Atomic Molecular Clusters 1 Introduction Introduction What are








- Slides: 47

Atomic & Molecular Clusters 1. Introduction

Introduction • • • What are clusters? Types of cluster Why study clusters? Cluster size effects Scaling Laws Spherical cluster approximation • Cluster-surface analogy • Nanoscience • Importance of theory in cluster science

What Are Clusters? • Aggregates of 2– 10 n (n 6 or 7) particles (atoms or molecules). • Constituent particles may be identical or they can be two or more different species. • Clusters may be studied in the gas phase, in a cluster “molecular” beam, adsorbed onto a surface or trapped in an inert matrix.

The Abundance of Clusters • Clusters are formed by most of the elements in the periodic table – even the noble gases! • Clusters of the coinage metals (copper, silver and gold) are found in stained glass windows. • Silver clusters are important in photography. • Molecular clusters are present in the atmosphere. • Carbon nanoclusters (e. g. C 60 and related fullerenes) may be present in soot and even in space.

Types of Cluster (1) Metal Clusters • s-block metals (e. g. alkali and alkaline earth metals) – bonding is “metallic” (delocalised and nondirectional) involving mainly the valence sorbitals. • sp-metals (e. g. aluminium) – bonding has some covalent character. • Transition metals – greater degree of covalency and directionality in the bonding - involving the valence d orbitals.

Types of Cluster (2) Semiconductor Clusters • Composed of elements (e. g. C, Si & Ge) which are semiconductors in the solid state. • Includes compound semiconductor (heteroatomic) clusters, with polar covalent-bonds (e. g. Gax. Asy). • Bonding is covalent – bonds are directional and strong.

Types of Cluster (3) Ionic Clusters • Heteroatomic clusters composed from atoms with large difference in electronegativity. • Bonding is predominately ionic (electrostatic). • Examples: alkali metal halides [Nax. Cly](x y)+ and magnesium oxides [Mgx. Oy]2(x y)+.

Types of Cluster (4) Rare Gas Clusters • Form at low temperatures. • Bound by weak van der Waals dispersion forces. • Inter-atomic attraction increases with increasing atomic mass (He Rn).

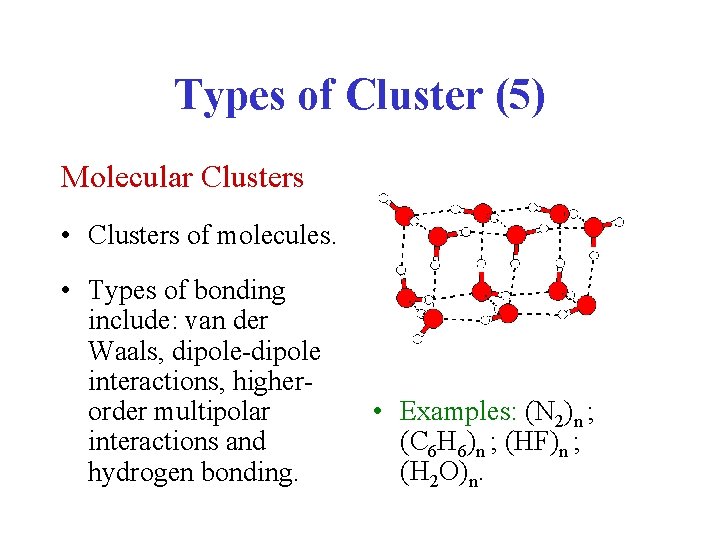
Types of Cluster (5) Molecular Clusters • Clusters of molecules. • Types of bonding include: van der Waals, dipole-dipole interactions, higherorder multipolar interactions and hydrogen bonding. • Examples: (N 2)n ; (C 6 H 6)n ; (HF)n ; (H 2 O)n.

Types of Cluster (6) Cluster Molecules • Rich chemistry of inorganic and organometallic clusters – developed over the second half of the 20 th century. • Generally thermodynamically and/or kinetically stable with respect to coalescence and can exist in the solid, liquid and vapour phases. • Examples: P 4 ; [B 12 H 12]2 - ; Os 6(CO)18.

Why Study Clusters? (1) • Clusters are of fundamental interest: due to their intrinsic properties because of their central position between molecular and condensed matter science. • Clusters span a wide range of particle size – from molecular (well separated, quantized states) to microcrystalline (quasi-continuous states). • Clusters constitute new materials (nano-particles) which may have properties that are distinct from those of discrete molecules or bulk matter.

Why Study Clusters? (2) • Fundamental interest in the evolution of geometric and electronic structures of clusters (and chemical and physical properties) with cluster size. • “How large must a cluster be before its properties resemble those of the bulk element? ” • (Answer depends on the type of cluster and which property). • The high ratio of surface to interior atoms in clusters means that there are many common features between clusters and bulk surfaces.

Cluster Size Effects (1) • To what extent do cluster properties resemble those of discrete molecules or infinite solids? • Can the study of large finite clusters tell us anything about the bonding or explain the properties of bulk solids? • How rapidly do cluster structures and other properties converge towards those of the bulk as the nuclearity (size) increases?

Cluster Size Effects (2) • Can the evolution of band structure with increasing cluster size be detected? • For clusters of metallic elements, at what cluster size is metallic conductivity first observed? • Can phase transitions be observed and are they of the same type found for bulk solids and surfaces? • By studying the geometric structures of clusters, how their structures change as a function of size, and cluster growth patterns, can we gain an understanding of crystal growth at the microscopic level?

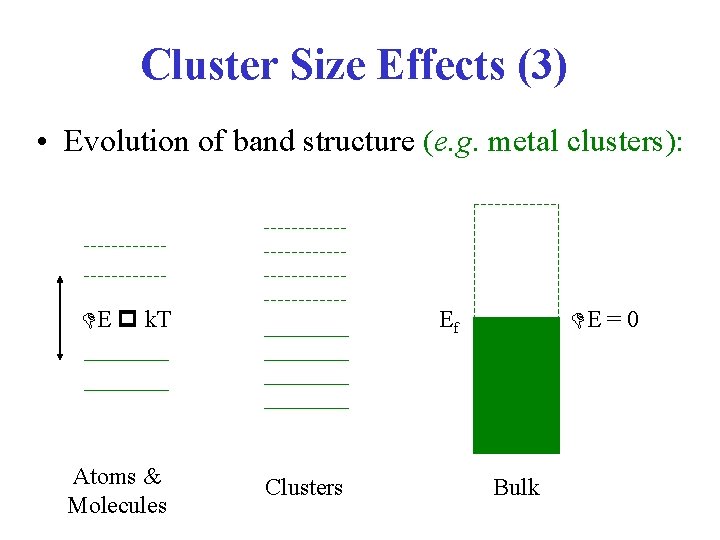
Cluster Size Effects (3) • Evolution of band structure (e. g. metal clusters): E k. T Atoms & Molecules E = 0 Ef Clusters Bulk

Cluster Size Effects (4) • Size-induced structural phase transitions

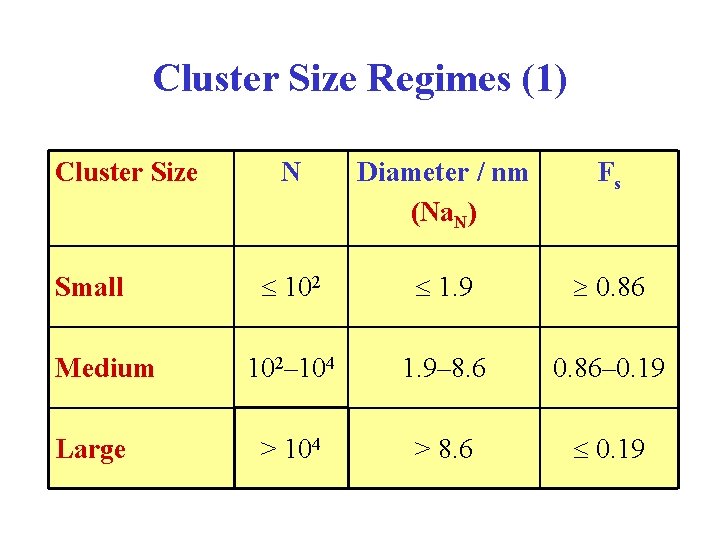
Cluster Size Regimes (1) Cluster Size Small Medium Large N Diameter / nm (Na. N) Fs 102 1. 9 0. 86 102– 104 1. 9– 8. 6 0. 86– 0. 19 > 104 > 8. 6 0. 19

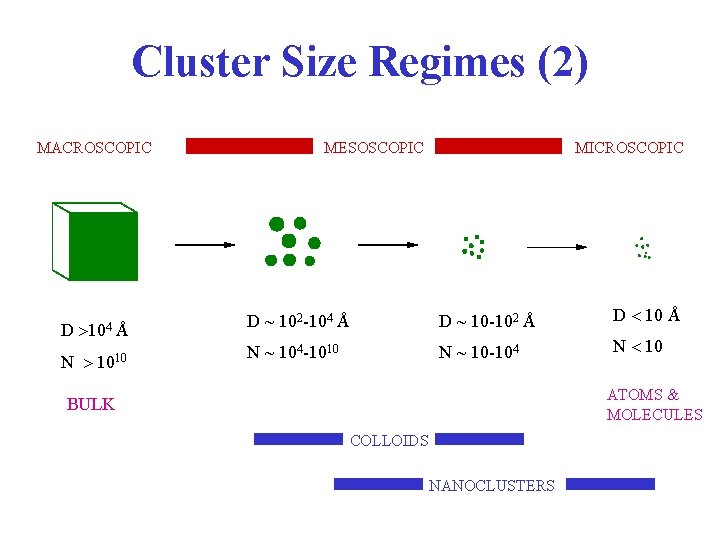
Cluster Size Regimes (2) MACROSCOPIC D 104 Å N 1010 MESOSCOPIC MICROSCOPIC D ~ 102 -104 Å D ~ 10 -102 Å D 10 Å N ~ 104 -1010 N ~ 10 -104 N 10 ATOMS & MOLECULES BULK COLLOIDS NANOCLUSTERS

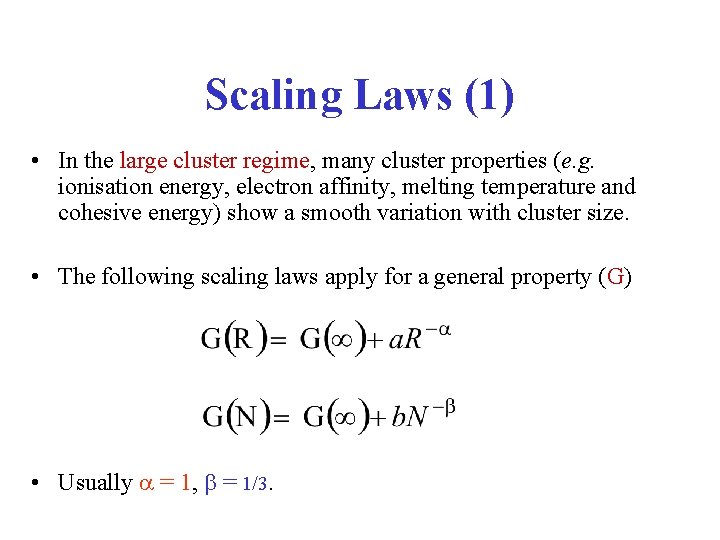
Scaling Laws (1) • In the large cluster regime, many cluster properties (e. g. ionisation energy, electron affinity, melting temperature and cohesive energy) show a smooth variation with cluster size. • The following scaling laws apply for a general property (G) • Usually = 1, = 1/3.

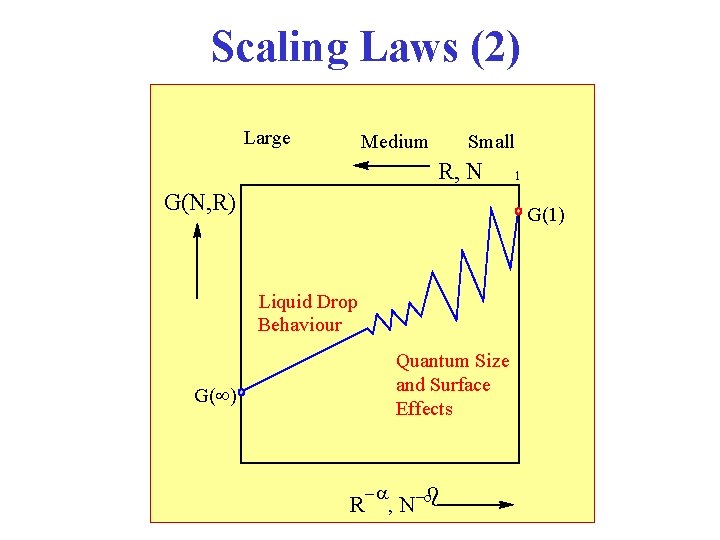
Scaling Laws (2) Large Medium Small R, N G(N, R) 1 G(1) Liquid Drop Behaviour Quantum Size and Surface Effects G( ) R , N b

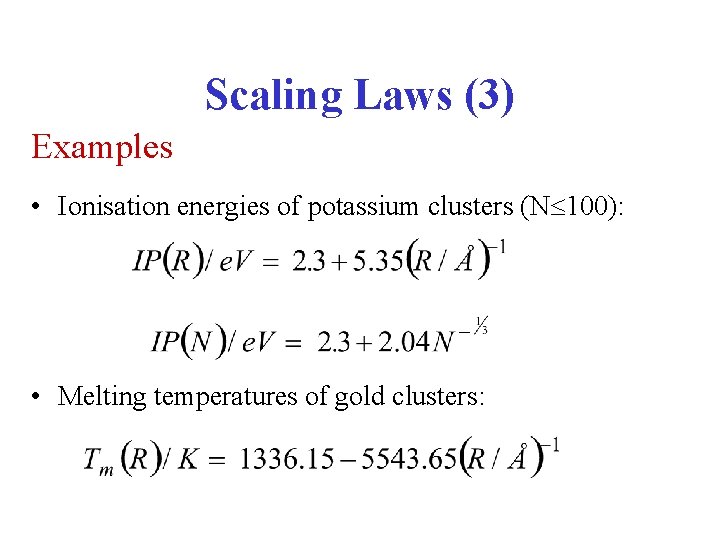
Scaling Laws (3) Examples • Ionisation energies of potassium clusters (N 100): • Melting temperatures of gold clusters:

Scaling Laws (4) Deviations from Scaling Laws • Large deviations (oscillations about the smooth trend) are observed for many properties in the medium and (especially) the small cluster size regimes. • Deviations arise due to Quantum Size Effects (electronic shell closings) and Surface Effects (geometric shell closings).

Spherical Cluster Approximation (1) • N-atom cluster modelled by sphere. • Cluster volume: Vc = NVa • Cluster radius: N 1/3 Ra Rc = • Cluster surface area: Ac = N 2/3 Aa • No. surface atoms: Ns = 4 N 2/3 • Fraction of surface atoms: Fs = Ns/N = 4 N 1/3 • Fs < 0. 01 (1%) for N > 64, 000 atoms.

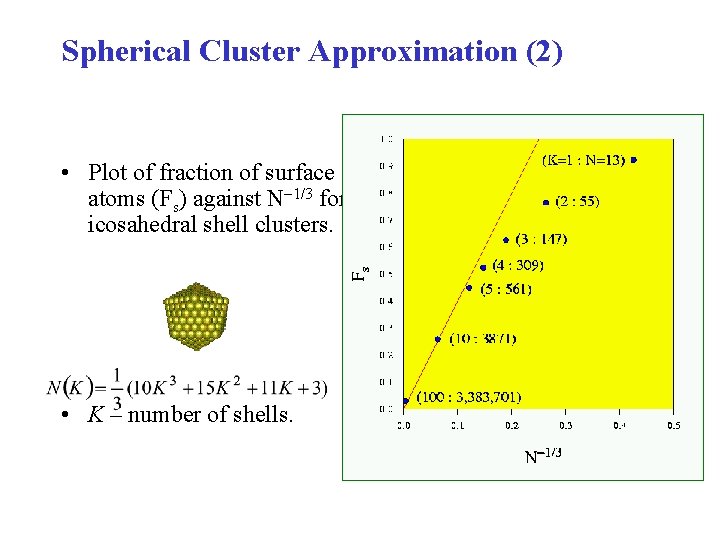
Spherical Cluster Approximation (2) • Plot of fraction of surface atoms (Fs) against N 1/3 for icosahedral shell clusters. • K – number of shells.

Cluster-Surface Analogy (1) • Clusters have a high percentage of their atoms on the surface. • There is a strong link between the chemistry and physics of clusters and that of the surfaces of bulk matter. • Cluster surface rearrangements, analogous to the reconstructions observed for bulk surfaces, which lower the cluster’s surface energy by forming additional surface bonds. • Clusters may be stabilised by the coordination of ligands to the surface.

Cluster-Surface Analogy (2) • The reactivity of under-coordinated surface atoms makes clusters of interest as models for heterogeneous catalysis on bulk metal surfaces. • Metal clusters (generally supported on an inert oxide substrate) can themselves be used as very finely dispersed metal for catalysis.

Nanoscience (1) • The study of particles and structures with dimensions of the order of a nanometre. Quantum dots etc. • Derives from an article by Richard Feynman (1960): “There’s Plenty of Room at the Bottom”. • Feynman challenged scientists to develop a new field of study where devices and machines could be constructed from components consisting of a small number of atoms. • Clusters will play an increasingly important role as building blocks in nanoscale devices.

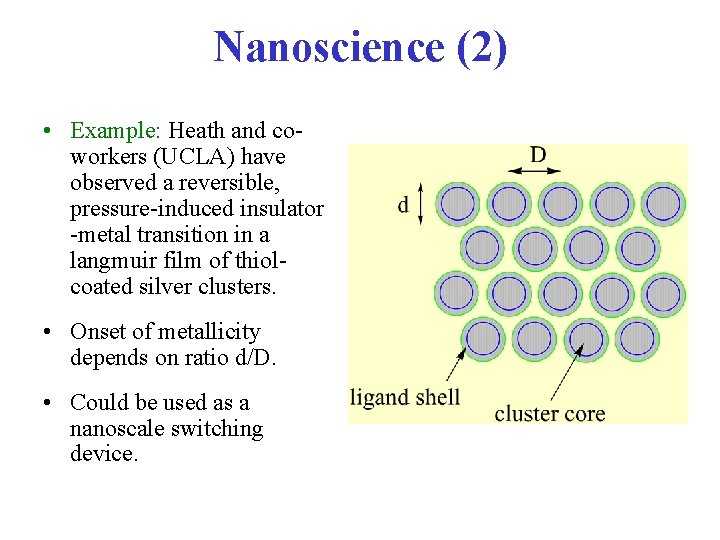
Nanoscience (2) • Example: Heath and coworkers (UCLA) have observed a reversible, pressure-induced insulator -metal transition in a langmuir film of thiolcoated silver clusters. • Onset of metallicity depends on ratio d/D. • Could be used as a nanoscale switching device.

The Importance of Theory • Many cluster properties (e. g. cluster geometries, binding energies and energy barriers) are not easily measured directly from experiment. • Theoretical models and computational methods have been very useful in helping to interpret spectroscopic (e. g. UV/visible and photoelectron) and mass spectrometric data. • Clusters constitute an exacting testing ground for theoretical methods – testing the range of validity of theoretical models derived from the extremes of atomic/molecular and solid state physics.

Atomic & Molecular Clusters 2. Experimental Methods Generic Cluster Experiment Generation (including Nucleation, Growth & Condensation) Sources Flow/Expansion Interaction/Investigation Detection Ionization Fragmentation Spectroscopy Reaction Ions Neutrals Photons

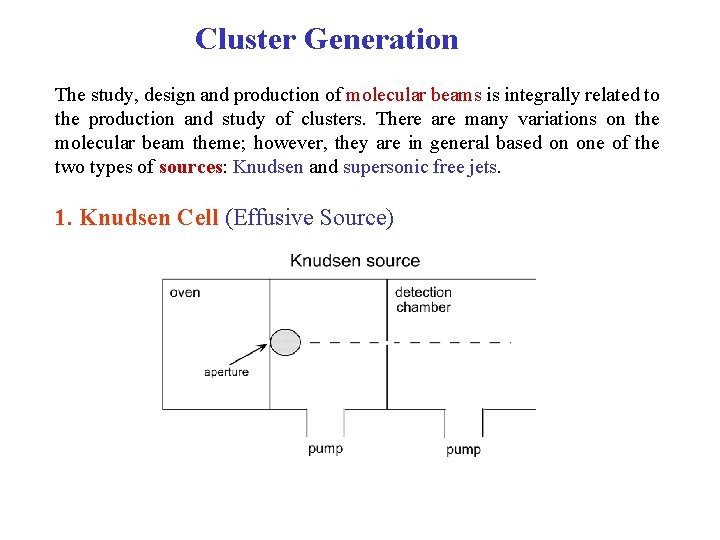
Cluster Generation The study, design and production of molecular beams is integrally related to the production and study of clusters. There are many variations on the molecular beam theme; however, they are in general based on one of the two types of sources: Knudsen and supersonic free jets. 1. Knudsen Cell (Effusive Source)

• Thermal source (Maxwell-Boltzmann distribution of energies). • Heat solid (or liquid) of low vapour pressure – mean free path of particles in cell > daperture – very few collisions before leaving cell • daperture small enough: solid–gas eqm. not perturbed. • Generates M 1 , M 2 , M 3. . MN in equilibrium distribution – cluster intensity: I(N) ≈ ae-b. N – broad energy and angular distribution – continuous source Advantages simple (cheap) I(N) Eb (small clusters) Disadvantages low cluster flux low nuclearity limited to volatile elements

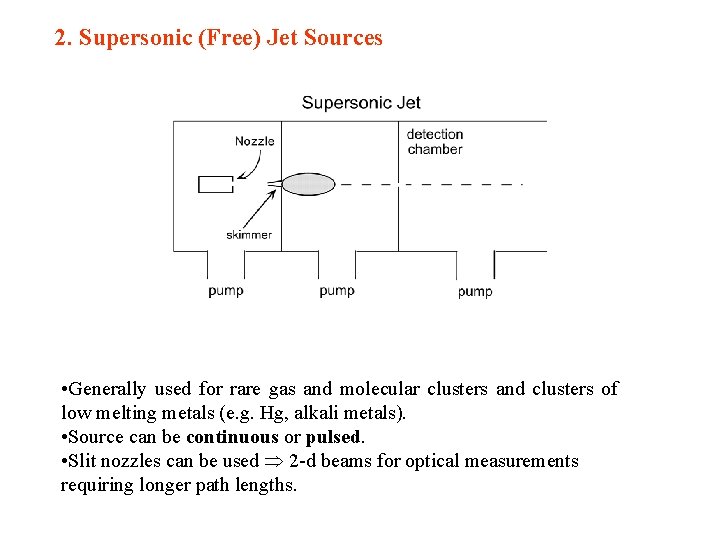
2. Supersonic (Free) Jet Sources • Generally used for rare gas and molecular clusters and clusters of low melting metals (e. g. Hg, alkali metals). • Source can be continuous or pulsed. • Slit nozzles can be used 2 -d beams for optical measurements requiring longer path lengths.

• Atomic vapour expanded from high pressure region (104– 107 Pa) into vacuum through a small nozzle (daperture ~ 0. 03– 1 mm). • Mean free path « d – many collisions during expansion – adiabatic + isenthalpic expansion – no collisions after expansion • Mean velocity v increases (therefore KE rises) • BUT random thermal motions reduced – very low relative velocities – low T in beam but high absolute velocity • Can study pure clusters or mixtures • Can pass beam through a pick-up chamber – pick up atoms, ions, electrons, molecules • Ionized clusters can be generated by introducing an electric discharge on the high or low pressure side of the aperture

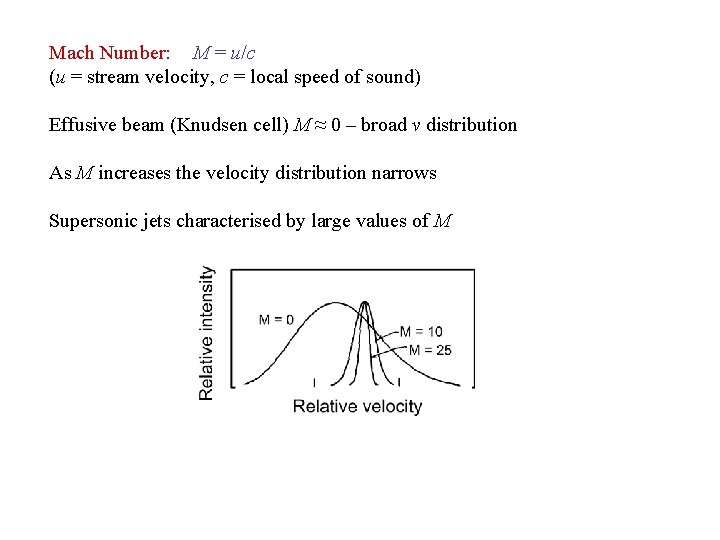
Mach Number: M = u/c (u = stream velocity, c = local speed of sound) Effusive beam (Knudsen cell) M ≈ 0 – broad v distribution As M increases the velocity distribution narrows Supersonic jets characterised by large values of M

Cluster Formation • 3 Factors influence clustering: – stagnation (or backing) pressure (p 0) – aperture cross section (daperture) – initial gas temperature (T 0) • The cluster content (number of clusters) and average cluster size N increase with increasing p 0 and daperture, and decrease with increasing T 0. • Low T 0, high collision rate cluster nucleation, growth and coalescence.

Nucleation, Growth and Condensation • Cluster Nucleation M + M M 2 + M – excess energy removed by third atom (KE) – dimer acts as site of further growth and condensation • Cluster Growth MN + M MN+1 • Cluster Condensation MN + MP MN+P (or MN+P-X + X M)

Cluster Temperature • It is difficult to measure and define T for an individual cluster. • If there is negligible clustering T is generally low. • BUT cluster formation causes an increase in T: – cluster formation is exothermic – internal energy increases due to heat of condensation of added atoms – in heavily clustered beams (high ratio clusters/atoms) the clusters are very hot (possibly molten).

Mechanisms for Cluster Cooling 1. Collisional Cooling By collision (and energy transfer) with other atoms in the beam: MN**(T 1) + A MN*(T 2<T 1) + A (inc. KE) A = M or rare gas (usually in excess). 2. Evaporative Cooling By evaporation (generally loss of one atom at a time for rare gas and metal clusters) – energy released as KE of emitted atom: MN**(T 1) MN-1*(T 2<T 1) + M (KE) The dominant cooling mechanism once free flight is achieved. 3. Radiative Cooling By emission of IR radiation: MN**(T 1) MN*(T 2<T 1) + h (IR) Inefficient and slow (compared with time scales of experiments).

Distribution of Cluster Sizes Depends on initial conditions (T 0, p 0), presence of carrier gas, size and shape of nozzle etc. Photoionization mass spectra for sodium in inert gas-seeded beams. A 0. 3 mm aperture cylindrical nozzle was used throughout. The oven temperature was 800°C, corresponding to a Na vapour pressure of 350 Torr. Inert gas backing pressures were 1. 3 atm in each case. Larger Na cluster ions were detected for heavier carrier gases. Note that expansion of Na vapour through the same nozzle without carrier gas results in comparatively small cluster abundances.

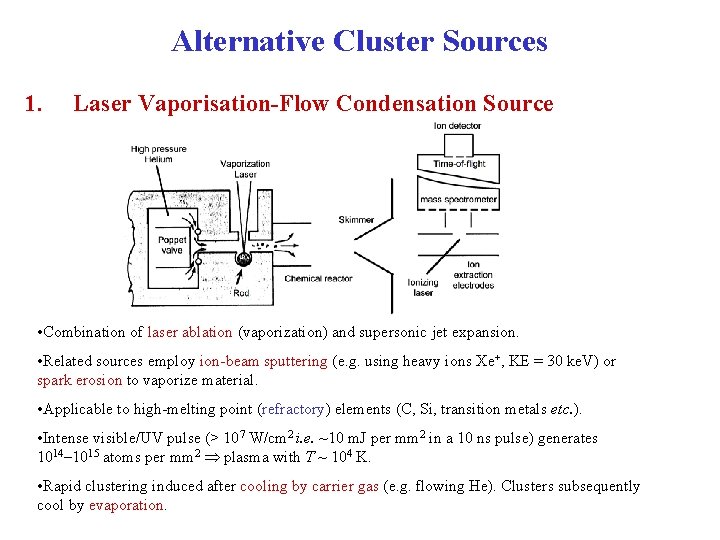
Alternative Cluster Sources 1. Laser Vaporisation-Flow Condensation Source • Combination of laser ablation (vaporization) and supersonic jet expansion. • Related sources employ ion-beam sputtering (e. g. using heavy ions Xe+, KE = 30 ke. V) or spark erosion to vaporize material. • Applicable to high-melting point (refractory) elements (C, Si, transition metals etc. ). • Intense visible/UV pulse (> 107 W/cm 2 i. e. ~10 m. J per mm 2 in a 10 ns pulse) generates 1014 1015 atoms per mm 2 plasma with T ~ 104 K. • Rapid clustering induced after cooling by carrier gas (e. g. flowing He). Clusters subsequently cool by evaporation.

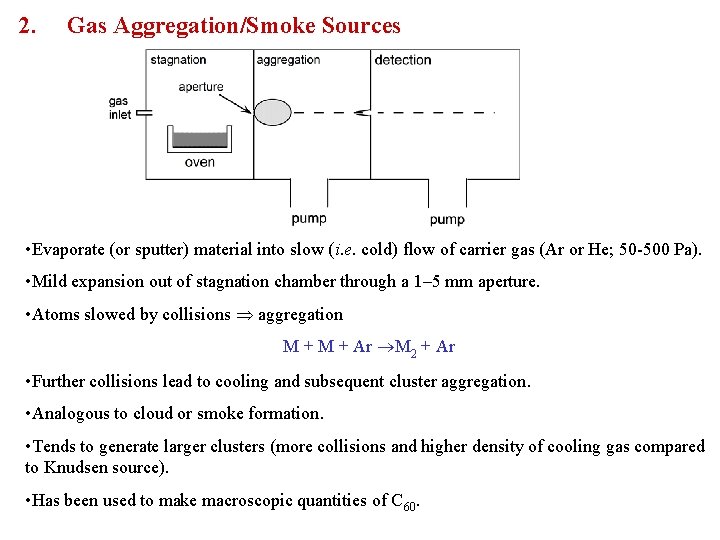
2. Gas Aggregation/Smoke Sources • Evaporate (or sputter) material into slow (i. e. cold) flow of carrier gas (Ar or He; 50 -500 Pa). • Mild expansion out of stagnation chamber through a 1– 5 mm aperture. • Atoms slowed by collisions aggregation M + Ar M 2 + Ar • Further collisions lead to cooling and subsequent cluster aggregation. • Analogous to cloud or smoke formation. • Tends to generate larger clusters (more collisions and higher density of cooling gas compared to Knudsen source). • Has been used to make macroscopic quantities of C 60.

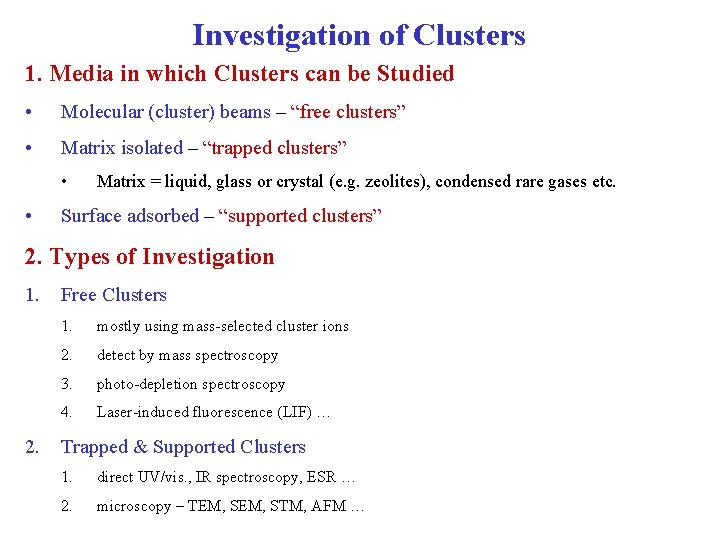
Investigation of Clusters 1. Media in which Clusters can be Studied • Molecular (cluster) beams – “free clusters” • Matrix isolated – “trapped clusters” • • Matrix = liquid, glass or crystal (e. g. zeolites), condensed rare gases etc. Surface adsorbed – “supported clusters” 2. Types of Investigation 1. 2. Free Clusters 1. mostly using mass-selected cluster ions 2. detect by mass spectroscopy 3. photo-depletion spectroscopy 4. Laser-induced fluorescence (LIF) … Trapped & Supported Clusters 1. direct UV/vis. , IR spectroscopy, ESR … 2. microscopy – TEM, STM, AFM …

3. Types of Experiment • • • Electron Removal • electron ejection from neutrals or anions • collect and study cluster cations (measure Q/M) or electrons (measure KE) Cluster Excitation • excite clusters (neutral or charged) by light irradiation or electron/atom impact • study radiation or particles (atoms/cluster fragments) emitted Cluster Reaction • reactions with small molecules (pick-up experiments) • cluster collisions (including collision-induced fragmentation)

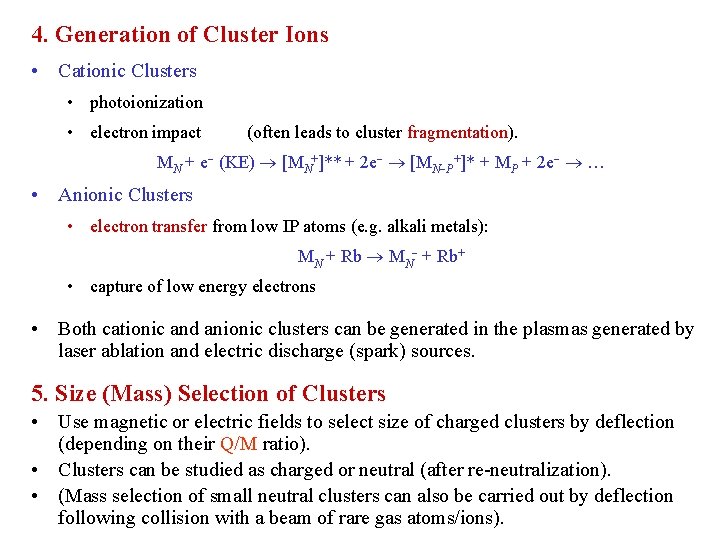
4. Generation of Cluster Ions • Cationic Clusters • photoionization • electron impact (often leads to cluster fragmentation). MN + e (KE) [MN+]** + 2 e [MN P+]* + MP + 2 e … • Anionic Clusters • electron transfer from low IP atoms (e. g. alkali metals): MN + Rb+ • capture of low energy electrons • Both cationic and anionic clusters can be generated in the plasmas generated by laser ablation and electric discharge (spark) sources. 5. Size (Mass) Selection of Clusters • Use magnetic or electric fields to select size of charged clusters by deflection (depending on their Q/M ratio). • Clusters can be studied as charged or neutral (after re-neutralization). • (Mass selection of small neutral clusters can also be carried out by deflection following collision with a beam of rare gas atoms/ions).

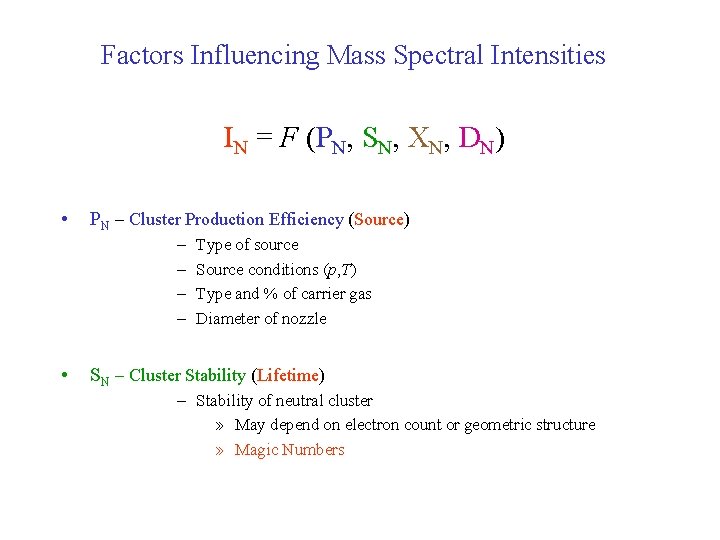
Factors Influencing Mass Spectral Intensities IN = F (PN, SN, XN, DN) • PN – Cluster Production Efficiency (Source) – – • Type of source Source conditions (p, T) Type and % of carrier gas Diameter of nozzle SN – Cluster Stability (Lifetime) – Stability of neutral cluster » May depend on electron count or geometric structure » Magic Numbers

• XN – Ionization Efficiency – Ionization probability (ionization cross section) – Fragmentation probability during ionization – Photoionization • “Softer” than electron impact ionization – Less likely to cause cluster fragmentation. – Near Threshold Photoionization • Most stable closed-shell clusters have high IPs and lower ionization cross sections – Dip in MS intensity for magic numbers. • DN – Detection Efficiency • Usually varies smoothly with cluster size