Atomic Molecular Clusters 3 Rare Gas Clusters Rare



- Slides: 61

Atomic & Molecular Clusters 3. Rare Gas Clusters • Rare gas (Rg) clusters are simple, but they illustrate important general points. • Note: at very low temperatures (< 2 K for 4 He), He clusters display quantum behaviour – superfluidity!

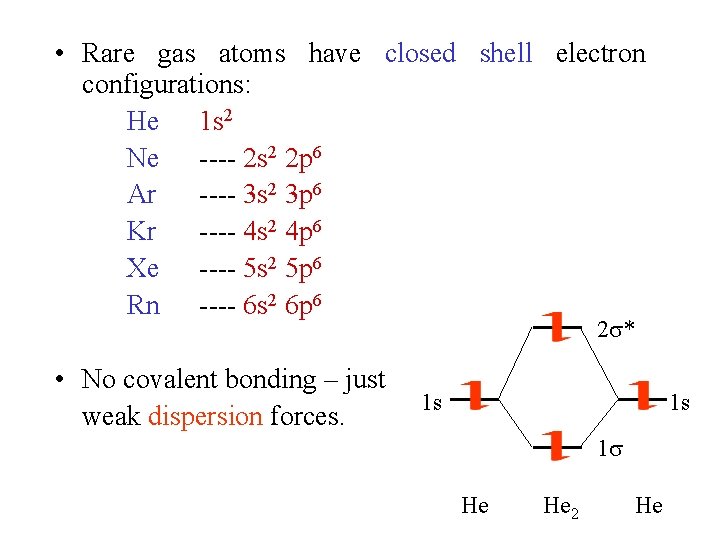
• Rare gas atoms have closed shell electron configurations: He 1 s 2 Ne ---- 2 s 2 2 p 6 Ar ---- 3 s 2 3 p 6 Kr ---- 4 s 2 4 p 6 Xe ---- 5 s 2 5 p 6 Rn ---- 6 s 2 6 p 6 2 * • No covalent bonding – just weak dispersion forces. 1 s 1 s 1 He He 2 He

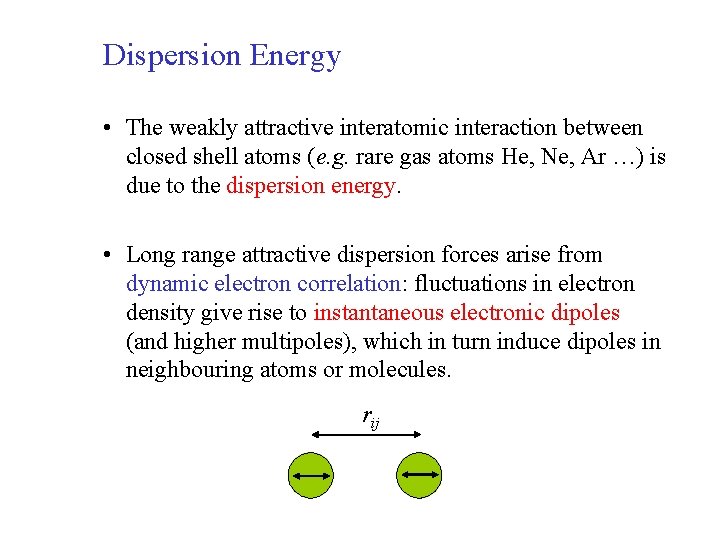
Dispersion Energy • The weakly attractive interatomic interaction between closed shell atoms (e. g. rare gas atoms He, Ne, Ar …) is due to the dispersion energy. • Long range attractive dispersion forces arise from dynamic electron correlation: fluctuations in electron density give rise to instantaneous electronic dipoles (and higher multipoles), which in turn induce dipoles in neighbouring atoms or molecules. rij

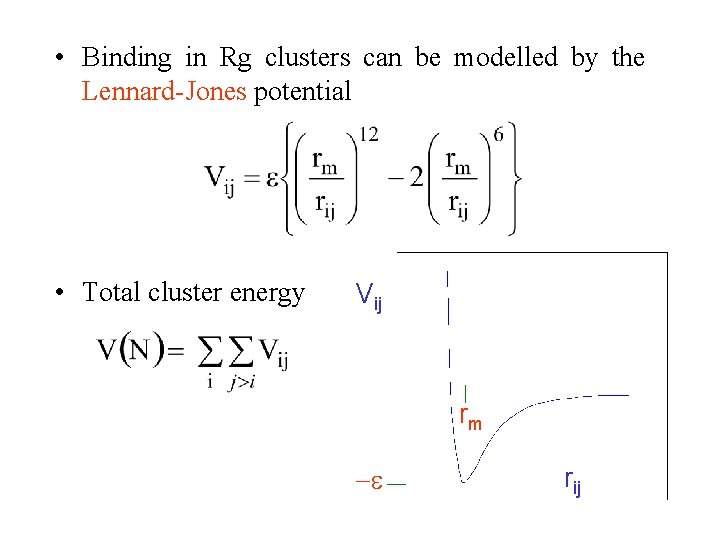
• Binding in Rg clusters can be modelled by the Lennard-Jones potential • Total cluster energy Vij rm rij

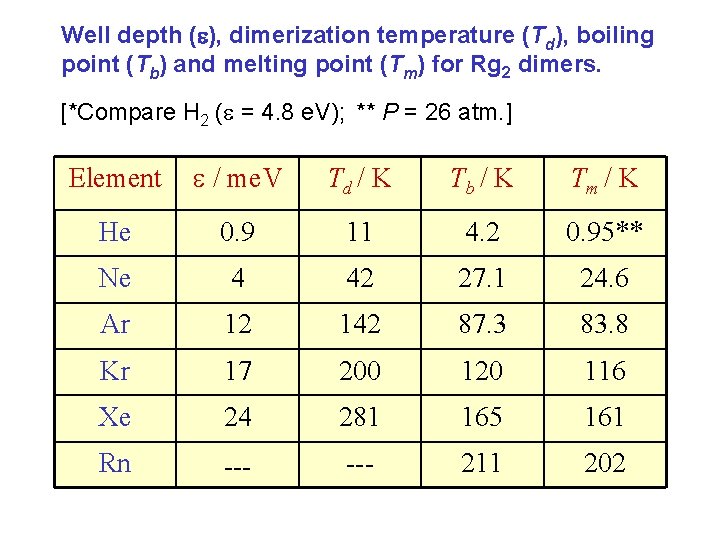
Well depth ( ), dimerization temperature (Td), boiling point (Tb) and melting point (Tm) for Rg 2 dimers. [*Compare H 2 ( = 4. 8 e. V); ** P = 26 atm. ] Element / me. V Td / K Tb / K Tm / K He 0. 9 11 4. 2 0. 95** Ne 4 42 27. 1 24. 6 Ar 12 142 87. 3 83. 8 Kr 17 200 120 116 Xe 24 281 165 161 Rn --- 211 202

Mass Spectroscopy of Rare Gas Clusters • • • Xe. N (N 150) – Echt (1981). He. N (N 32) – Stephens & King (1983). Ar. N and Kr. N (N 60) – Ding and Hesslich (1983). Ne. N (N 90) – Märk (1989). Rg. N (Rg = Ar, Kr, Xe; N 1000) – Friedman & Buehler. Mass spectrum of Ar clusters

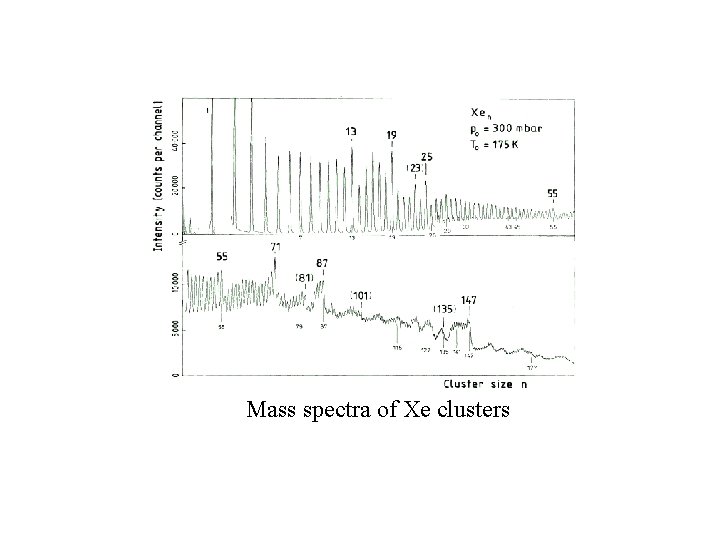
Mass spectra of Xe clusters

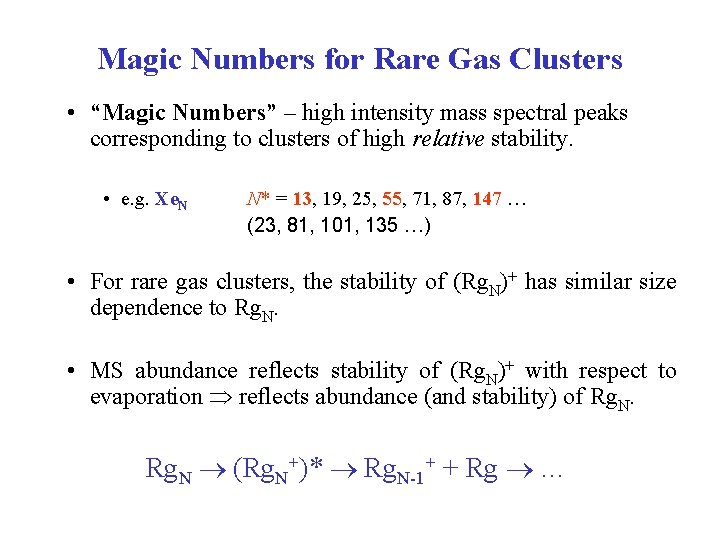
Magic Numbers for Rare Gas Clusters • “Magic Numbers” – high intensity mass spectral peaks corresponding to clusters of high relative stability. • e. g. Xe. N N* = 13, 19, 25, 55, 71, 87, 147 … (23, 81, 101, 135 …) • For rare gas clusters, the stability of (Rg. N)+ has similar size dependence to Rg. N. • MS abundance reflects stability of (Rg. N)+ with respect to evaporation reflects abundance (and stability) of Rg. N (Rg. N+)* Rg. N-1+ + Rg …

Geometric Shell Structure and Magic Numbers • Enhanced stability of magic number clusters (relative to their neighbours) is due to packing effects – complete geometric shells (i. e. complete shells of concentric polyhedra) have low surface energies (and therefore low total energies). • Geometric shell structure is commonly found for rare gas and large metal clusters. • Rare gas clusters up to several hundreds (or thousands) of atoms adopt icosahedral packing.

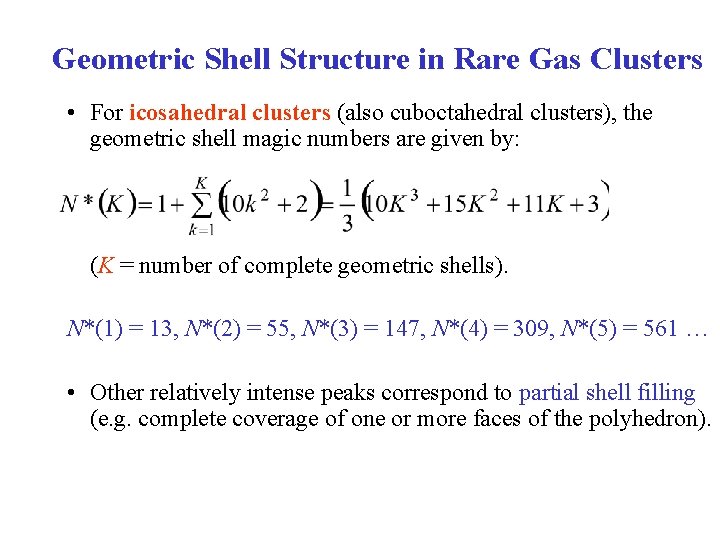
Geometric Shell Structure in Rare Gas Clusters • For icosahedral clusters (also cuboctahedral clusters), the geometric shell magic numbers are given by: (K = number of complete geometric shells). N*(1) = 13, N*(2) = 55, N*(3) = 147, N*(4) = 309, N*(5) = 561 … • Other relatively intense peaks correspond to partial shell filling (e. g. complete coverage of one or more faces of the polyhedron).

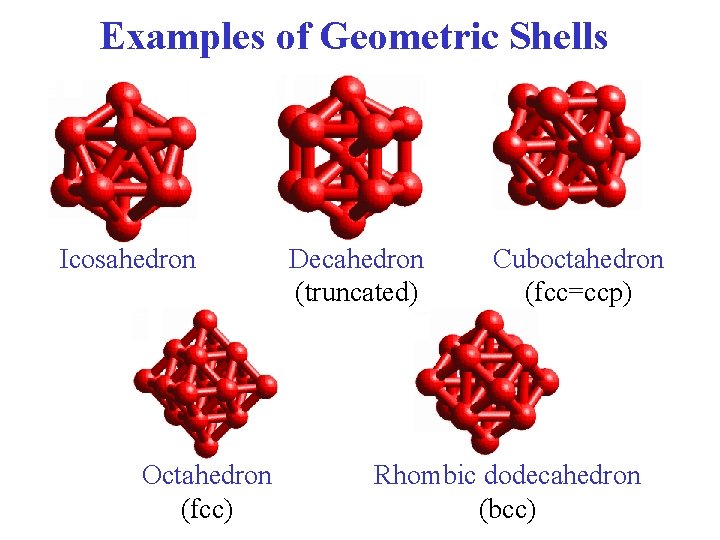
Examples of Geometric Shells Icosahedron Octahedron (fcc) Decahedron (truncated) Cuboctahedron (fcc=ccp) Rhombic dodecahedron (bcc)

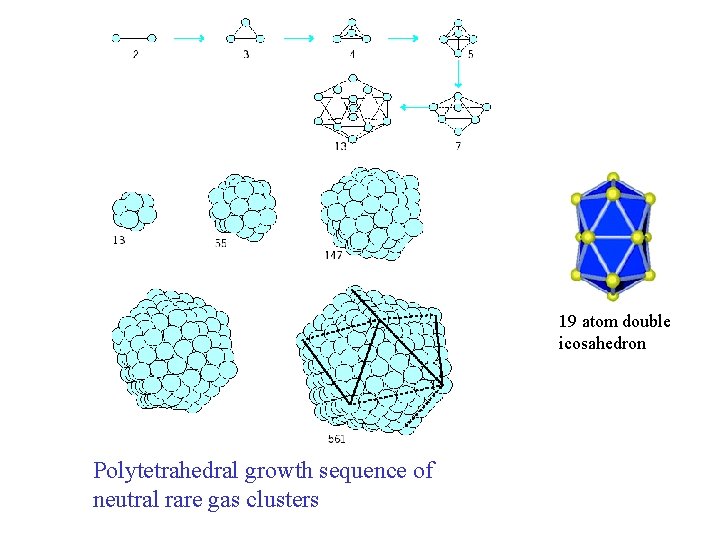
19 atom double icosahedron Polytetrahedral growth sequence of neutral rare gas clusters

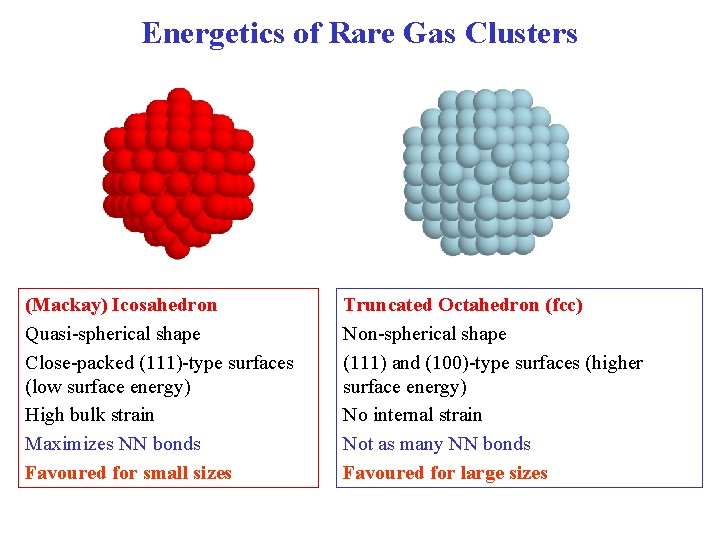
Energetics of Rare Gas Clusters (Mackay) Icosahedron Quasi-spherical shape Close-packed (111)-type surfaces (low surface energy) High bulk strain Maximizes NN bonds Favoured for small sizes Truncated Octahedron (fcc) Non-spherical shape (111) and (100)-type surfaces (higher surface energy) No internal strain Not as many NN bonds Favoured for large sizes

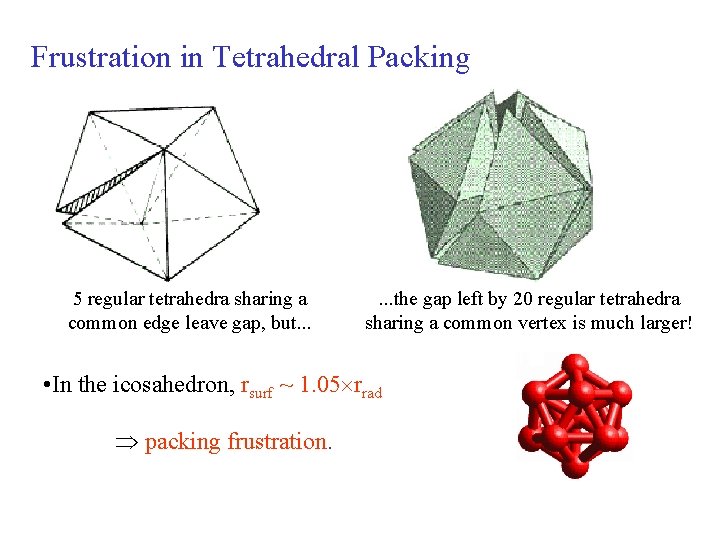
Frustration in Tetrahedral Packing 5 regular tetrahedra sharing a common edge leave gap, but. . . the gap left by 20 regular tetrahedra sharing a common vertex is much larger! • In the icosahedron, rsurf ~ 1. 05 rrad packing frustration.

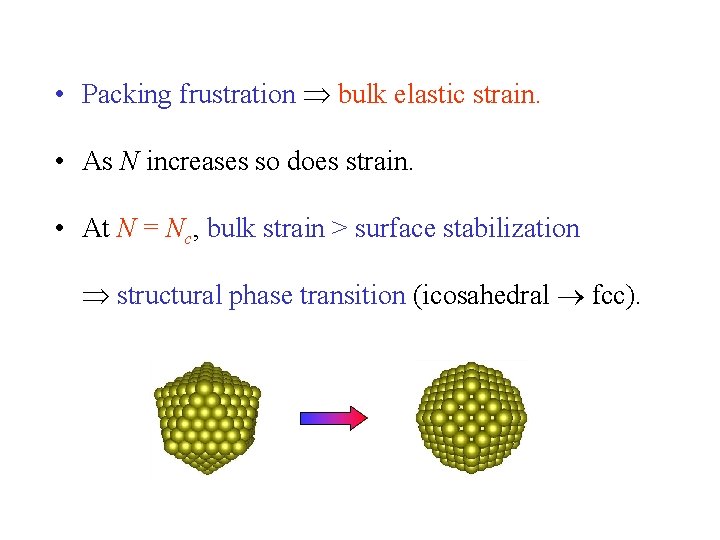
• Packing frustration bulk elastic strain. • As N increases so does strain. • At N = Nc, bulk strain > surface stabilization structural phase transition (icosahedral fcc).

Electron Diffraction Experiments • Electron Diffraction studies (Farges-1983, Lee 1987): 800 Nc 3500 • For N 800, electron diffraction patterns indicate icosahedral geometric shell structures. • Smaller clusters (up to 50– 60 atoms) have the “polytetrahedral” structures, predicted by calculations using the Lennard-Jones potential. • Theoretical Calculations: Nc 10, 000.

Why Do Experiment and Theory Differ? • Calculations are carried out at a cluster temperature of 0 K but cluster temperatures in the electron diffraction experiments were 38 4 K. • The high energy (40– 50 ke. V) electrons used in the diffraction experiments may cause fragmentation of larger clusters, which may have fcc structures, and which are responsible for the observed diffraction patterns.

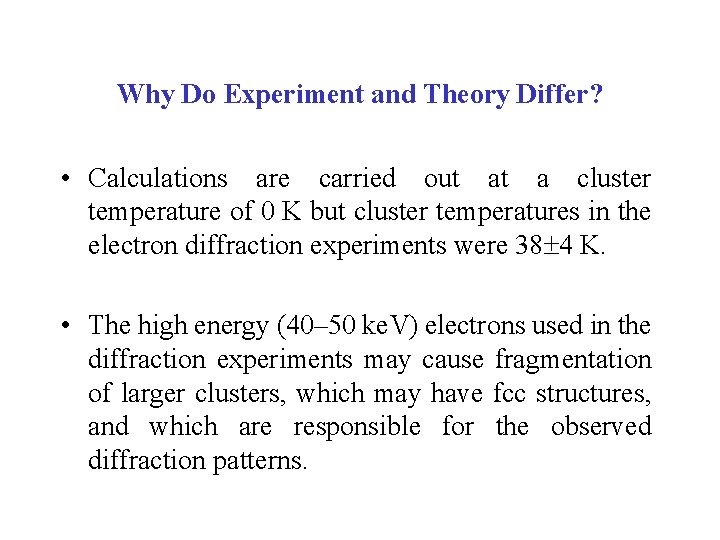
Charged and Excited Rare Gas Clusters Ar 2+ Ar 2* Ar 2 (Å)

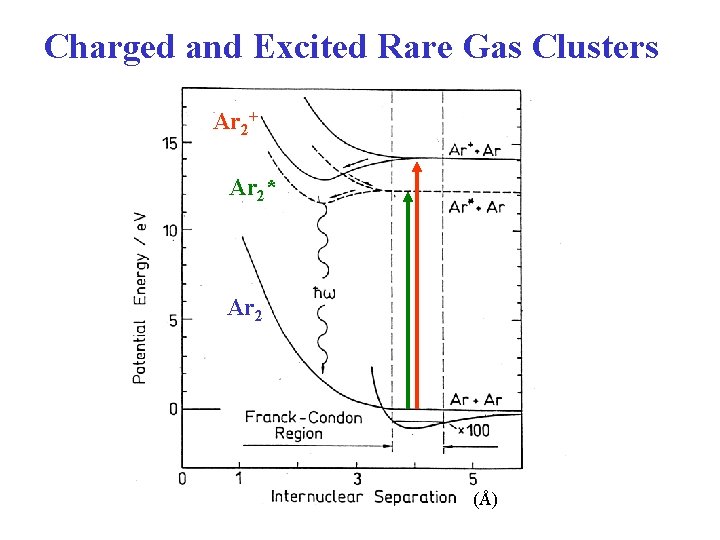
Charged Rare Gas Clusters • Ionization leads to a significant increase in bond strength (decrease in Rg–Rg bond length) due to covalent bonding. • He 2+ (1 )2(2 *)2 bond order = 0 (1 )2(2 *)1 bond order = 0. 5 • Ar 2 bond order = 0 12 me. V Ar 2+ bond order = 0. 5 1. 5 e. V re(Ar 2+) is 30% smaller than re(Ar 2). 1 me. V 2. 5 e. V

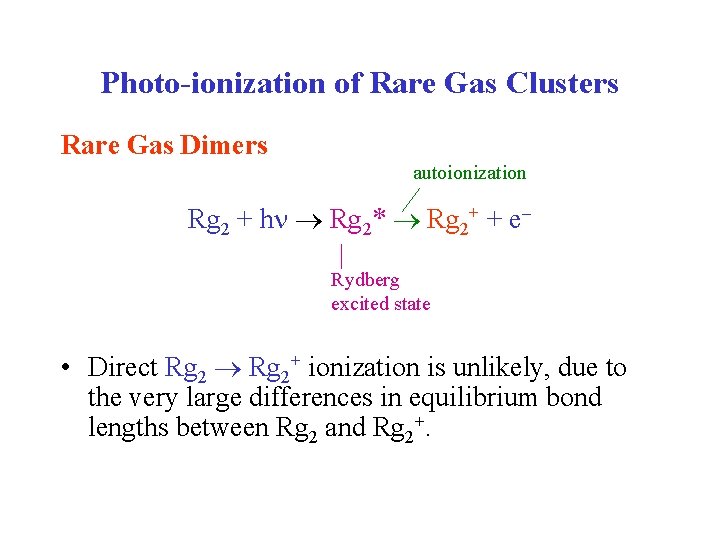
Photo-ionization of Rare Gas Clusters Rare Gas Dimers autoionization Rg 2 + h Rg 2* Rg 2+ + e Rydberg excited state • Direct Rg 2+ ionization is unlikely, due to the very large differences in equilibrium bond lengths between Rg 2 and Rg 2+.

Larger Charged Clusters • Delocalization of charge requires large geometry changes of neighbouring atoms “self localization (trapping)” of charge over small core units. • Ne. N+ = (Ne 2+)Ne. N 2 97% of positive charge resides on “Ne 2+” core. • In heavier Rg clusters, charge may be localized on linear Rg 2+, Rg 3+, Rg 4+ cores.

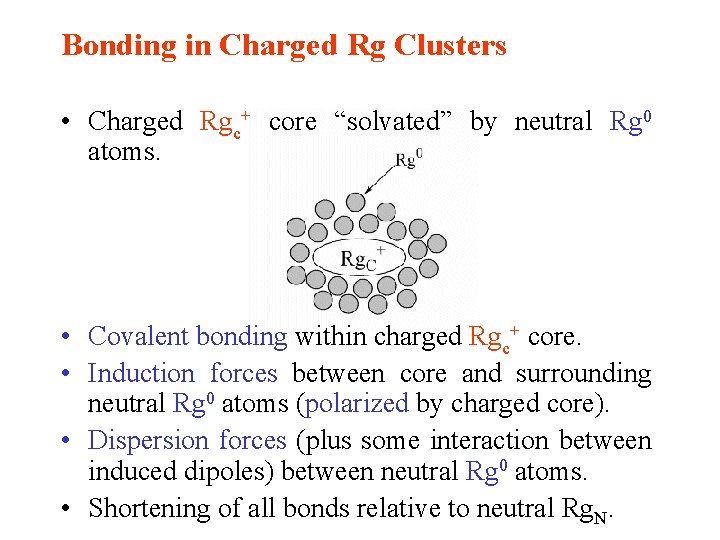
Bonding in Charged Rg Clusters • Charged Rgc+ core “solvated” by neutral Rg 0 atoms. • Covalent bonding within charged Rgc+ core. • Induction forces between core and surrounding neutral Rg 0 atoms (polarized by charged core). • Dispersion forces (plus some interaction between induced dipoles) between neutral Rg 0 atoms. • Shortening of all bonds relative to neutral Rg. N.

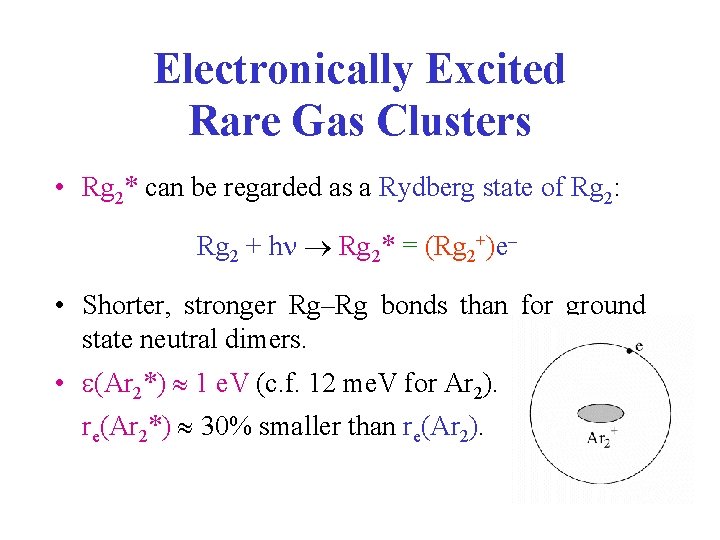
Electronically Excited Rare Gas Clusters • Rg 2* can be regarded as a Rydberg state of Rg 2: Rg 2 + h Rg 2* = (Rg 2+)e • Shorter, stronger Rg–Rg bonds than for ground state neutral dimers. • (Ar 2*) 1 e. V (c. f. 12 me. V for Ar 2). re(Ar 2*) 30% smaller than re(Ar 2).

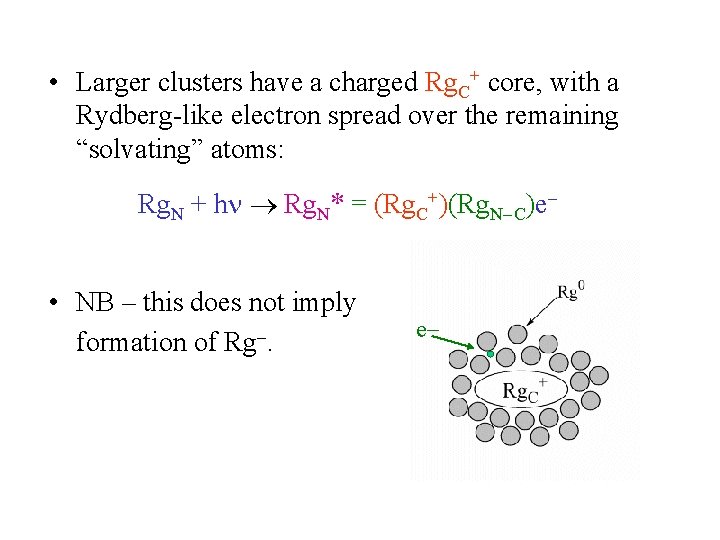
• Larger clusters have a charged Rg. C+ core, with a Rydberg-like electron spread over the remaining “solvating” atoms: Rg. N + h Rg. N* = (Rg. C+)(Rg. N C)e • NB – this does not imply formation of Rg. e

Photoabsorption Spectra of Rg. N+ Clusters • Charged Rgc+ core is the chromophore. rapid electronic-vibrational energy transfer (Rg. C+)(Rg. N C) + h (Rg. C+)*(Rg. N C) [(Rg. C+)(Rg. N C)]# electronically excited core evaporation (Rg. C+)(Rg. N C-M) + MRg • Photodepletion Spectroscopy – scan (UV-vis. ) and map out absorption spectrum by monitoring decrease of intensity of Rg. N+ peak in MS.

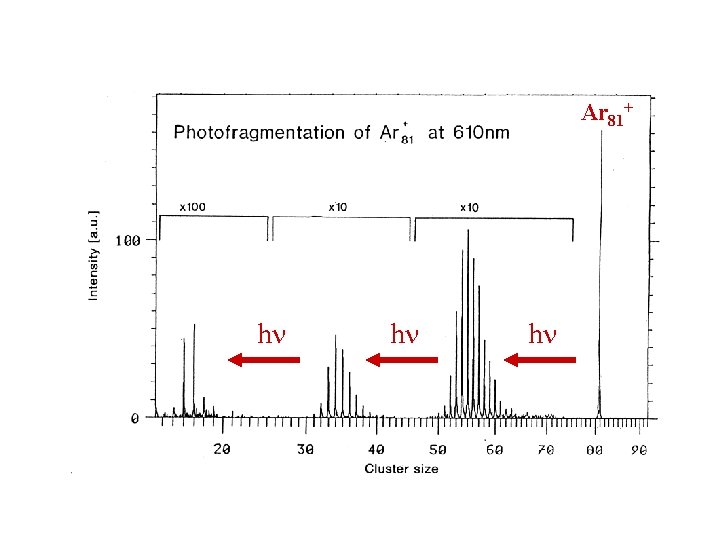
Photofragmentation Spectra of Rg. N+ Clusters • Mass select a particular Rg. N+ cluster. • Irradiate at constant frequency (e. g. h = 2 e. V). • Vary photon flux and record mass spectrum due to fragmentation. • As photon flux , more photons are absorbed and greater fragmentation is observed (the initial photofragments are themselves fragmented etc. ): Rg. N+ + h Rg. A+ + Rg. B+ + … + x. Rg + h …

Ar 81+ h h h

Helium Clusters: Superfluid Droplets • Because of weak vd. W interactions and large zero point energy, quantum effects dominate the physics of He at low T. • He is the only element that is known to remain liquid (at ambient pressure) down to 0 K. Can only be solidified at P > 25 atmospheres (~ 2. 5 106 Pa). • He is an ordinary, viscous liquid (He-I) just below its boiling point (4. 2 K), but for T < 2. 18 K (for 4 He) or T < 3 10 3 K (for 3 He) a phase transition occurs to the superfluid (He-II) state, which has zero viscosity, high heat conduction and quantized circulation. • For 4 He (a boson with nuclear spin I = 0), superfluidity is due to Bose condensation. • For 3 He (a fermion with I = ½), superfluidity may be due to the formation of quasi-Bose particles.

Superfluididy in He Clusters (Droplets) • Droplets of 4 He first observed by Kamerlingh- Onnes (1908). • Becker (1961) used molecular beam techniques to generate 4 He droplets (liquid. He clusters with thousands of atoms). • Gspann (1977) produced a beam of 3 He droplets. • Under exptl. conditions, 4 He clusters are produced with T 0. 38 K, and 3 He clusters are produced with T 0. 15 K. • Comparison with the bulk superfluid temperatures leads to the prediction that 4 He clusters should be superfluid liquid droplets at

• Calculations indicate that superfluidity should be exhibited for 4 He. N clusters with N 69 atoms. • Calculations on mixed 3 He/4 He droplets indicate that spontaneous isotopic separation occurs, producing a droplet with a 4 He core surrounded by 3 He. This has been observed experimentally.

Stabilities of He Clusters • 4 He N clusters calculated to be stable for all sizes – binding energy per He atom rises smoothly from 1. 3 10 3 K for 4 He 2 to 7. 2 K for bulk 4 He (bulk binding energy is reached for clusters with N 104). • 3 He N clusters with N (unbound) < 29 atoms are unstable – total zero point energy exceeds the cluster dissociation wel depth. • For larger 3 He clusters, large oscillations are observed in the binding energy per atom until convergence is reached on the bulk value (2. 7 K) – due to nuclear-spin pairing effects (the 3 He nucleus is a fermion) • Lower binding energy of bulk liquid 3 He is consistent 3

Doped He Droplets • He clusters are loaded with dopant atoms and molecules (D) by a “pick-up” experiment, where preformed He clusters are passed through a chamber containing vaporized dopant atoms or molecules. • As the strength of the D He interaction is greater than the He He interaction, adsorption is accompanied by the evaporation of many (often thousands) of He atoms: He. N + D (D)He. N# (D)He. M + (N M)He • Energy transfer from dopant molecules to the He droplet is very rapid evaporation of He atoms cooling of the adsorbed dopant molecule. • Therefore, liquid He droplets act as ideal matrices (“nanolaboratories”) for performing spectroscopy on very cold molecules.

• Open-shell dopant atoms (e. g. alkali metals) and molecules (e. g. O 2) lie on the surface of liquid helium droplets – due to strong repulsive interactions between the unpaired electrons and He atoms. • Closed-shell atoms and molecules (and most cations) are found at the centre of the He droplet – Cations have strong attractive interactions with neighbouring He atoms, leading to an increase of the density relative to bulk He. • In mixed 3 He/4 He clusters, dopant molecules such as SF 6 are observed to preferentially occupy the 4 He core.

Spectroscopy of Dopants in Helium Droplets • Scoles and Toennies have performed spectroscopic measurements on atoms and molecules doped into He droplets. • They have used photodepletion spectroscopy to measure electronic, vibrational and rotational spectra: (Mol)He. N + h (Mol*)He. N (Mol)He. N# (Mol)He. M# + (N M)He • In liquid 4 He droplets the spectral lines are very sharp, with line widths as narrow as 100 MHz (0. 03 cm 1).

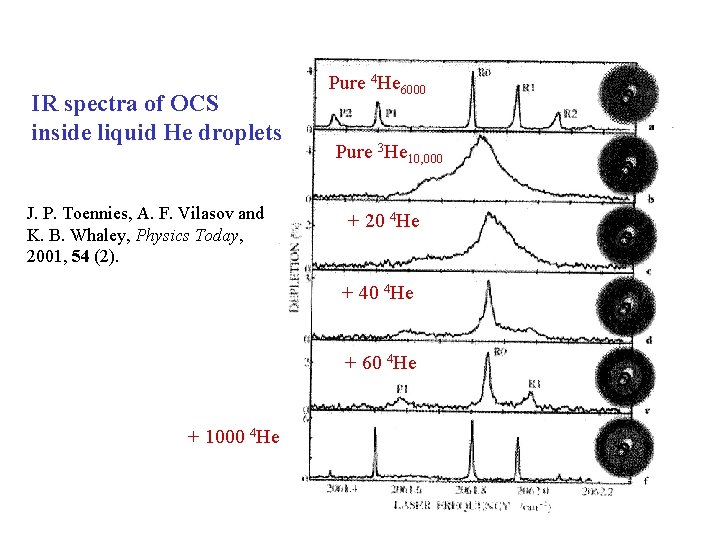
• Scoles and Toennies have detected sharp, well resolved rotational fine structure in the IR spectra of molecules such as SF 6 and OCS in 4 He droplets (N ~ 6, 000) N – indicates free rotation of the molecule in the superfluid (zero viscosity) 4 He droplet. • Under analogous conditions, 3 He droplets are not superfluid – their temperature (0. 15 K) is significantly higher than the bulk superfluid temperature of liquid 3 He (0. 003 K) – see broad peaks in the IR spectrum of OCS ( = 0. 1 cm 1).

• BUT – the addition of ~60 4 He atoms to (OCS)3 He. N (N ~ 12, 000) results in a sharpening of the spectral lines and reappearance of rotational fine structure – the 60 4 He atoms lie at the core of the droplet and solvate the OCS molecule. • The temperature of the cluster (~0. 15 K) is below the superfluid temperature of bulk 4 He (2. 18 K) – the 4 He core of the droplet is superfluid, though the 3 He mantle is not.

IR spectra of OCS inside liquid He droplets J. P. Toennies, A. F. Vilasov and K. B. Whaley, Physics Today, 2001, 54 (2). Pure 4 He 6000 Pure 3 He 10, 000 + 20 4 He + 40 4 He + 60 4 He + 1000 4 He

Atomic & Molecular Clusters 4. Molecular Clusters • Clusters of discrete molecules. • Strong covalent bonds within each molecule. • Weaker intermolecular forces between molecules. • Typical Binding Energy Eb(Mol)N ~ 10 Eb(Rg)N

Why Study Molecular Clusters? • Models of solvation. • Study of localization and transfer of charge and excitation. • Study of fragmentation patterns – exploring reactions. • Models for atmospheric reactions (e. g. taking place within or on the surface of water droplets). • Use of size-controlled molecular clusters as “nanolaboratories” – investigate fundamental reactions in a controlled manner, at the molecular level • Biomolecular clusters – clusters of biophysically relevant molecules (e. g. experimental conformational studies of solvated polypeptides as models for in vivo proteins).

Intermolecular Interactions • Dipole-dipole forces – between permanent dipoles (polar molecules) + + – e. g. (HCl)N, (ICl)N I • Higher order multipoles Cl I Cl – e. g. (CO 2)N, (C 6 H 6)N - quadrupoles • Induction forces – dipoles induced by charged or polar molecules – e. g. (HCl)(C 6 H 6) • (London) Dispersion forces – present in all molecular clusters – interactions between fluctuating electron distributions (as in rare gas clusters). • Binding energy Eb 100 me. V/molecule

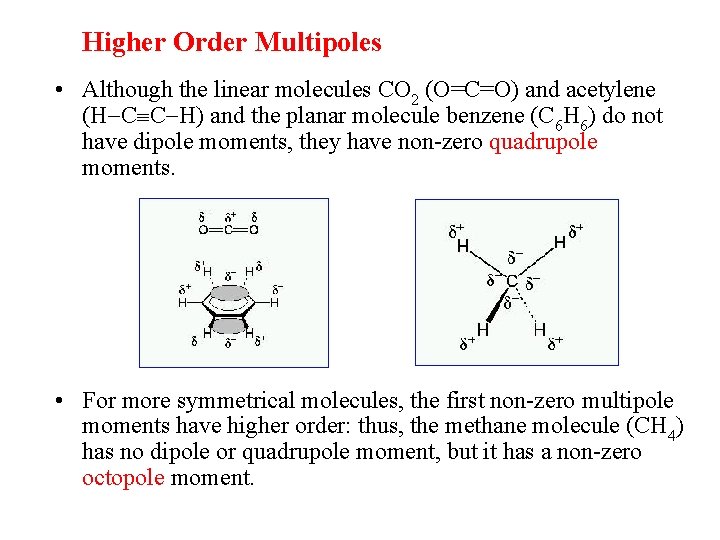
Higher Order Multipoles • Although the linear molecules CO 2 (O=C=O) and acetylene (H C C H) and the planar molecule benzene (C 6 H 6) do not have dipole moments, they have non-zero quadrupole moments. • For more symmetrical molecules, the first non-zero multipole moments have higher order: thus, the methane molecule (CH 4) has no dipole or quadrupole moment, but it has a non-zero octopole moment.

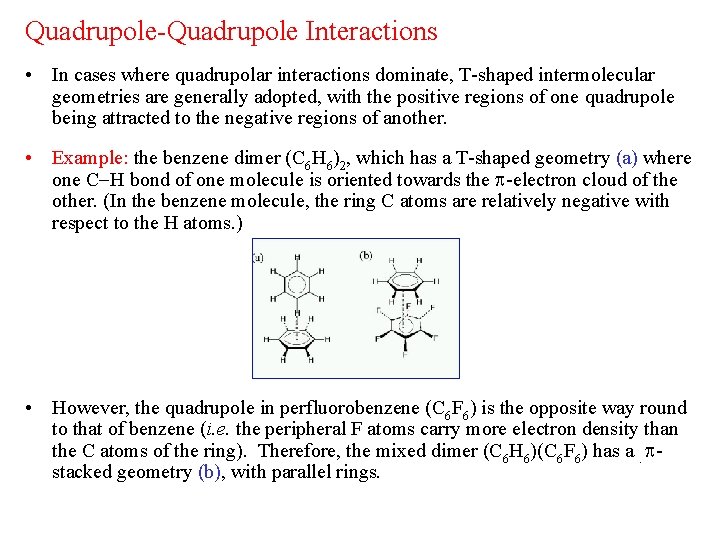
Quadrupole-Quadrupole Interactions • In cases where quadrupolar interactions dominate, T-shaped intermolecular geometries are generally adopted, with the positive regions of one quadrupole being attracted to the negative regions of another. • Example: the benzene dimer (C 6 H 6)2, which has a T-shaped geometry (a) where one C H bond of one molecule is oriented towards the -electron cloud of the other. (In the benzene molecule, the ring C atoms are relatively negative with respect to the H atoms. ) • However, the quadrupole in perfluorobenzene (C 6 F 6) is the opposite way round to that of benzene (i. e. the peripheral F atoms carry more electron density than the C atoms of the ring). Therefore, the mixed dimer (C 6 H 6)(C 6 F 6) has a. stacked geometry (b), with parallel rings.

Hydrogen Bonding • A hydrogen bond is a short-ranged attractive interaction of the form X H : Y, where a hydrogen atom is covalently bound to one electronegative atom (X = N, O, F etc. ) and interacts with a second electronegative atom (Y: ), which has an accessible lone-pair of electrons. X H – hydrogen bond donor. Y: – hydrogen bond acceptor. • Very important in water clusters, biological molecules etc. • Eb 300 me. V/H-bond

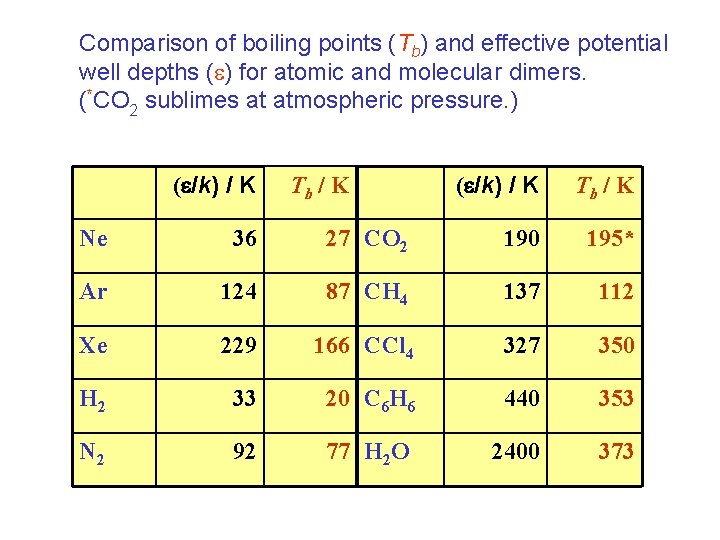
Comparison of boiling points (Tb) and effective potential well depths ( ) for atomic and molecular dimers. (*CO 2 sublimes at atmospheric pressure. ) ( /k) / K Tb / K Ne 36 27 CO 2 190 195* Ar 124 87 CH 4 137 112 Xe 229 166 CCl 4 327 350 H 2 33 20 C 6 H 6 440 353 N 2 92 77 H 2 O 2400 373

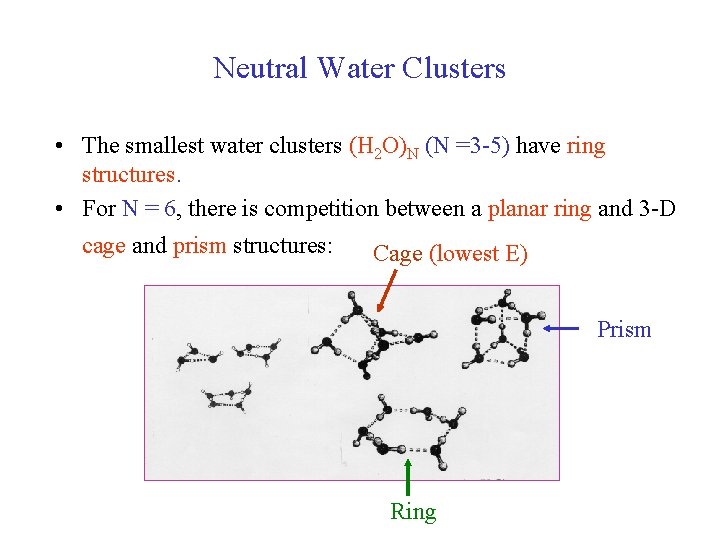
Neutral Water Clusters • The smallest water clusters (H 2 O)N (N =3 -5) have ring structures. • For N = 6, there is competition between a planar ring and 3 -D cage and prism structures: Cage (lowest E) Prism Ring

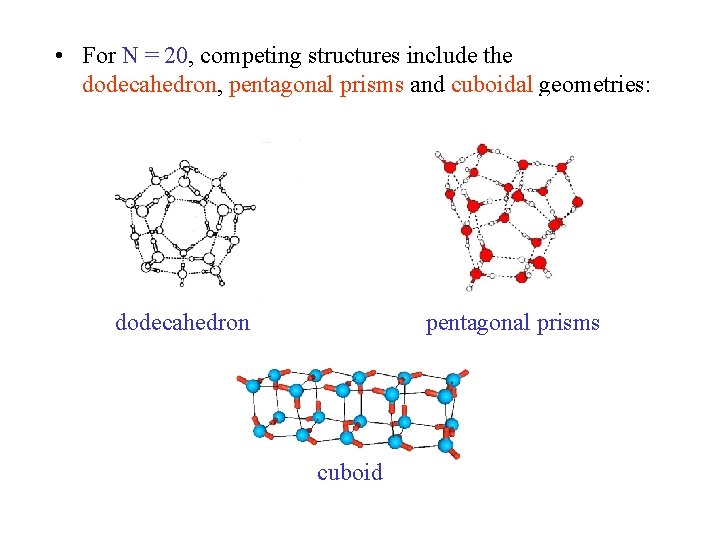
• For N = 20, competing structures include the dodecahedron, pentagonal prisms and cuboidal geometries: dodecahedron pentagonal prisms cuboid

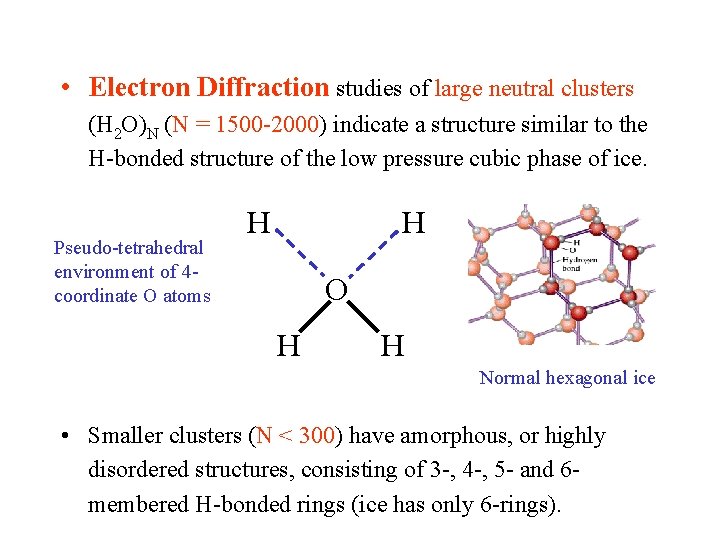
• Electron Diffraction studies of large neutral clusters (H 2 O)N (N = 1500 -2000) indicate a structure similar to the H-bonded structure of the low pressure cubic phase of ice. Pseudo-tetrahedral environment of 4 coordinate O atoms H H O H H Normal hexagonal ice • Smaller clusters (N < 300) have amorphous, or highly disordered structures, consisting of 3 -, 4 -, 5 - and 6 membered H-bonded rings (ice has only 6 -rings).

Infra Red Spectra • Large clusters (up to N ~ 10, 000) have spectra similar to crystalline ice. • Smaller clusters (N ~ 100) have spectra similar to amorphous ice.

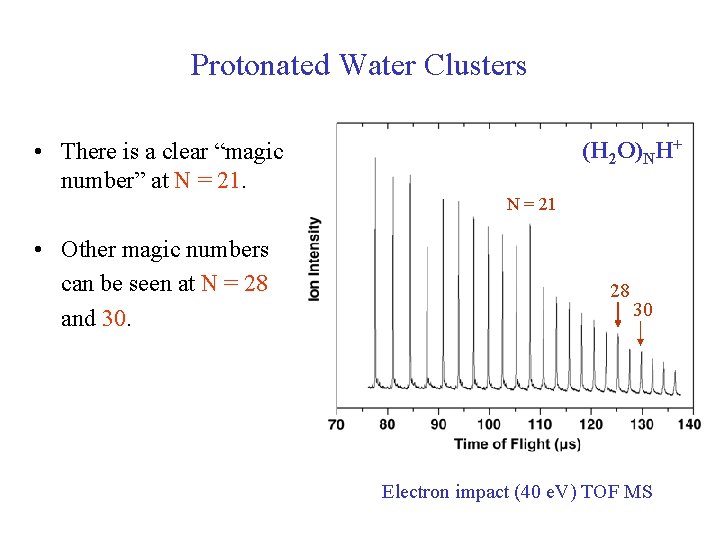
Protonated Water Clusters (H 2 O)NH+ • There is a clear “magic number” at N = 21 • Other magic numbers can be seen at N = 28 and 30. 28 30 Electron impact (40 e. V) TOF MS

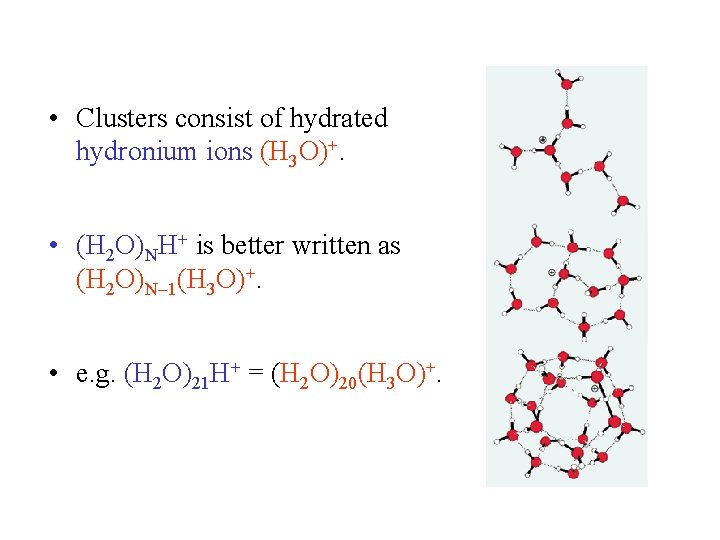
• Clusters consist of hydrated hydronium ions (H 3 O)+. • (H 2 O)NH+ is better written as (H 2 O)N 1(H 3 O)+. • e. g. (H 2 O)21 H+ = (H 2 O)20(H 3 O)+.

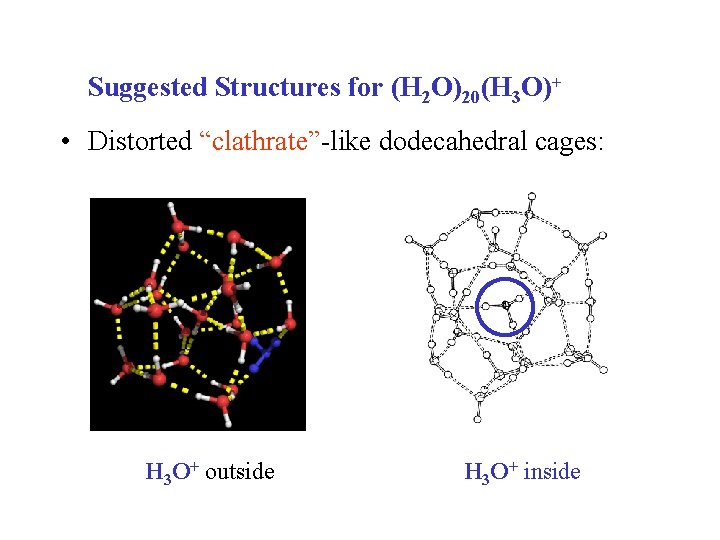
Suggested Structures for (H 2 O)20(H 3 O)+ • Distorted “clathrate”-like dodecahedral cages: H 3 O+ outside H 3 O+ inside

Electron Impact Studies of Water Clusters 1. High Energy Electrons (40 e. V) • • Ionization accompanied by fragmentation. Main products = protonated water clusters. (H 2 O)N + e (H 2 O)MH+ + … 2. Medium Energy Electrons (6 -14 e. V) • • Electron capture accompanied by fragmentation. Main products = water-hydroxide clusters. (H 2 O)N + e (H 2 O)M(OH) + … • Electron Affinity EA ~ 1. 8 e. V

3. Low Energy Electrons (< 1 e. V) • • Electron capture. Products = anionic water clusters = “solvated electrons”. (H 2 O)N + e (H 2 O)N + … • For colder H 2 O clusters, (H 2 O)N is stabilized for smaller values of N. • Cooling achieved by supersonic expansion of a low concentration of water clusters (~ 2%) in Ar.

• At higher cluster T (or using more energetic electrons), the anionic cluster is generated in an excited state. It relaxes by evaporating and fragmenting H 2 O molecules: [(H 2 O)N ]* (H 2 O)M(OH) + … • Electron Affinity of (H 2 O)N increases (i. e. [(H 2 O)N ] is more stable) as N increases – due to better electron solvation.

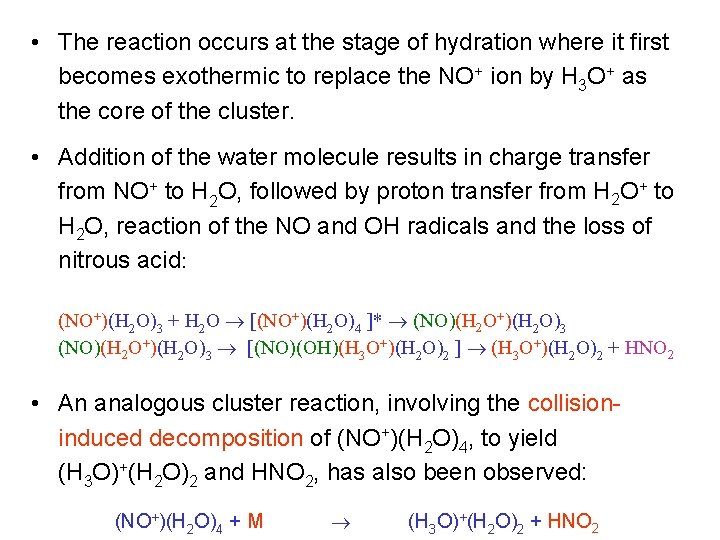
Reactions of Molecular Clusters 1. Cluster-Promoted Reactions • There are many examples where reactivity is initiated or promoted by clustering, and where the degree of clustering (cluster size) influences the favoured reaction channel. • Example: NO+ does not react with a single water molecule, but the cluster (NO+)(H 2 O)3 undergoes the following bimolecular reaction with a further water molecule: (NO+)(H 2 O)3 + H 2 O (H 3 O+)(H 2 O)2 + HNO 2

• The reaction occurs at the stage of hydration where it first becomes exothermic to replace the NO+ ion by H 3 O+ as the core of the cluster. • Addition of the water molecule results in charge transfer from NO+ to H 2 O, followed by proton transfer from H 2 O+ to H 2 O, reaction of the NO and OH radicals and the loss of nitrous acid: (NO+)(H 2 O)3 + H 2 O [(NO+)(H 2 O)4 ]* (NO)(H 2 O+)(H 2 O)3 [(NO)(OH)(H 3 O+)(H 2 O)2 ] (H 3 O+)(H 2 O)2 + HNO 2 • An analogous cluster reaction, involving the collisioninduced decomposition of (NO+)(H 2 O)4, to yield (H 3 O)+(H 2 O)2 and HNO 2, has also been observed: (NO+)(H 2 O)4 + M (H 3 O)+(H 2 O)2 + HNO 2

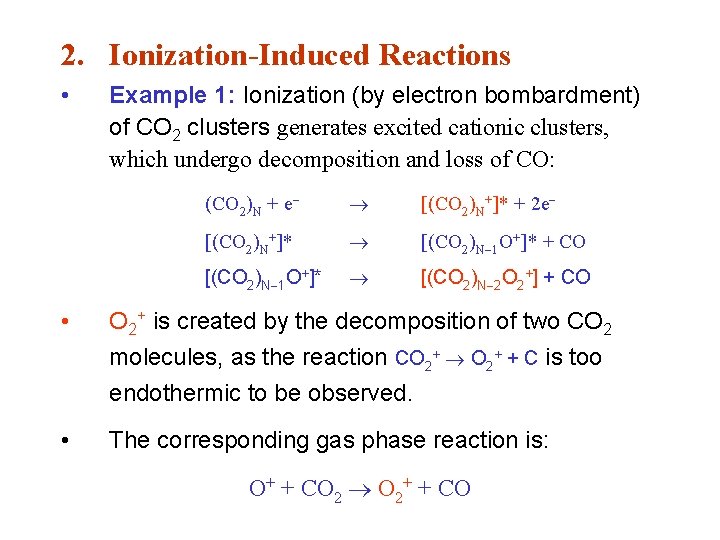
2. Ionization-Induced Reactions • Example 1: Ionization (by electron bombardment) of CO 2 clusters generates excited cationic clusters, which undergo decomposition and loss of CO: (CO 2)N + e [(CO 2)N+]* + 2 e [(CO 2)N+]* [(CO 2)N 1 O+]* + CO [(CO 2)N 1 O+]* [(CO 2)N 2 O 2+] + CO • O 2+ is created by the decomposition of two CO 2 molecules, as the reaction CO 2+ + C is too endothermic to be observed. • The corresponding gas phase reaction is: O+ + CO 2 O 2+ + CO

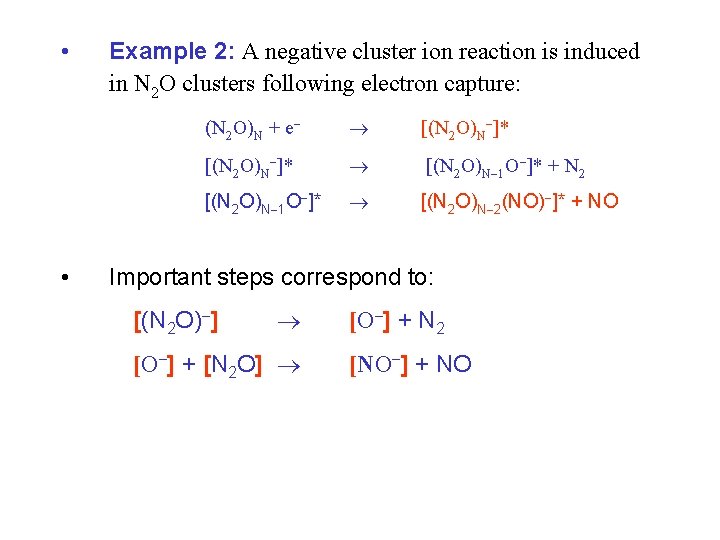
• • Example 2: A negative cluster ion reaction is induced in N 2 O clusters following electron capture: (N 2 O)N + e [(N 2 O)N ]* [(N 2 O)N 1 O ]* + N 2 [(N 2 O)N 1 O ]* [(N 2 O)N 2(NO) ]* + NO Important steps correspond to: [(N 2 O) ] [O ] + [N 2 O] [O ] + N 2 [NO ] + NO

3. Cluster-Hindered Reactions • The opposite of cluster-promoted reactions. • The presence of “solvent” molecules in the cluster hinders or blocks a particular reaction channel. . • Example: The photodissociation of the CO 3 anion: CO 3 + h CO 2 + O is blocked in small (CO 3 )(H 2 O)N clusters (N = 1– 3), where the preferred reaction channel is the loss of water from the cluster.

4. Ion-Molecule Reactions • Ion-molecule reactions often have high reaction rates, due to low (or zero) activation barriers. • They are responsible for many important processes – e. g. in the Earth’s atmosphere (and those of other planets) and in interstellar space.

Atmospheric Cluster Chemistry • In the ionosphere, the cation NO+ is present in high abundance, due to photolysis of “NOx” pollutants. • It is believed that NO+ is a nucleation site for the stepwise growth of small water clusters, as far as the addition of three water molecules: NO+ + 3 H 2 O (NO+)(H 2 O)3 • The next water molecule to be added, results in charge transfer from NO+ to H 2 O, fragmentation of a water molecule and the loss of nitrous acid (as described previously).