Ch 1 The Chemical World Dr Namphol Sinkaset








- Slides: 13

Ch. 1: The Chemical World Dr. Namphol Sinkaset Chem 152: Introduction to General Chemistry

I. Chapter Outline I. III. IV. Introduction Atoms and Molecules The Scientific Method Succeeding in Chemistry

I. Layman’s View of Chemicals

I. Educated View of Chemicals

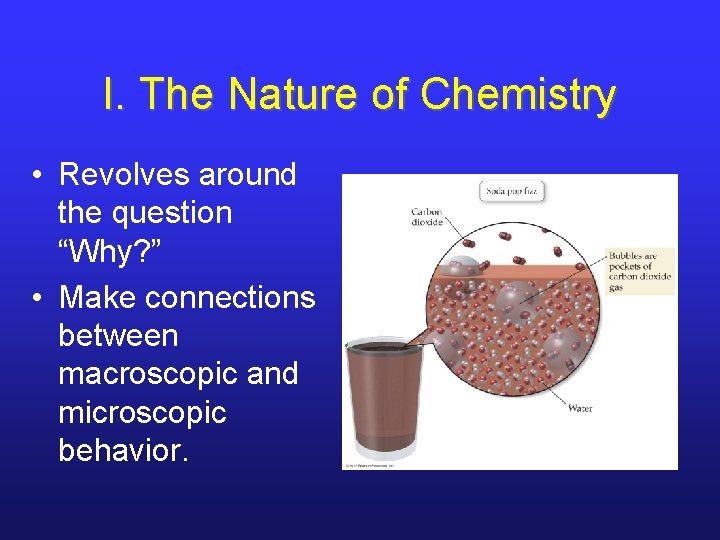
I. The Nature of Chemistry • Revolves around the question “Why? ” • Make connections between macroscopic and microscopic behavior.

II. Atoms and Molecules • • • Microscopic view refers to atoms. Atoms can bind together to form molecules. chemistry: the science that seeks to understand what matter does by studying what atoms/molecules do

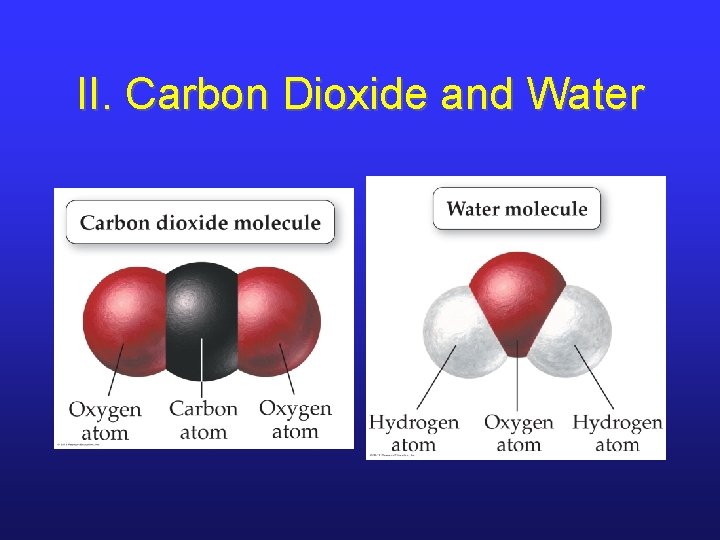
II. Carbon Dioxide and Water

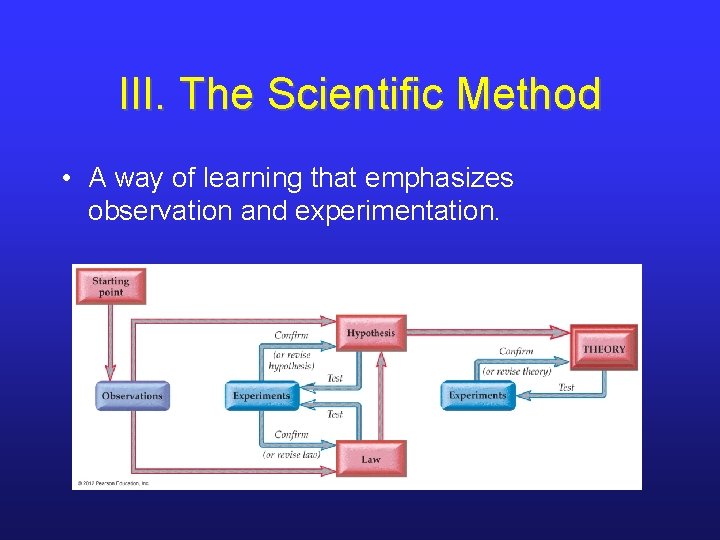
III. The Scientific Method • A way of learning that emphasizes observation and experimentation.

III. It Begins w/ Observations • Scientists begin their study by making an observation or measurement. • Observation can be simple description or complex measurement. • e. g. Fleming noticed bacteria-free rings around a certain mold. • e. g. Lavoisier measured masses before and after combustion reactions.

III. A Hypothesis is Formed and Tested • Observations lead to preliminary interpretation or explanation. • The hypothesis should be falsifiable. • Hypotheses are tested via experiments. • experiment: highly controlled observations designed to validate/invalidate a hypothesis. • Results may lead to revision of hypothesis.

III. Scientific Laws • Many similar observations may be developed into a scientific law. • scientific law: statement that synthesizes past observations and predicts future ones • Laws can be tested with experiments as well.

III. Theories • One or more well-established hypotheses can be formulated into a theory. • theory: models of nature that provide a broader and deeper explanation of observations and laws • A lot of work goes into formulating a theory; they are the height of scientific understanding…not “just a theory. ”

IV. Keys to Success • Curiosity – you need to be interested in finding out the “why” of things. • Math – you need to have the math skills to deal with and calculate quantities in chemistry. • Hard work – you need to be able to commit the time and effort to be successful.
 Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Namphol sinkaset
Namphol sinkaset Section 2 classifying chemical reactions worksheet answers
Section 2 classifying chemical reactions worksheet answers Empirical formula and molecular formula pogil
Empirical formula and molecular formula pogil Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 1 chemical changes
Chemical reactions section 1 chemical changes Modern chemistry chapter 7 test
Modern chemistry chapter 7 test Chapter 18 chemical reactions balancing chemical equations
Chapter 18 chemical reactions balancing chemical equations Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em





















