Ch 4 Atoms and Elements Dr Namphol Sinkaset














- Slides: 45

Ch. 4: Atoms and Elements Dr. Namphol Sinkaset Chem 152: Introduction to General Chemistry

I. Chapter Outline I. Introduction II. Atomic Theory III. The Nuclear Atom IV. Elements V. The Periodic Table VI. Ions VII. Isotopes VIII. Atomic Mass

I. Introduction • Atoms are the building blocks of everything we experience. • What we smell, what we feel, what we see. • In this chapter, we trace the history of the atom and learn about its makeup.

II. The Greeks • From out perspective, matter can be infinitely divided. • However, Leucippus and Democritus (5 th century B. C. ) believed there was a limit. § Eventually, you will reach something that was “atomos” or “indivisible. ” • Unfortunately, their idea was not accepted.

II. Revival of the Atom • The idea of the atom lay dormant for over 2000 years. • John Dalton revived the idea in order to explain 3 natural laws that puzzled everyone at the time. • Dalton’s Atomic Theory worked so well that it was quickly accepted.

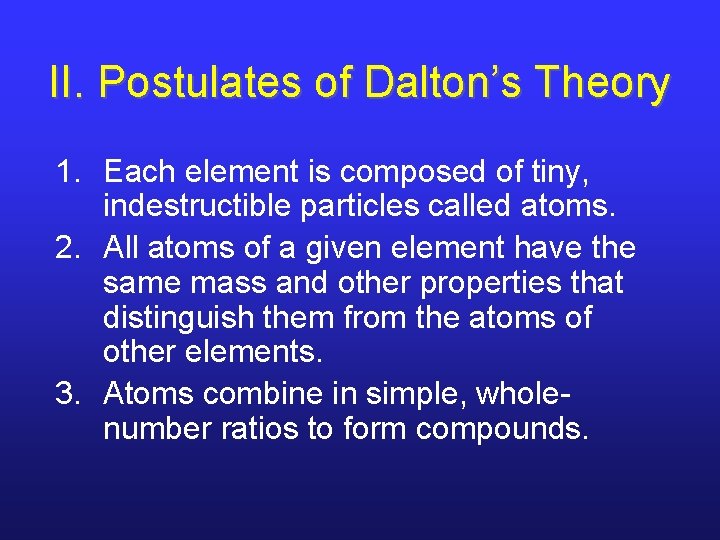
II. Postulates of Dalton’s Theory 1. Each element is composed of tiny, indestructible particles called atoms. 2. All atoms of a given element have the same mass and other properties that distinguish them from the atoms of other elements. 3. Atoms combine in simple, wholenumber ratios to form compounds.

II. Atoms • Today, overwhelming evidence points towards the existence of atoms. • Atoms can be imaged and arranged!

III. Not “Atomos” • Dalton’s theory treated atoms as permanent, indestructible building blocks that composed everything. • However, J. J. Thomson discovered electrons, which were much smaller than an atom and negatively charged! • Since atoms are neutral, where’s the positive charge?

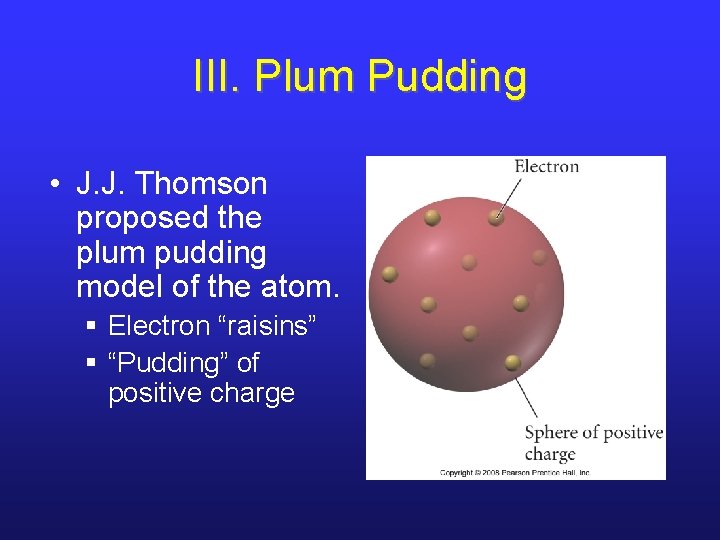
III. Plum Pudding • J. J. Thomson proposed the plum pudding model of the atom. § Electron “raisins” § “Pudding” of positive charge

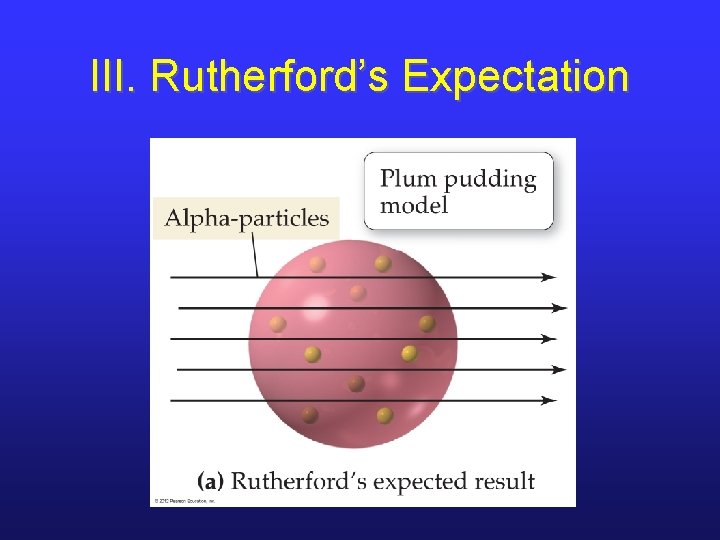
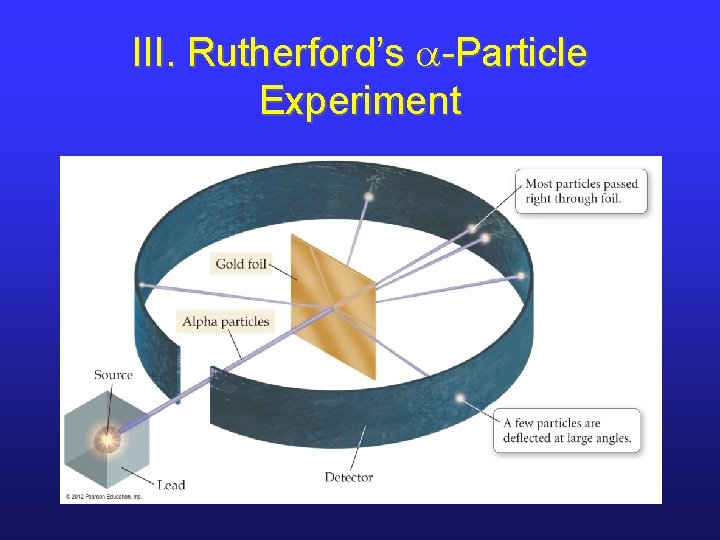
III. Rutherford likes Plum Pudding • Ernest Rutherford was a student of J. J. Thomson. • He tried to prove the plum pudding model by shooting a-particles at gold foil. • Note that a-particles are 7000 x more massive than an electron and have a positive charge.

III. Rutherford’s Expectation

III. Rutherford’s a-Particle Experiment

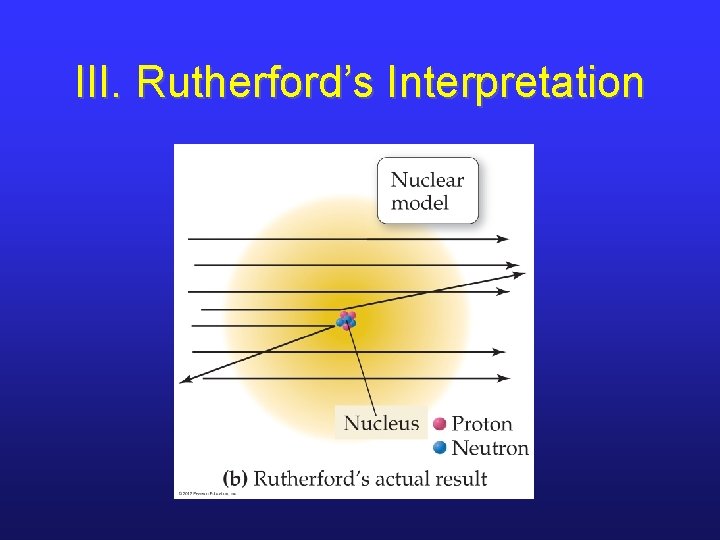
III. Conclusions from Rutherford’s Experiment • Most of an atom’s mass and all of its positive charge exist in a nucleus. • Most of an atom is empty space, throughout which tiny electrons are dispersed. • By having equal numbers of positivelycharged particles (protons) and electrons, an atom remains electrically neutral.

III. Rutherford’s Interpretation

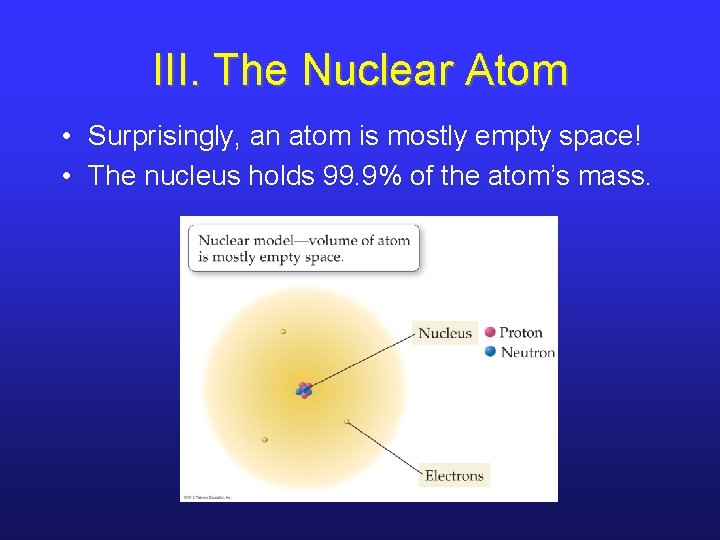
III. The Nuclear Atom • Surprisingly, an atom is mostly empty space! • The nucleus holds 99. 9% of the atom’s mass.

III. Components of an Atom • Protons. Positively-charged particles in the nucleus. Mass of 1. 67262 x 10 -27 kg or 1. 0073 amu. • Neutrons. Neutral particles in the nucleus. Mass of 1. 67493 x 10 -27 kg or 1. 0087 amu. • Electrons. Negatively-charged particles. Mass of 9. 1 x 10 -31 kg or 0. 00055 amu.

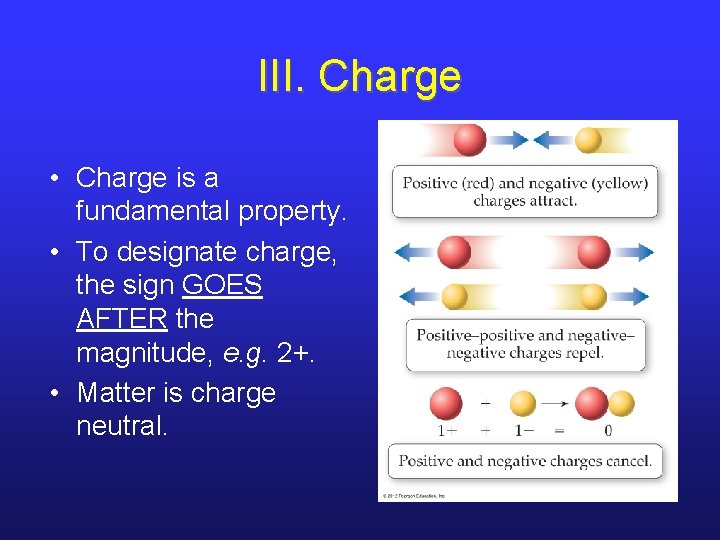
III. Charge • Charge is a fundamental property. • To designate charge, the sign GOES AFTER the magnitude, e. g. 2+. • Matter is charge neutral.

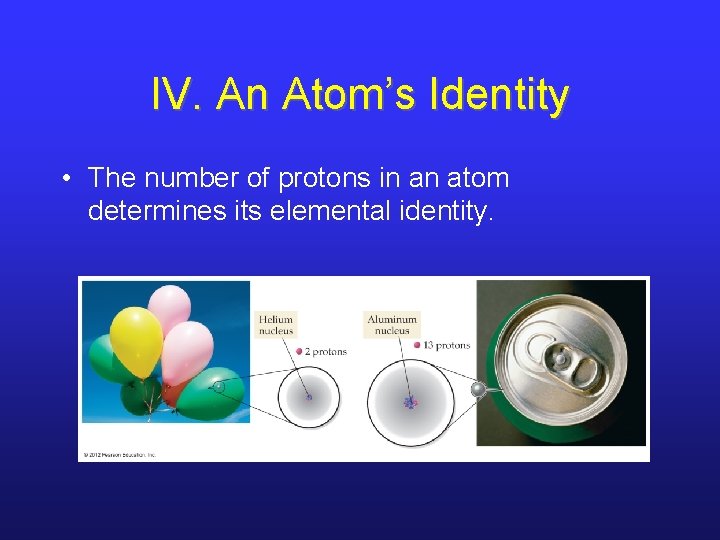
IV. An Atom’s Identity • The number of protons in an atom determines its elemental identity.

IV. Referring to Elements • Since each element has a unique # of protons, we could refer to elements using Z, the atomic number, which equals the # of protons in an atom. § e. g. The Z = 2 element. • More commonly, we use an element’s name or chemical symbol. § e. g. The element helium, or He.

IV. Chemical Symbols • Chemical symbols are a one or two letter abbreviation of an element’s name. • First letter always capitalized; second letter is LOWERCASE. • Some symbols are based on historical names: e. g. Au from aurum.

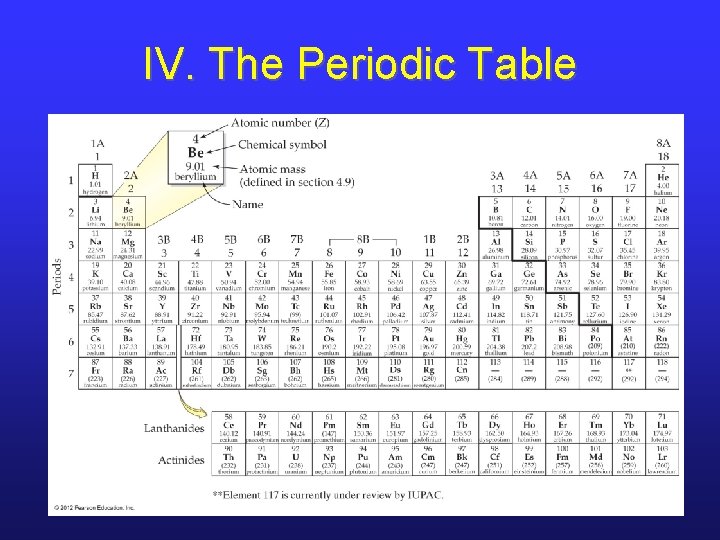
IV. The Periodic Table

IV. Sample Problem • Find the name and atomic number of the following elements. a) b) c) d) e) V N Hg Rh Mo

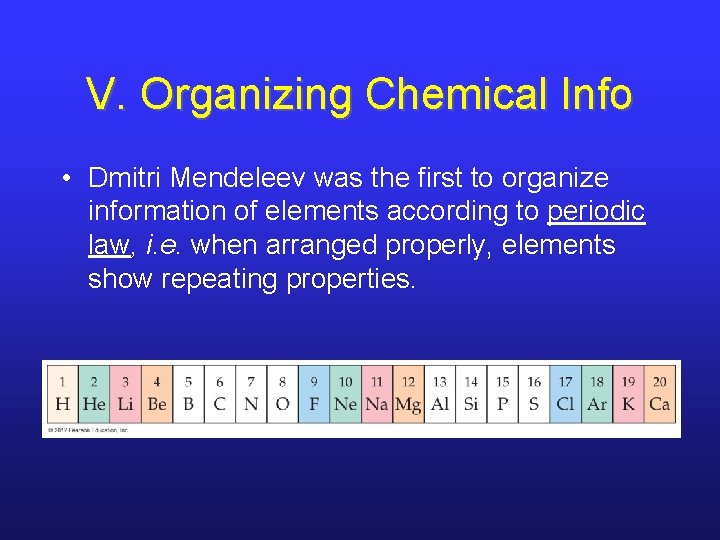
V. Organizing Chemical Info • Dmitri Mendeleev was the first to organize information of elements according to periodic law, i. e. when arranged properly, elements show repeating properties.

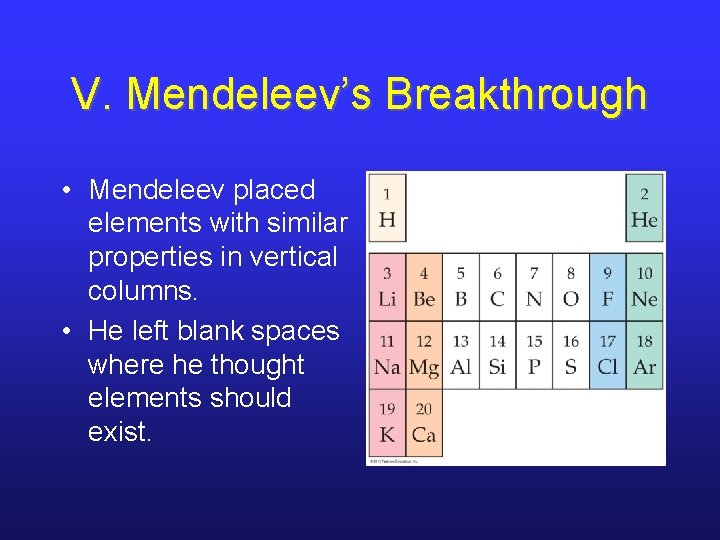
V. Mendeleev’s Breakthrough • Mendeleev placed elements with similar properties in vertical columns. • He left blank spaces where he thought elements should exist.

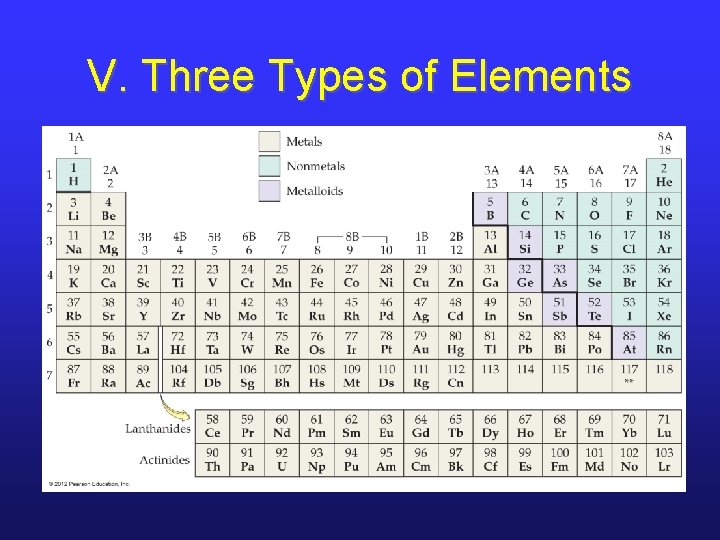
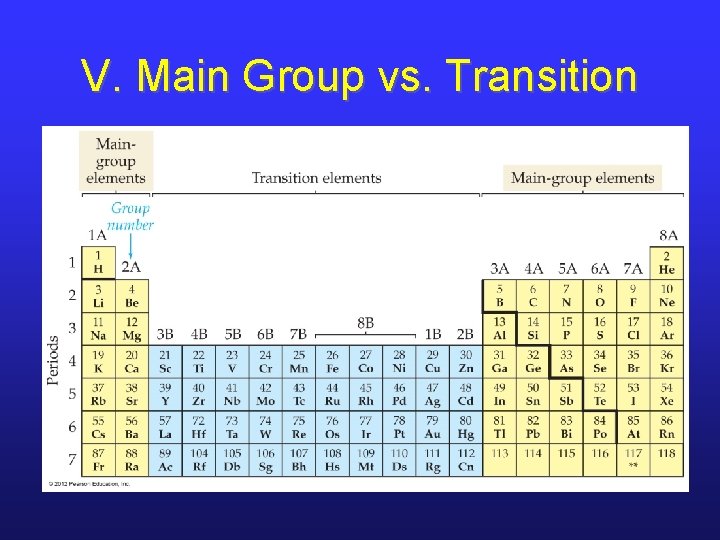
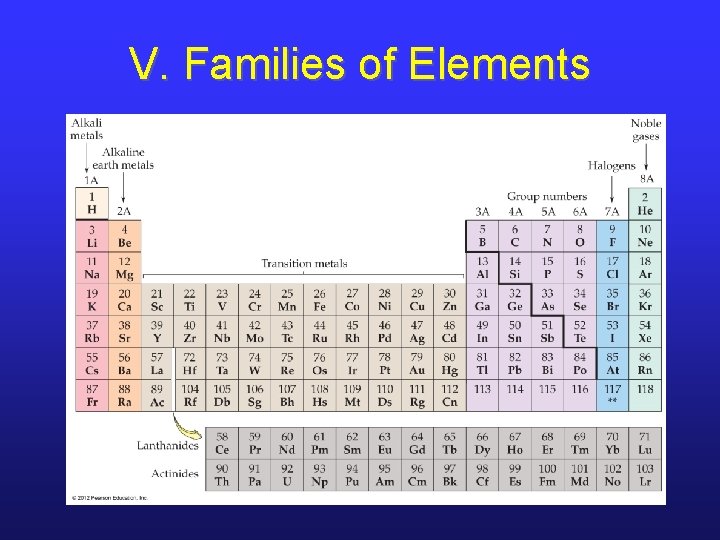
V. Three Types of Elements

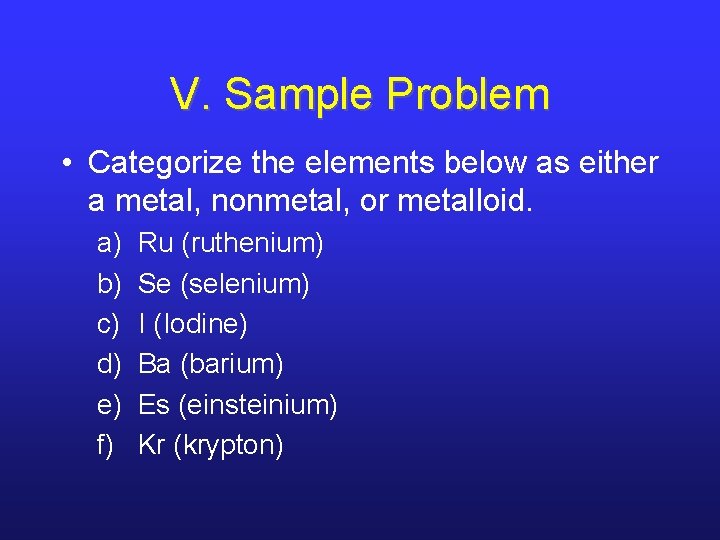
V. Sample Problem • Categorize the elements below as either a metal, nonmetal, or metalloid. a) b) c) d) e) f) Ru (ruthenium) Se (selenium) I (Iodine) Ba (barium) Es (einsteinium) Kr (krypton)

V. Main Group vs. Transition

V. Families of Elements

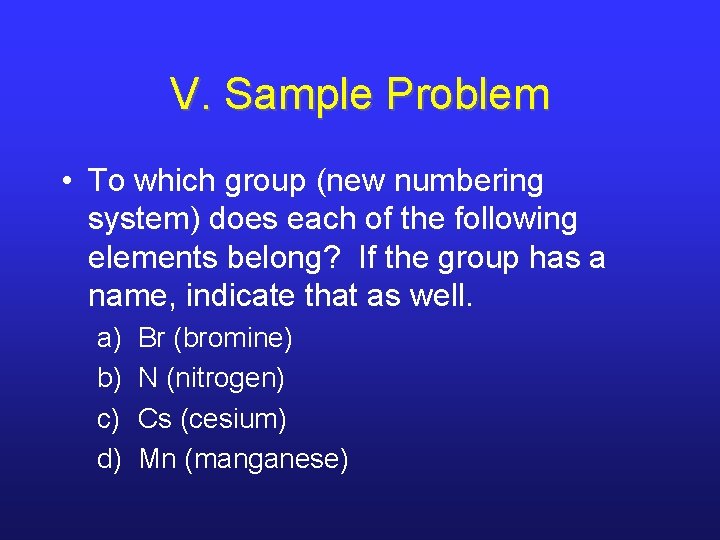
V. Sample Problem • To which group (new numbering system) does each of the following elements belong? If the group has a name, indicate that as well. a) b) c) d) Br (bromine) N (nitrogen) Cs (cesium) Mn (manganese)

VI. Atoms Can Lose/Gain e-’s • In chemical reactions, it’s common for atoms to lose or gain electrons and become ions. • ion: a particle that has a charge • Examples: § Na Na+ + e§ I + e - I-

VI. Origin of the Charge • The charge arises from the different number of protons and electrons in the atom. § Ion Charge = # protons - # electrons • A neutral Na atom has 11 protons and 11 electrons. If it loses and electron… § Ion Charge = 11 – 10 = 1+

VI. Cations and Anions • An ion is fundamentally different than a neutral atom, so it needs a different name. • cation: a positively-charged ion • anion: a negatively-charged ion • Note that cations and ions have different properties than their parent atoms, e. g. Na vs. Na+.

VI. Sample Problem • Determine the charges of the ions described below. a) b) c) d) A chromium atom that has lost 3 electrons. A sulfur atom that has gained 2 electrons. An iron atom (Fe) that has 24 electrons. A phosphorus atom (P) that has 18 electrons.

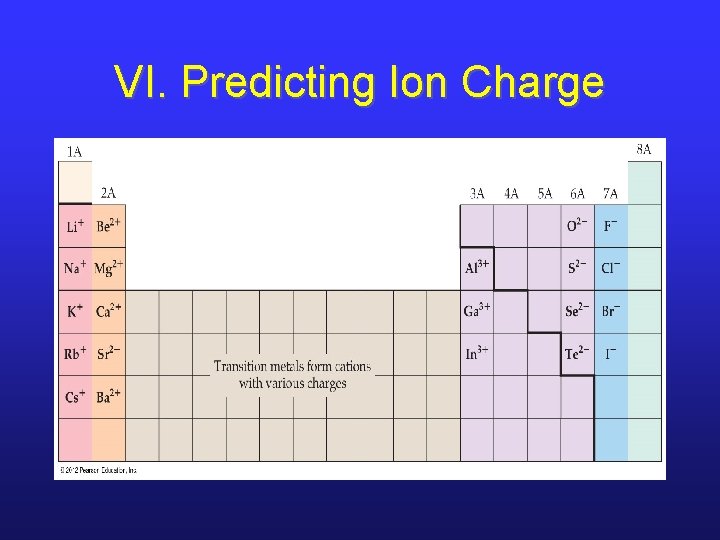
VI. Ions and the Periodic Table • The charge of an ion can be predicted by the position of its parent element on the periodic table IF it’s a main group element. • Simply count the number of spaces to the nearest noble gas (forward or backward). • If you go forward, it’s an anion; if you go backward, it’s a cation.

VI. Predicting Ion Charge

VI. Sample Problem • What are the ions that form from atoms of the following elements? § § aluminum (Al) tellurium (Te) rubidium (Rb) oxygen (O)

VII. Isotopes • Protons are the only thing that determines the identity of an atom. • Therefore, it’s possible for atoms of the same element to have different masses due to differing number of neutrons. • isotopes: atoms with the same number of protons, but different numbers of neutrons

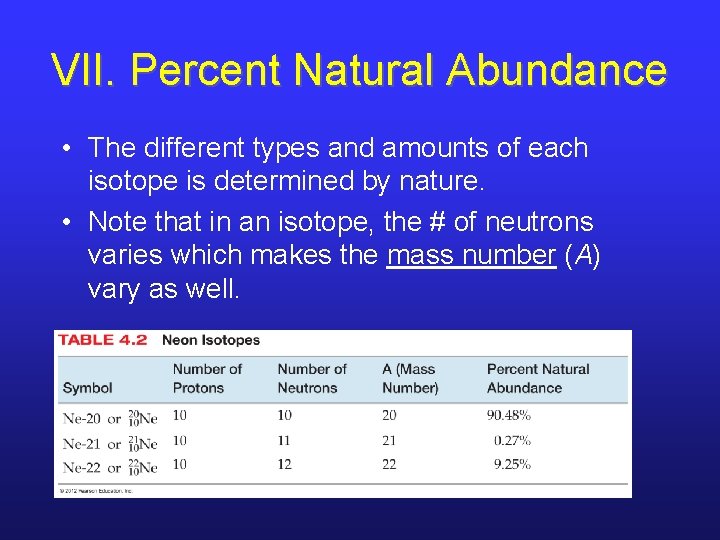
VII. Percent Natural Abundance • The different types and amounts of each isotope is determined by nature. • Note that in an isotope, the # of neutrons varies which makes the mass number (A) vary as well.

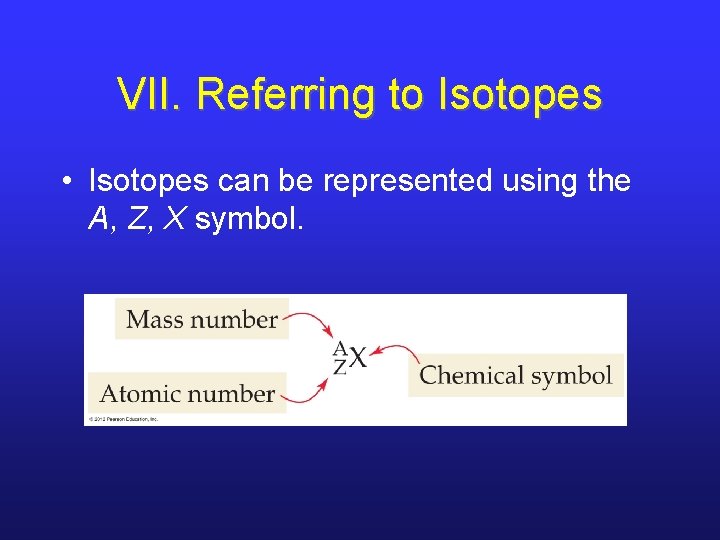
VII. Referring to Isotopes • Isotopes can be represented using the A, Z, X symbol.

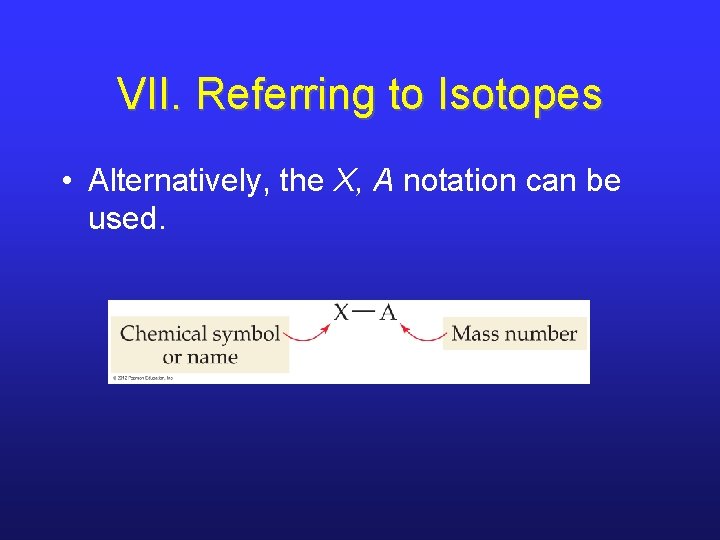
VII. Referring to Isotopes • Alternatively, the X, A notation can be used.

VII. Sample Problem • How many protons and neutrons are in a potassium isotope with a mass number of 39? What are three ways to represent this isotope?

VIII. What’s the Mass of an Atom? • It depends! • Are we talking about the mass of a specific atom, i. e. a given isotope? § If so, it’s just approximately the mass number. • Are we talking about in general? § Then it’s more complicated…

VIII. Atomic Mass • Not all atoms of the same element have the same mass, but we can calculate an average. • The atomic mass is the weighted average mass of an element which accounts for all isotopes and their percent natural abundances.

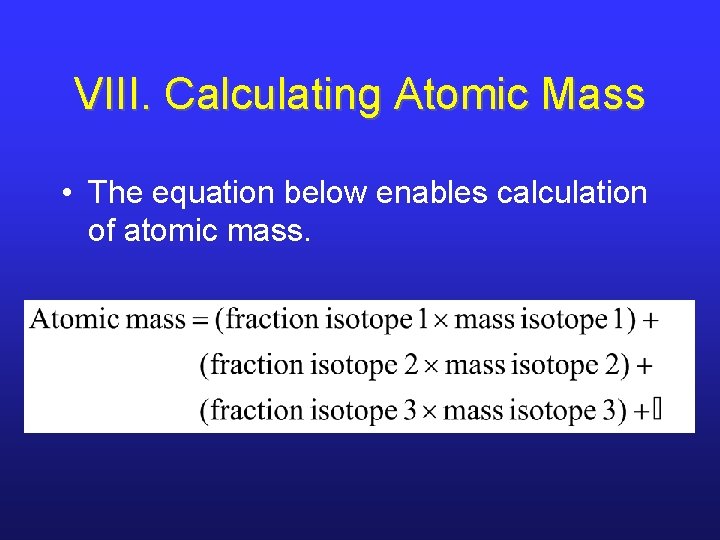
VIII. Calculating Atomic Mass • The equation below enables calculation of atomic mass.

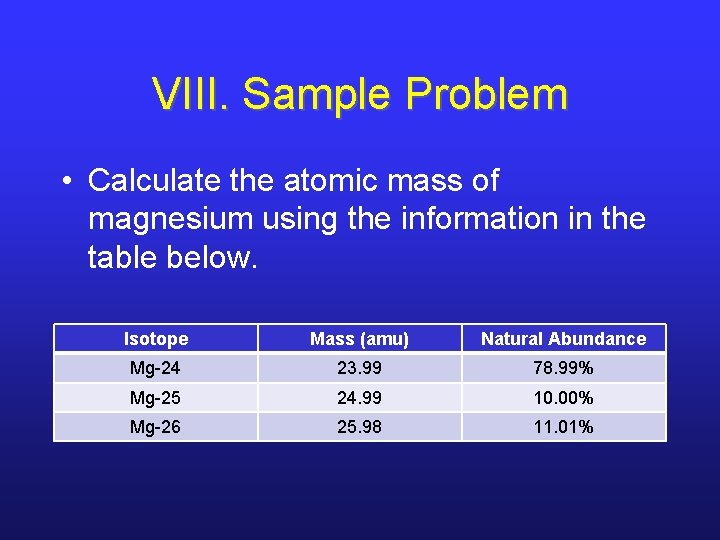
VIII. Sample Problem • Calculate the atomic mass of magnesium using the information in the table below. Isotope Mass (amu) Natural Abundance Mg-24 23. 99 78. 99% Mg-25 24. 99 10. 00% Mg-26 25. 98 11. 01%