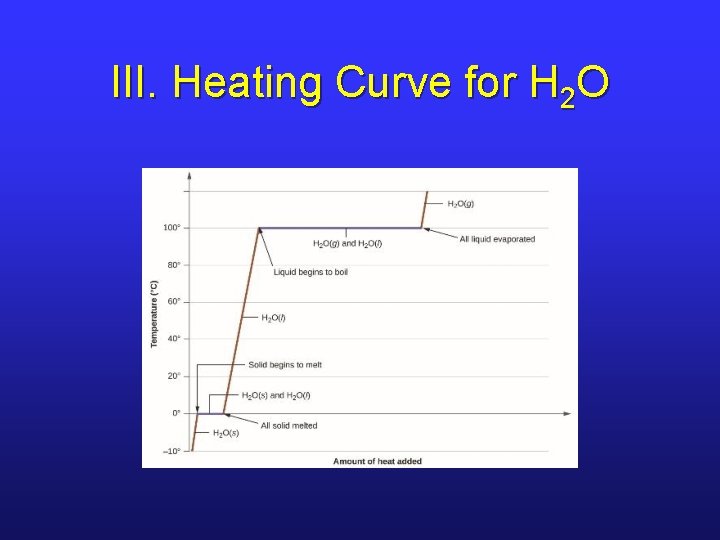
III. Energies of Phase Changes • The enthalpies involved in a phase change depends on the amount of substance and the substance itself. • We look at a heating curve for H 2 O at 1. 00 atm pressure. • Note that there are sloping regions and flat regions in the curve. (Why? )

III. Heating Curve for H 2 O

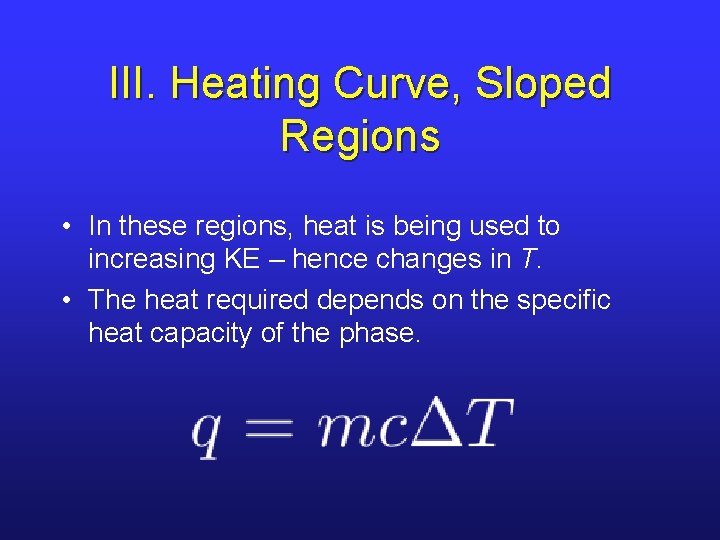
III. Heating Curve, Sloped Regions • In these regions, heat is being used to increasing KE – hence changes in T. • The heat required depends on the specific heat capacity of the phase.

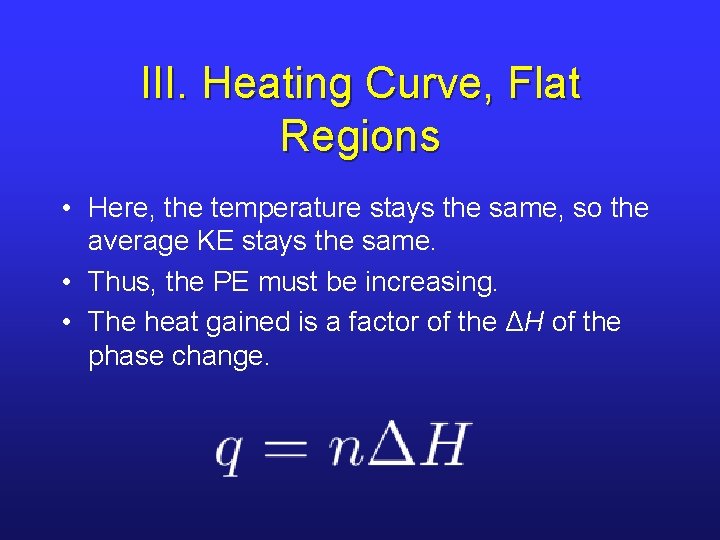
III. Heating Curve, Flat Regions • Here, the temperature stays the same, so the average KE stays the same. • Thus, the PE must be increasing. • The heat gained is a factor of the ΔH of the phase change.

IV. Phase Diagrams • Measurements of phase transitions over a variety of different temperatures and pressures are used to construct phase diagrams. • Phase diagrams allow predictions of the phase in which a substance will exist under specific conditions.

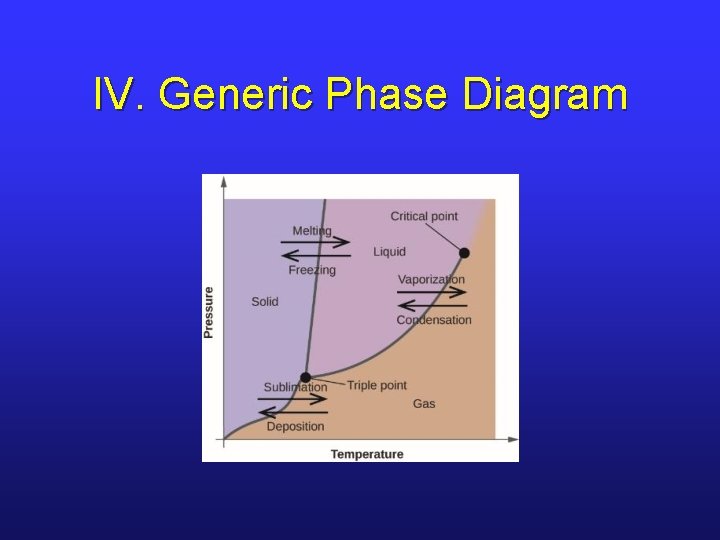
IV. Generic Phase Diagram

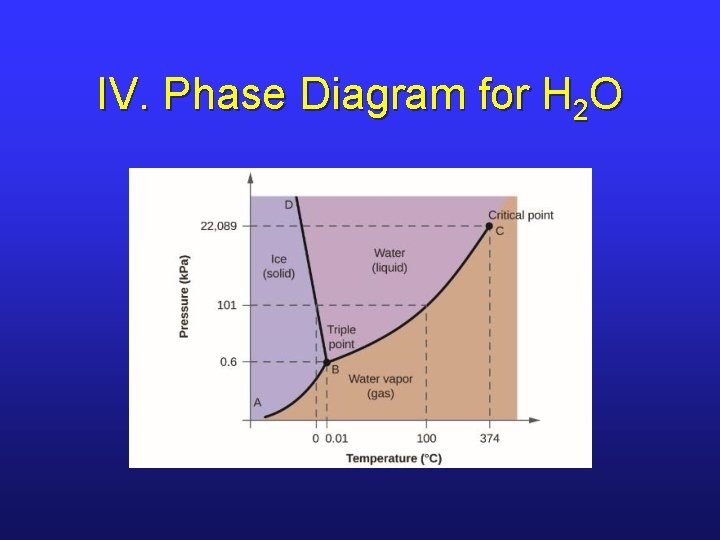
IV. Phase Diagram for H 2 O

IV. The Critical Point • In a sealed container, as T of liquid is heated, more and more vapor is formed, and P increases. • At the critical temperature, a supercritical fluid forms; liquid can’t exist above this temperature.
 Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Solids liquids and gases section 2 properties of fluids
Solids liquids and gases section 2 properties of fluids Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Properties of solid
Properties of solid States of matter diagram
States of matter diagram The properties of solids liquids and gases
The properties of solids liquids and gases Solid liquid gas examples
Solid liquid gas examples Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Liquids and solids menu
Liquids and solids menu Lesson outline lesson 1 solids liquids and gases answer key
Lesson outline lesson 1 solids liquids and gases answer key Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Kesler science.com
Kesler science.com Red liquid element
Red liquid element How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Liquid information
Liquid information Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Why are gases easier to compress than solids or liquids?
Why are gases easier to compress than solids or liquids? Filtering solids from liquids
Filtering solids from liquids The liquids that form the foundation of sauces and soups
The liquids that form the foundation of sauces and soups Thickened liquids that complement other foods
Thickened liquids that complement other foods Molecular theory of gases and liquids
Molecular theory of gases and liquids Liquids dielectrics are mainly used as
Liquids dielectrics are mainly used as Miscible and immiscible liquids worksheet
Miscible and immiscible liquids worksheet Rocks changed by temperature pressure and hot liquids
Rocks changed by temperature pressure and hot liquids Properties of liquid matter
Properties of liquid matter What are solids
What are solids Ionic liquids green chemistry ppt
Ionic liquids green chemistry ppt Class k fire
Class k fire Phonetic transcription
Phonetic transcription The monophasic liquid are prepared with which solvent
The monophasic liquid are prepared with which solvent Baking liquid
Baking liquid Pull examples
Pull examples Why are liquids incompressible
Why are liquids incompressible Viscosity of liquids
Viscosity of liquids Miscible and immiscible definitions
Miscible and immiscible definitions Why are liquids incompressible
Why are liquids incompressible Long pile and alternate shovel method
Long pile and alternate shovel method Metal that liquifies at room temperature
Metal that liquifies at room temperature Kinetic molecular theory of liquids
Kinetic molecular theory of liquids