Ch 11 Solutions and Colloids Dr Namphol Sinkaset

























- Slides: 57

Ch. 11: Solutions and Colloids Dr. Namphol Sinkaset Chem 201: General Chemistry II

I. Chapter Outline I. III. IV. V. VI. Introduction Solution Formation Electrolytes Solubility Solution Concentration Colligative Properties

I. Solutions • solution: homogeneous mixture of 2 or more substances or components • solute: substance present in smaller amount • solvent: substance present in greater amount

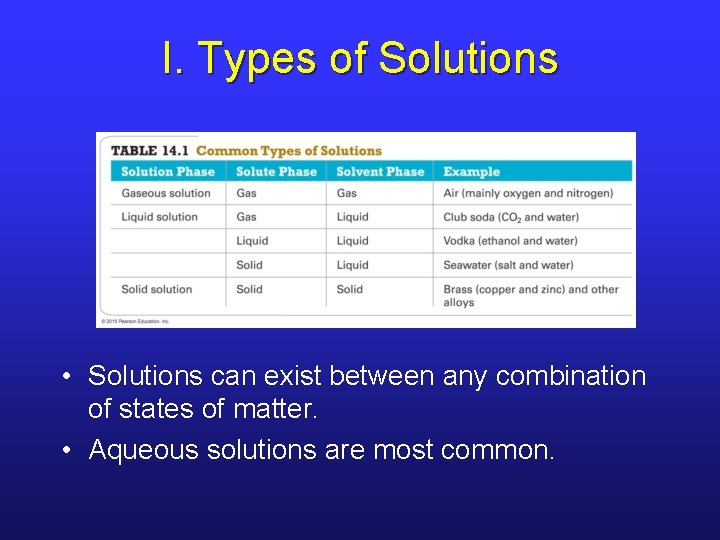
I. Types of Solutions • Solutions can exist between any combination of states of matter. • Aqueous solutions are most common.

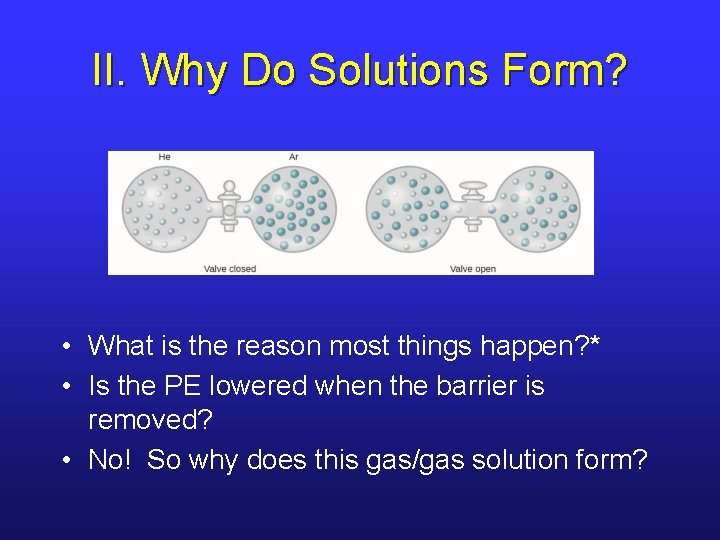
II. Why Do Solutions Form? • What is the reason most things happen? * • Is the PE lowered when the barrier is removed? • No! So why does this gas/gas solution form?

II. Spontaneous Processes • Solution formation is an example of a process that occurs under specific conditions w/out the need of outside energy. • Two things favor (but don’t guarantee) spontaneity: § A decrease in internal energy of the system § An increase in entropy of the system

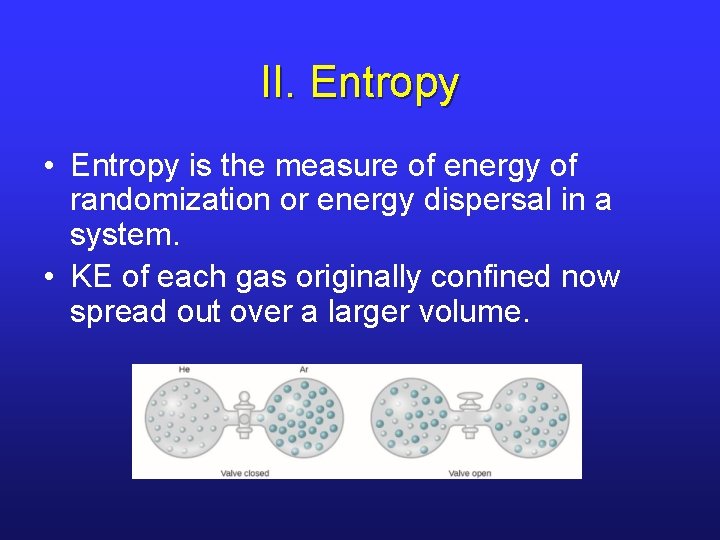
II. Entropy • Entropy is the measure of energy of randomization or energy dispersal in a system. • KE of each gas originally confined now spread out over a larger volume.

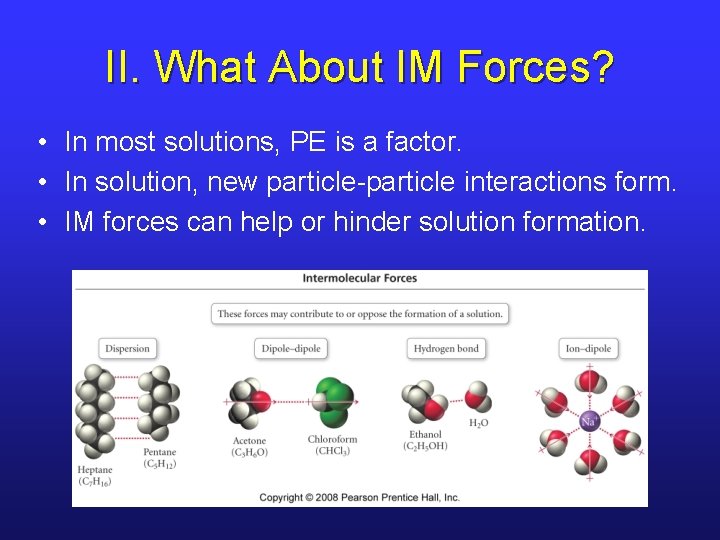
II. What About IM Forces? • In most solutions, PE is a factor. • In solution, new particle-particle interactions form. • IM forces can help or hinder solution formation.

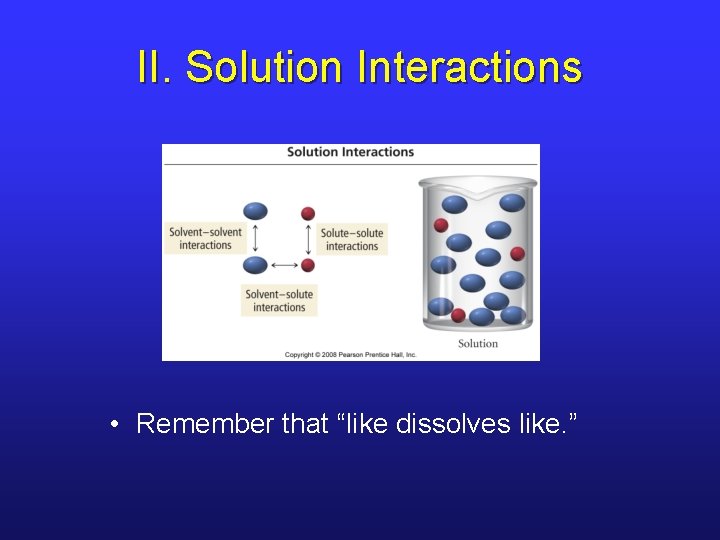
II. Solution Interactions • Remember that “like dissolves like. ”

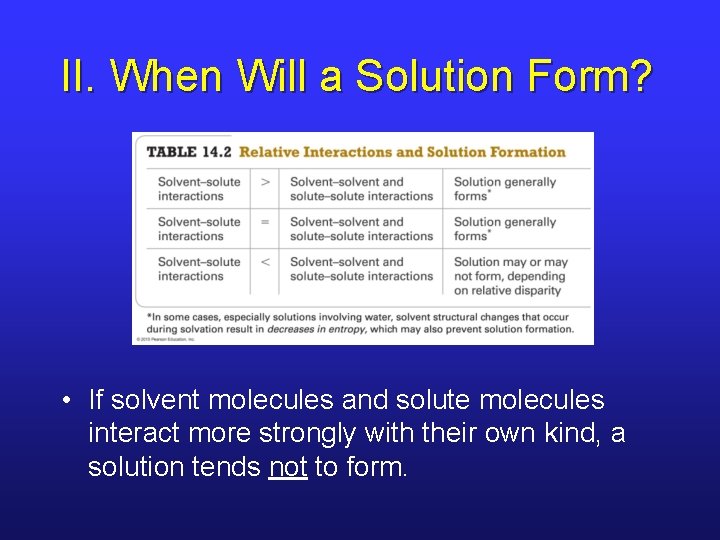
II. When Will a Solution Form? • If solvent molecules and solute molecules interact more strongly with their own kind, a solution tends not to form.

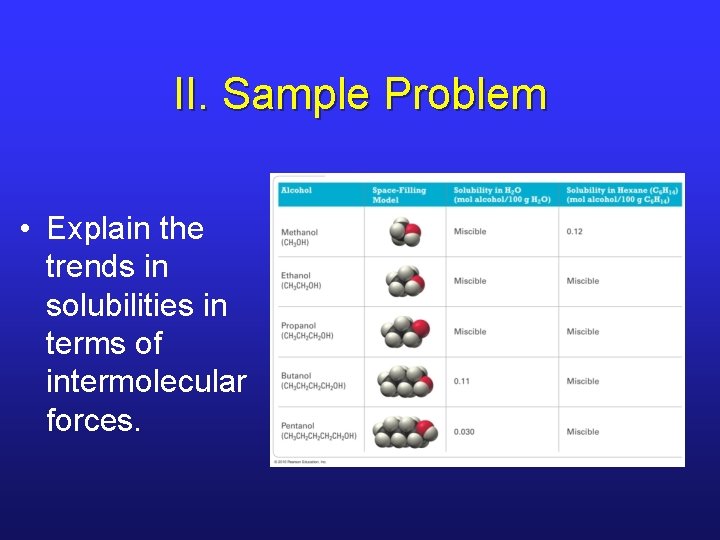
II. Sample Problem • Explain the trends in solubilities in terms of intermolecular forces.

II. Energetics of Solution Formation • Although not a reaction, we see energy changes when a solution forms. • The energy change observed depends on relative strengths of IM forces. • The enthalpy of solution, ΔHsoln, can be exo or endo and can be estimated using a Hess’s Law type of calculation.

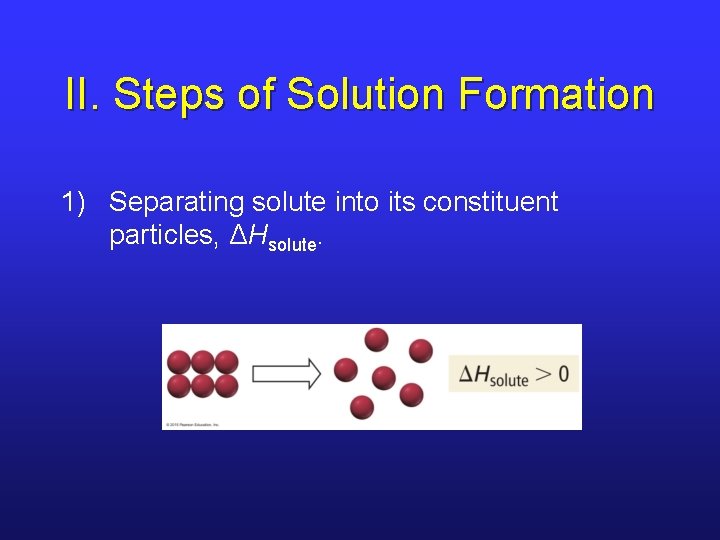
II. Steps of Solution Formation 1) Separating solute into its constituent particles, ΔHsolute.

II. Steps of Solution Formation 2) Separating solvent particles to make room for solute particles, ΔHsolvent.

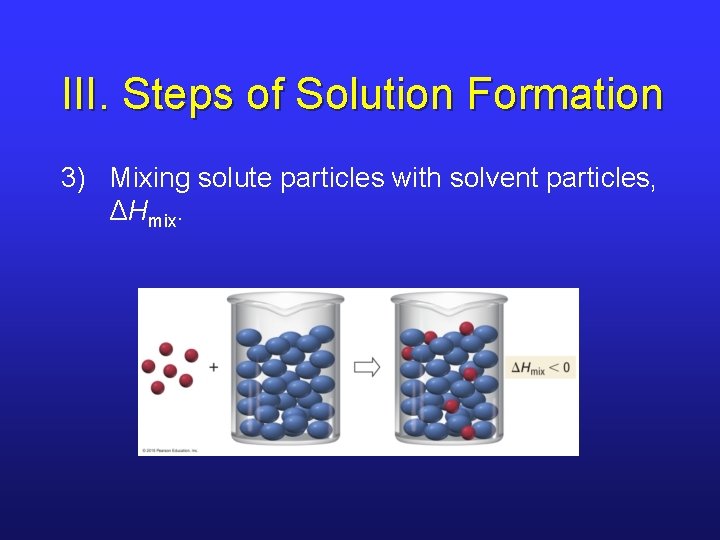
III. Steps of Solution Formation 3) Mixing solute particles with solvent particles, ΔHmix.

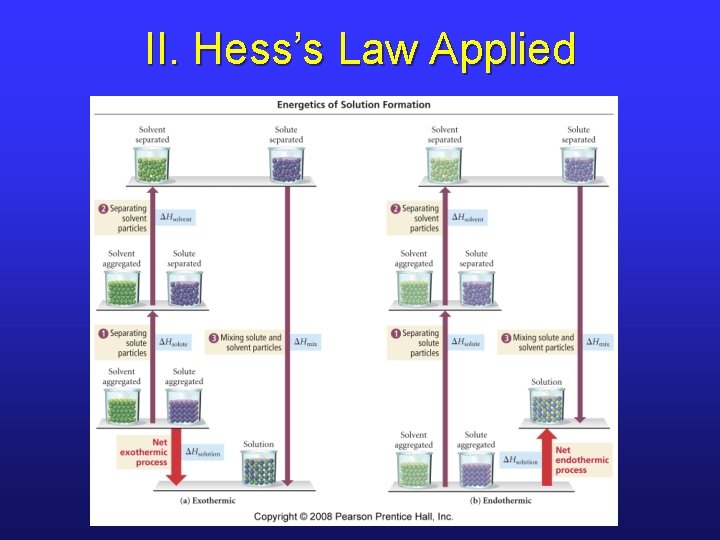
II. Steps of Solution Formation • Hess’s Law allows us to break up a process into a series of steps. • Therefore, the 3 -step path for solution formation yields the following equation. * ΔHsoln = ΔHsolute + ΔHsolvent + ΔHmix

II. Hess’s Law Applied

III. Electrolytes • Substances that form ions in aqueous solution are known as electrolytes and those that do not are known as nonelectrolytes. • If ions are formed near 100% efficiency, the substance is a strong electrolyte; otherwise, it’s a weak electrolyte.

III. Solution Conductivity • Electrical conductance can be used to discriminate between strong and weak electrolytes.

III. Ionic Electrolytes • Ion-dipole attraction is important in the dissolving process. • Note how entropy plays a role as well. • What about insoluble ionic compounds? *

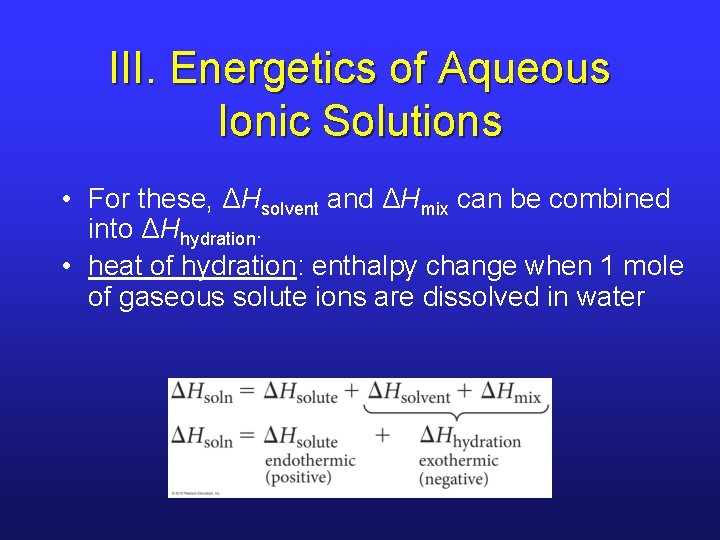
III. Energetics of Aqueous Ionic Solutions • For these, ΔHsolvent and ΔHmix can be combined into ΔHhydration. • heat of hydration: enthalpy change when 1 mole of gaseous solute ions are dissolved in water

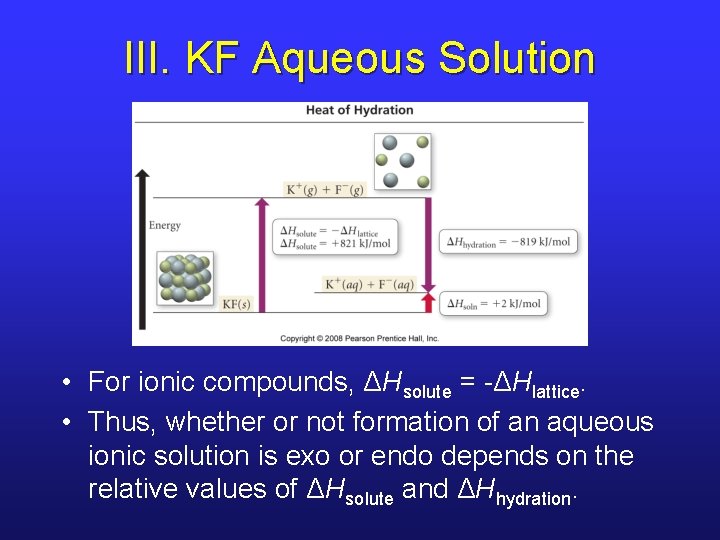
III. KF Aqueous Solution • For ionic compounds, ΔHsolute = -ΔHlattice. • Thus, whether or not formation of an aqueous ionic solution is exo or endo depends on the relative values of ΔHsolute and ΔHhydration.

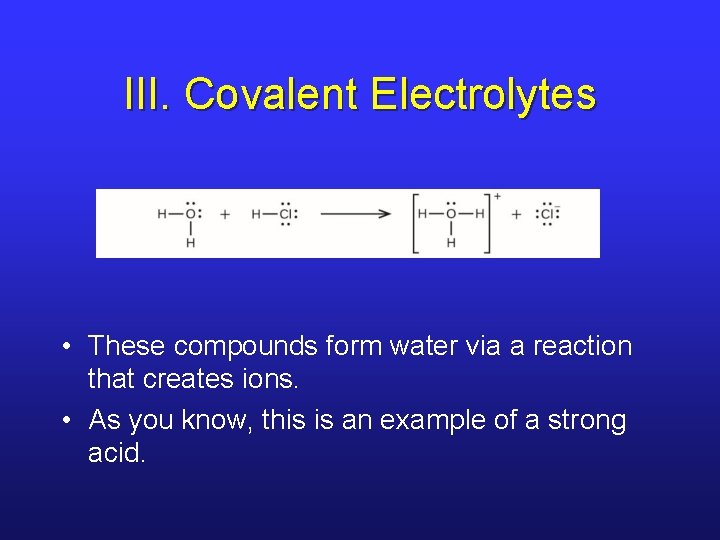
III. Covalent Electrolytes • These compounds form water via a reaction that creates ions. • As you know, this is an example of a strong acid.

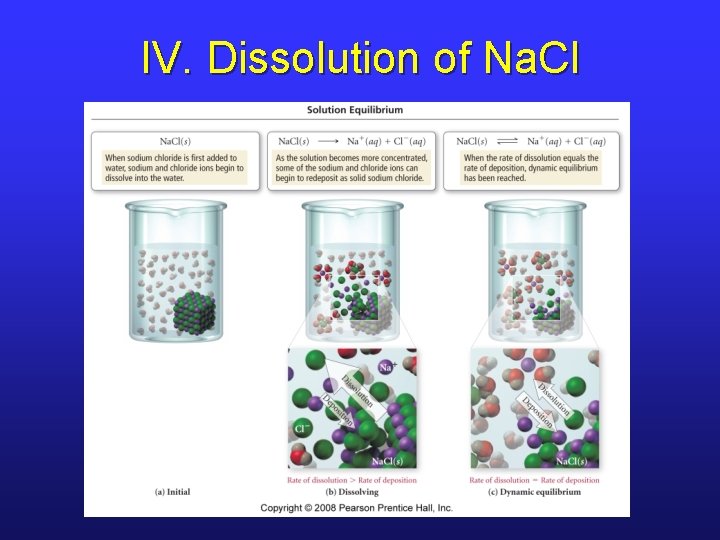
IV. Solution Solubility • Initially, when solid is placed in a solvent, solid particles rapidly go into solution. • Eventually, there will be excess solute particles in solution, and they will begin to redeposit. • At dynamic equilibrium, the rates of dissolution and deposition are equal.

IV. Dissolution of Na. Cl

IV. Solubility Terminology • Solubility is the maximum concentration that can be achieved under a set of conditions. • Equilibrium between solid and solvated particles occurs if solution is saturated, i. e. when solution’s concentration is equal to its solubility. • If there’s less than this equilibrium amount, the solution is unsaturated. • Some solutions can be made to be supersaturated.

IV. Solubility of Gases • Gas solubility in water depends on intermolecular forces, temperature and external pressure. • Gas solubility decreases with temp. • Gas solubility increases with higher external pressure.

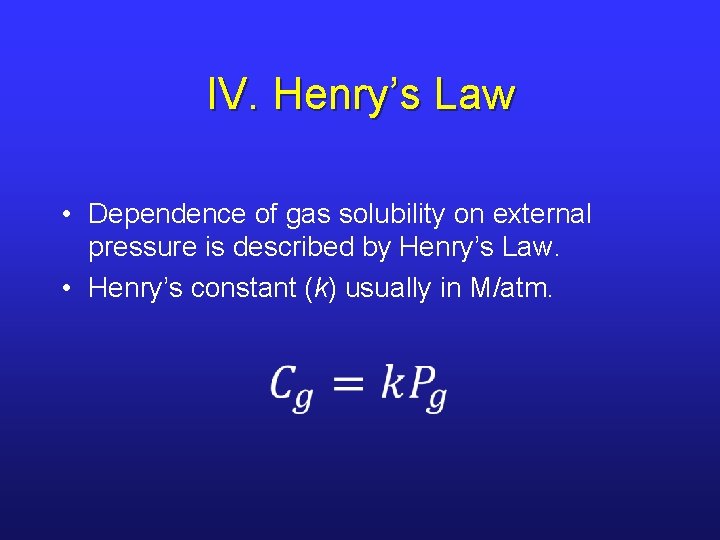
IV. Henry’s Law • Dependence of gas solubility on external pressure is described by Henry’s Law. • Henry’s constant (k) usually in M/atm. •

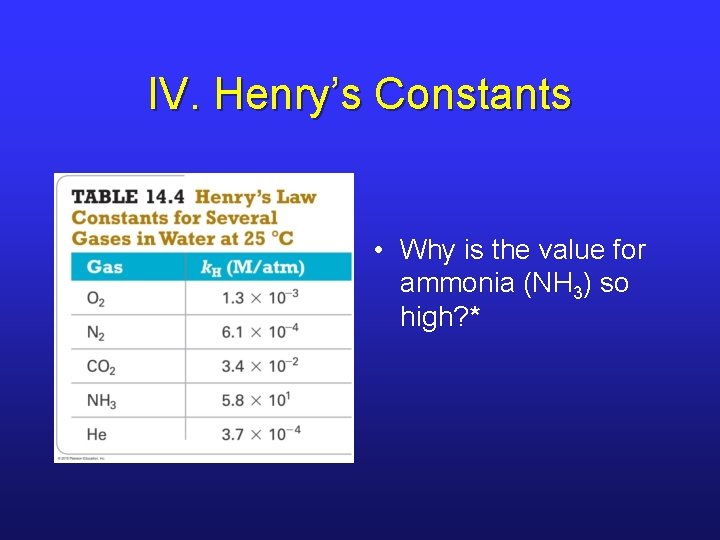
IV. Henry’s Constants • Why is the value for ammonia (NH 3) so high? *

IV. Liquid/Liquid Solutions • Liquids that mix in all proportions are miscible. • Also have immiscible and partially miscible. • “Like dissolves like” often used to explain. *

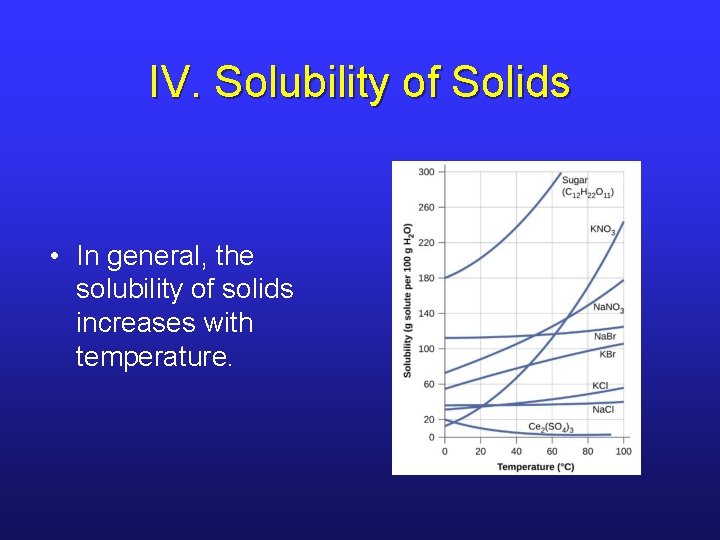
IV. Solubility of Solids • In general, the solubility of solids increases with temperature.

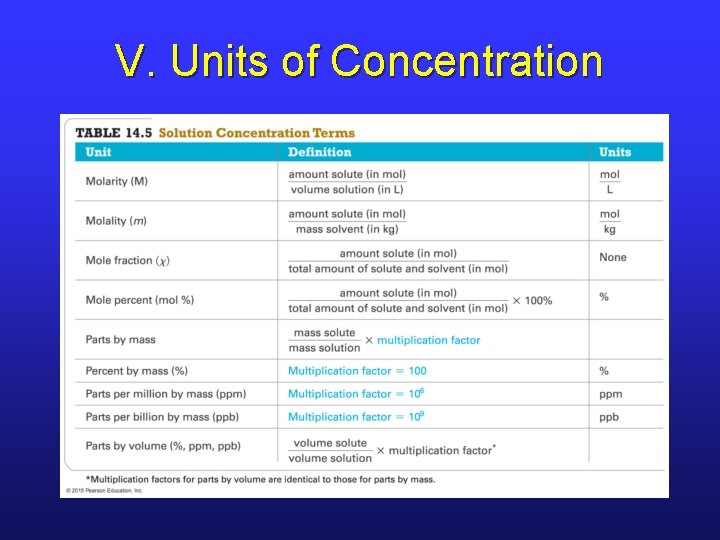
V. Solution Concentration • One of the most important aspects about a solution is its concentration. • There are many different units of concentration, and we need to be able to convert between all of them. • Which unit we use depends on what type of problem we are solving.

V. Units of Concentration

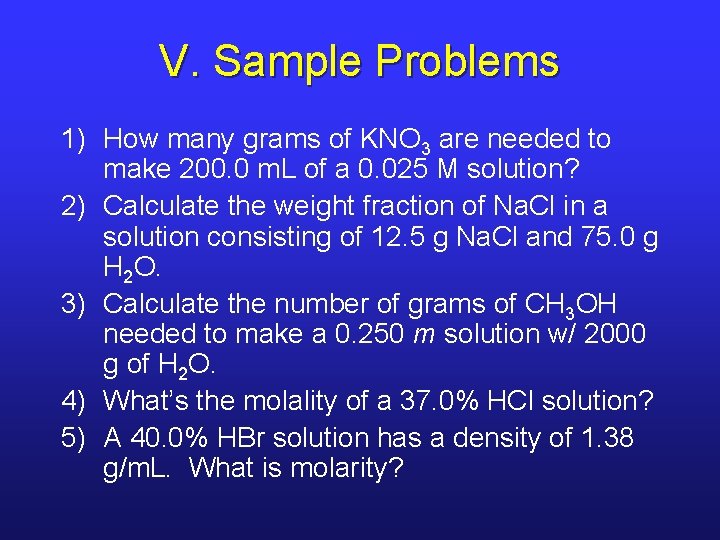
V. Sample Problems 1) How many grams of KNO 3 are needed to make 200. 0 m. L of a 0. 025 M solution? 2) Calculate the weight fraction of Na. Cl in a solution consisting of 12. 5 g Na. Cl and 75. 0 g H 2 O. 3) Calculate the number of grams of CH 3 OH needed to make a 0. 250 m solution w/ 2000 g of H 2 O. 4) What’s the molality of a 37. 0% HCl solution? 5) A 40. 0% HBr solution has a density of 1. 38 g/m. L. What is molarity?

VI. Colligative Properties • • Salt is added to ice in an ice cream maker. Icy roads are salted in winter. Antifreeze is mixed with water. These are practical uses of colligative properties. • colligative properties: properties that depend on number of dissolved particles, not the type particle.

VI. Colligative Properties • We will look at 3 colligative properties: § § Vapor pressure lowering Boiling point elevation Freezing point depression Osmotic pressure

VI. Vapor Pressure • Vapor pressure is the pressure of a gas in dynamic equilibrium w/ its liquid. • It’s a measure of how many molecules go into the gas phase.

VI. Vapor Pressure of Solutions • All liquid solutions of nonvolatile solutes have lower vapor pressures than the pure solvents. • Why? *

VI. Raoult’s Law • The vapor pressure of a solution can be calculated using Raoult’s Law.

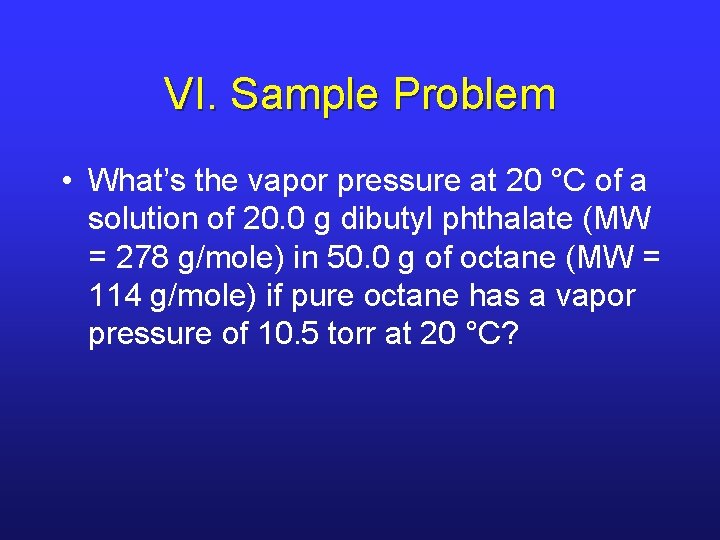
VI. Sample Problem • What’s the vapor pressure at 20 °C of a solution of 20. 0 g dibutyl phthalate (MW = 278 g/mole) in 50. 0 g of octane (MW = 114 g/mole) if pure octane has a vapor pressure of 10. 5 torr at 20 °C?

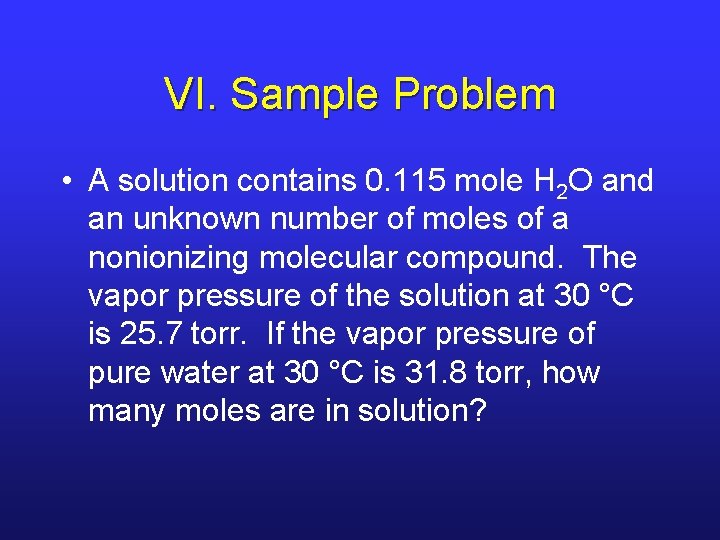
VI. Sample Problem • A solution contains 0. 115 mole H 2 O and an unknown number of moles of a nonionizing molecular compound. The vapor pressure of the solution at 30 °C is 25. 7 torr. If the vapor pressure of pure water at 30 °C is 31. 8 torr, how many moles are in solution?

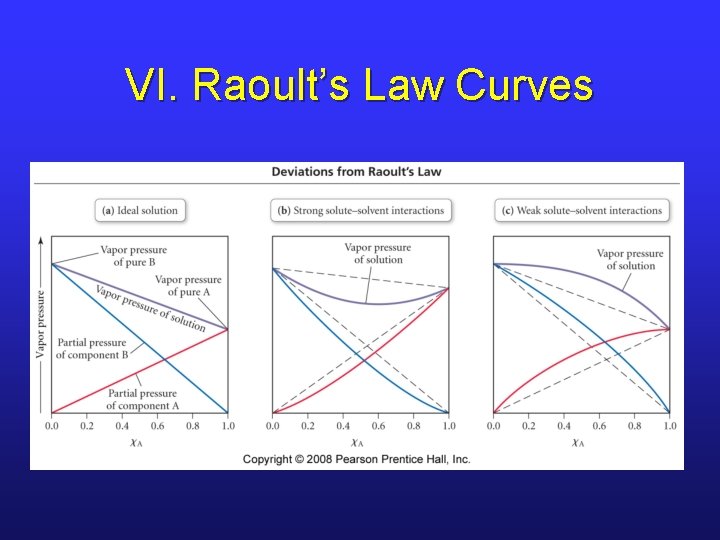
VI. Ideal and Nonideal Liquid/Liquid Solutions • Ideal solutions obey Raoult’s law at all concentrations for both solute and solvent. • In an ideal solution, ΔHsoln = 0, which means forces of attraction between all molecules are identical. • We can calculate vapor pressures of each component…

VI. Rauolt’s Law for 2 Liquids • Stronger or weaker solute-solvent interactions will cause deviations as shown in the curves in the next slide…

VI. Raoult’s Law Curves

VI. Changes in BP • The boiling point is defined as the temperature at which the vapor pressure equals the atmospheric pressure. • Since vapor pressures are lowered in solutions, it makes sense that the boiling point of a solution must be higher.

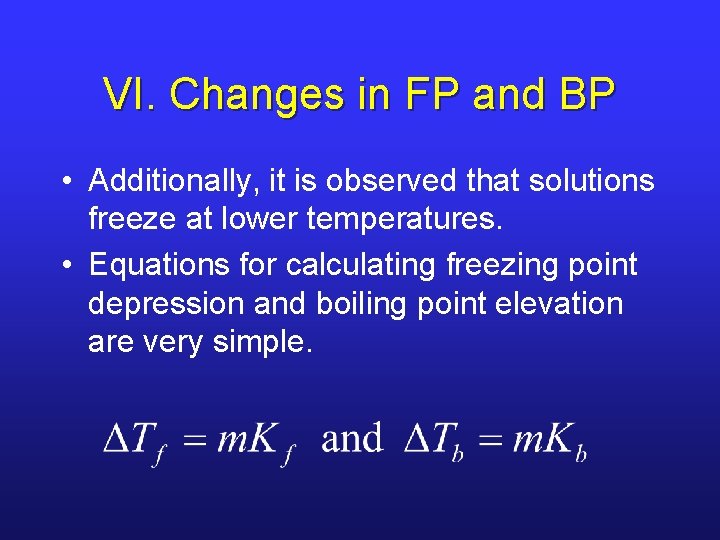
VI. Changes in FP and BP • Additionally, it is observed that solutions freeze at lower temperatures. • Equations for calculating freezing point depression and boiling point elevation are very simple.

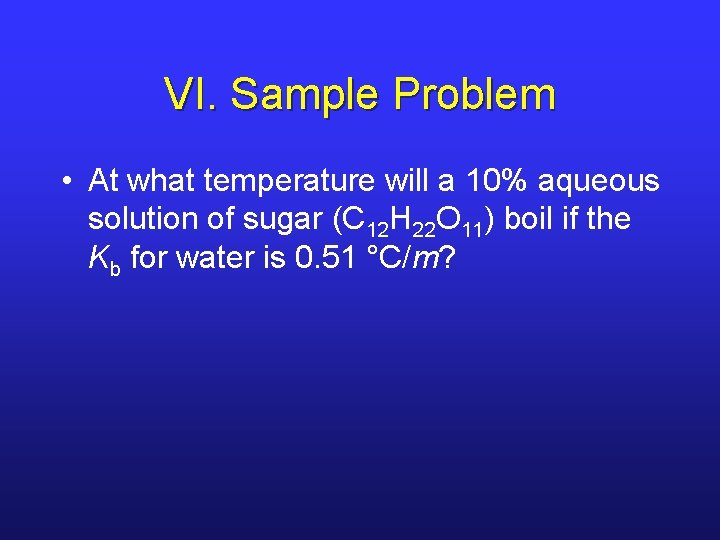
VI. Sample Problem • At what temperature will a 10% aqueous solution of sugar (C 12 H 22 O 11) boil if the Kb for water is 0. 51 °C/m?

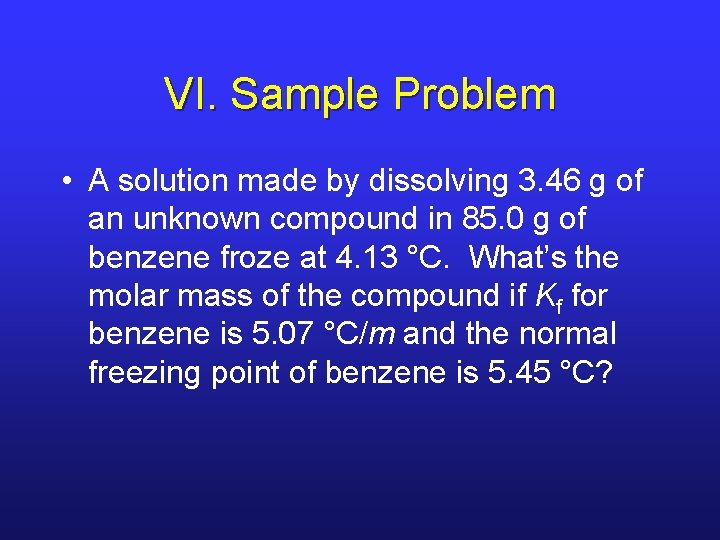
VI. Sample Problem • A solution made by dissolving 3. 46 g of an unknown compound in 85. 0 g of benzene froze at 4. 13 °C. What’s the molar mass of the compound if Kf for benzene is 5. 07 °C/m and the normal freezing point of benzene is 5. 45 °C?

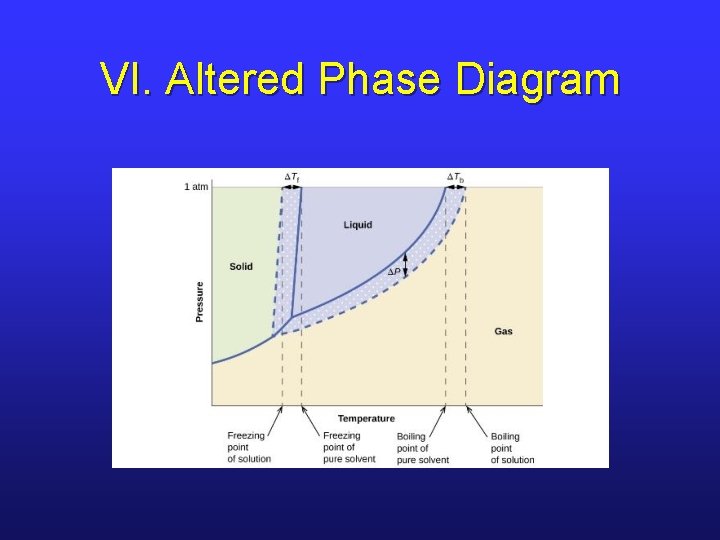
VI. Altered Phase Diagram

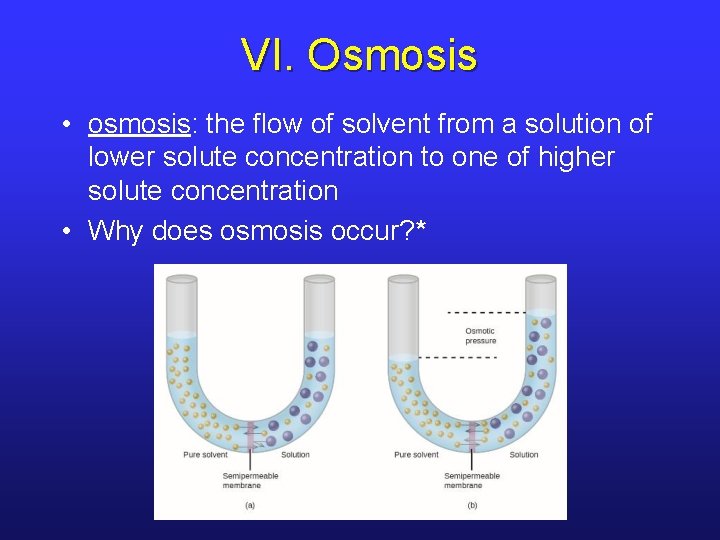
VI. Osmosis • osmosis: the flow of solvent from a solution of lower solute concentration to one of higher solute concentration • Why does osmosis occur? *

VI. Osmotic Pressure • The flow of solvent can be stopped by applying external pressure. This pressure is called the osmotic pressure. • The “gas” value of R is used.

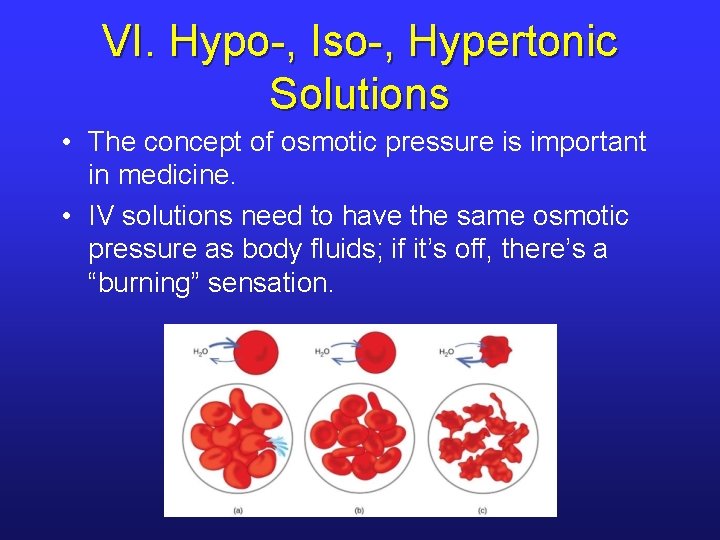
VI. Hypo-, Iso-, Hypertonic Solutions • The concept of osmotic pressure is important in medicine. • IV solutions need to have the same osmotic pressure as body fluids; if it’s off, there’s a “burning” sensation.

VI. Ionic Solutions • Since colligative properties only depend on number, we need to consider how many particles form when ionics form a solution. • We have to adjust the molality/molarity values used in the equations. • Problem – ionics do not dissociate completely!

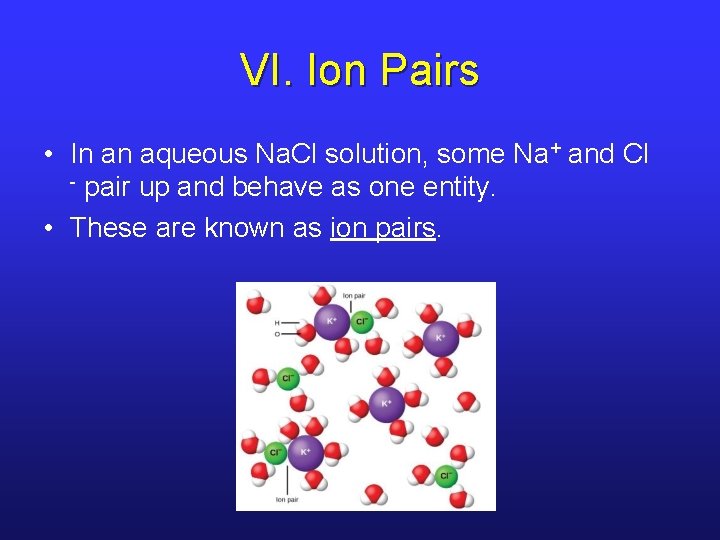
VI. Ion Pairs • In an aqueous Na. Cl solution, some Na+ and Cl - pair up and behave as one entity. • These are known as ion pairs.

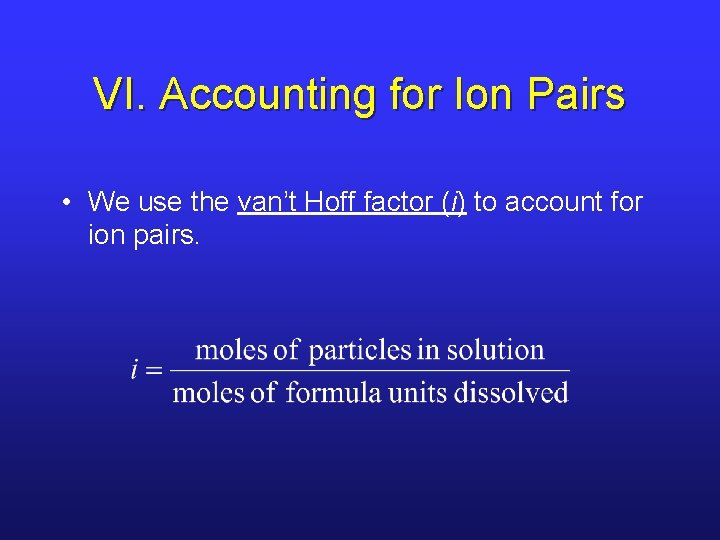
VI. Accounting for Ion Pairs • We use the van’t Hoff factor (i) to account for ion pairs.

VI. van’t Hoff Factors

VI. Equations for Ionic Solutions • Incorporating van’t Hoff factors is easy…