Active Chemistry Fall Final Reveiw Active Chemistry Fall











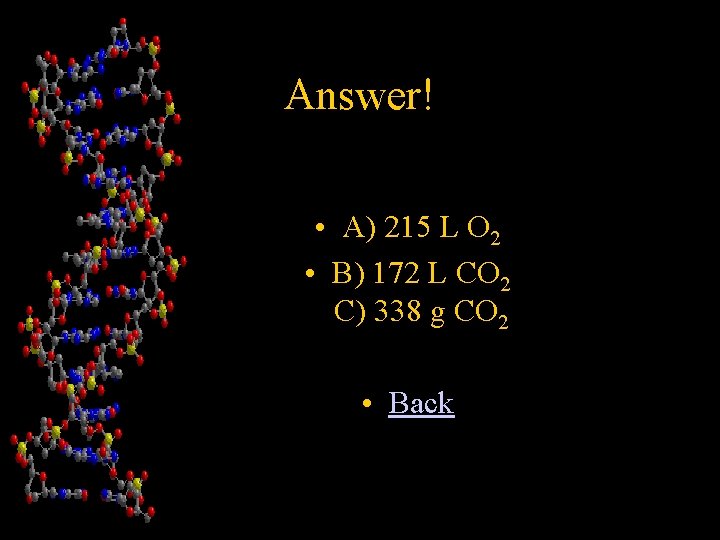


- Slides: 86

Active Chemistry Fall Final Reveiw

Active Chemistry Fall Final Reveiw Name that Acid! Acid or Base? ! You Decide! Acids and Metals! Acids and Carbonates! Indicators! Acid Essentials! Reaction Satisfaction! 100 100 200 200 300 300 400 400 500 500

Chapter 1 Which one of the following is classified as a compound? • a) H 2(g) b) CO 2(g) • c) Cl 2(g) d) I 2(s) • Answer!

Answer! • Back

Chapter 1 • Which substance cannot be broken down by a chemical change? • a. water • c. borax • b. propane • d. Calcium • Answer!

Answer! • D • Back

Chapter 1 Salt water is an example of a _____. • a. Heterogeneous mixture • c. Substance • b. Solution • d. Molecular Compound • Answer!

Answer! • Back

Chapter 1 If 3 liquids with different densities were placed in a beaker, what would you expect to happen? • Answer!

Answer! Most Dense Liquid will settle to the bottom Least Dense liquid will be the liquid on top Liquid with a density between the most and least dense liquids will settle in the middle • Back

Chapter 1 The state of matter for an object that has a definite volume but not a definite shape is • a. solid • b. gas • c. liquid • d. plasma

Answer! • C • Back

Chapter 1 • Which of the following pairings is incorrect? • a. Melting – endothermic (absorbing energy) • b. Vaporization – exothermic (releasing energy) • c. Condensation – exothermic (releasing energy) • d. Sublimation – endothermic (absorbing energy)

Answer! B Back

Chapter 1 • In lab, we combined PVA with Borax to form a polymer that resulted in the formation of slime. This new polymer was formed by crosslinking PVA and Borax. What would you predict to be the effect on a liquid polymer if it was modified to have less crosslinking? • a. deeper colors • b. greater conductivity • c. less viscous • d. more viscous • Answer!

Answer! C • Back

Chapter 1 A wooden splint soaked with potassium chloride solution produces a purple color when placed in the flame of a Bunsen burner. The Bunsen burner provides the • a. energy to move electrons in the metal ion to a higher energy level • b. energy to move electrons in the nonmetal ion to a lower energy level • c. energy to move electrons in the metal ion to a lower energy level • d. energy for combustion • Answer!

Answer! • A • Back

Chapter 1 The density of a 25 m. L sample of ethanol is 0. 79 g/m. L. What is the mass of the sample of ethanol? • a. 20. g • b. 25 g • c. 32 g • d. 0. 032 g • Answer!

Answer! • A • Back

Chapter 1 • Which arrangement correctly orders mixtures in terms of increasing particle size? • a. solution, colloid, suspension • b. solution, suspension, colloid • c. colloid, solution, suspension • d. colloid, suspension, solution • Answer!

Answer! • A • Back

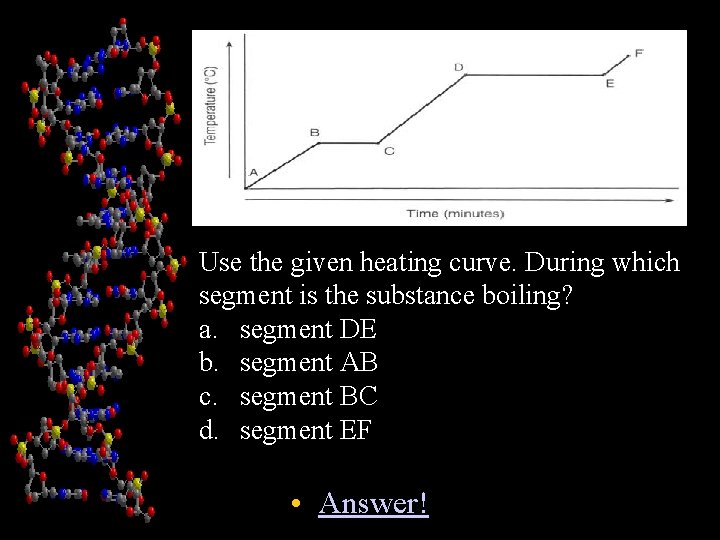
Chapter 1 Use the given heating curve. During which segment is the substance boiling? a. segment DE b. segment AB c. segment BC d. segment EF • Answer!

Answer! • A • Back

Chapter 1 We know that when you squeeze orange peels, you produce ethylene gas. This organic compound is classified as a(n) • a. hydrocarbon • b. alcohol • c. carbohydrate • d. amino acid • Answer!

Answer! • A • Back

Chapter 1 • • An electrically charged atom or group of atoms that has acquired a net charge is an a. electron b. element c. ion d. molecule • Answer!

Answer! • C • Back

Chapter 1 What is the measurement 1042 L rounded off to two significant digits? • a. 1. 0 x 103 L • b. 1050 L • c. 1040 L • d. 1. 1 x 103 L • Answer!

Answer! • A • Back

Chapter 1 How many significant figures are in the measurement 811. 40 grams? • a. two • b. four • c. three • d. five

Answer! • D • Back

Chapter 2 Which of the following is a chemical property? A. shiny silver color B. magnetic C. density D. reacts with HCl • Answer!

Answer! • D • Back

Chapter 2 When placed in distilled water, the p. H of metal oxides will be A. acidic - less than 7 B. basic – greater than 7 C. acidic - greater than 7 D. basic - less than 7 • Answer!

Answer! • Back

Chapter 2 A periodic table resembling the one used today was first developed by _____. A. Dalton B. Aristotle C. Proust D. Mendeleev

Answer! • D • Back

Chapter 2 All of the following are nonmetals except A. Phosphorus B. Strontium C. Iodine D. Carbon • Answer!

Answer! • Strontium • Back

Acids and Carbonates! 6. 02 X 1023 atoms of silicon have a mass of ______ grams • A. 20. 18 • C. 28. 09 B. 22. 99 D. 30. 97 • Answer!

Answer! • C • Back

Chapter 2 During the process of deposition, a _______ change is occurring. • A. physical • B. chemical C. compositional • D. mass • Answer!

Answer! • A • Back

Chapter 2 Which of the following is the least heavy? A. carbon B. hydrogen C. Calcium D. helium • Answer!

Answer! • Back

Chapter 2 Calculate the energy of light given that its wavelength is 525 nm (5. 25 X 10 -7 m) (Show all work: variable set up, values of constants, equations, and units for each number and final answer expressed with units rounded to 3 numbers in scientific notation) • Answer!
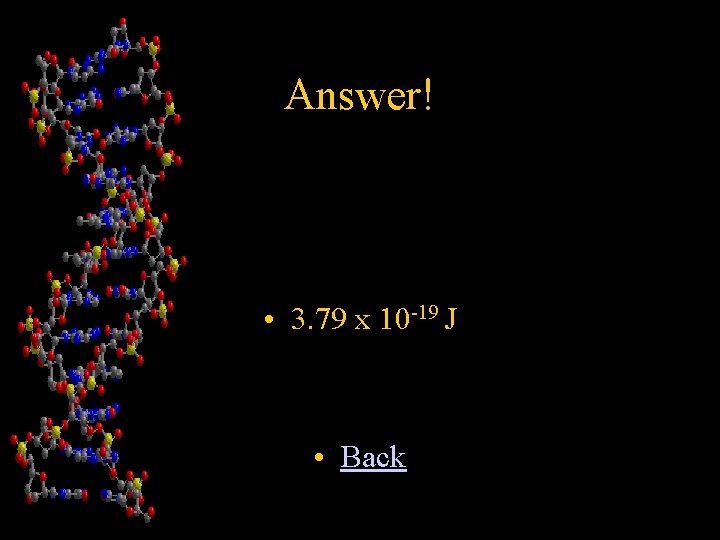
Answer! • 3. 79 x 10 -19 J • Back

Chapter 2 The two main parts of an atom are its _________. A. B. C. D. Nucleus and electron cloud Nucleons and protons Isotopes and neutrons Protons and electron cloud E. Protons and electrons • Answer!

Answer! • A • Back

Chapter 2 • A mercury atom has 80 protons, 120 neutrons, and 80 electrons. What is the atomic number of this atom? A. B. C. D. 120 80 200 40 • Answer!

Answer! • Back

Chapter 2 Which element would have properties most similar to an element with an atomic number of 113? a) Uuq c) beryllium b) boron d) carbon • Answer!

Answer! • Back

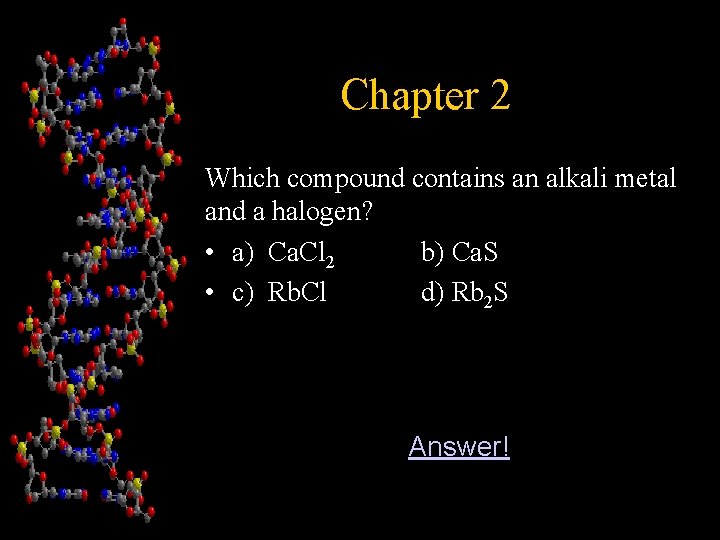
Chapter 2 Which compound contains an alkali metal and a halogen? • a) Ca. Cl 2 b) Ca. S • c) Rb. Cl d) Rb 2 S Answer!

Answer! • C • Back

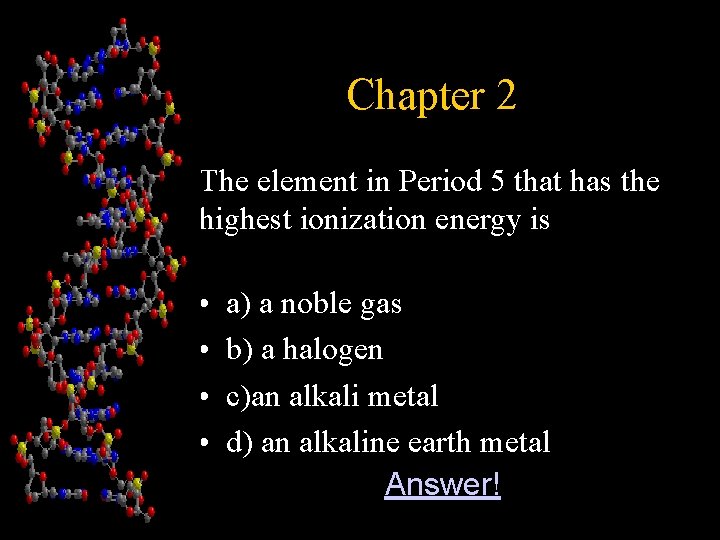
Chapter 2 The element in Period 5 that has the highest ionization energy is • • a) a noble gas b) a halogen c)an alkali metal d) an alkaline earth metal • Answer!

Answer! • A • Back

Chapter 2 • What is the electron configuration of arsenic? Answer!

Answer! • ? ? ? • Back

Chapter 2 Covalent compounds ______ • A. Share electrons between elements • B. Are formed between two or more nonmetals • C. Use prefixes when naming the compounds • D. All of the above Answer!

Answer! • D • Back

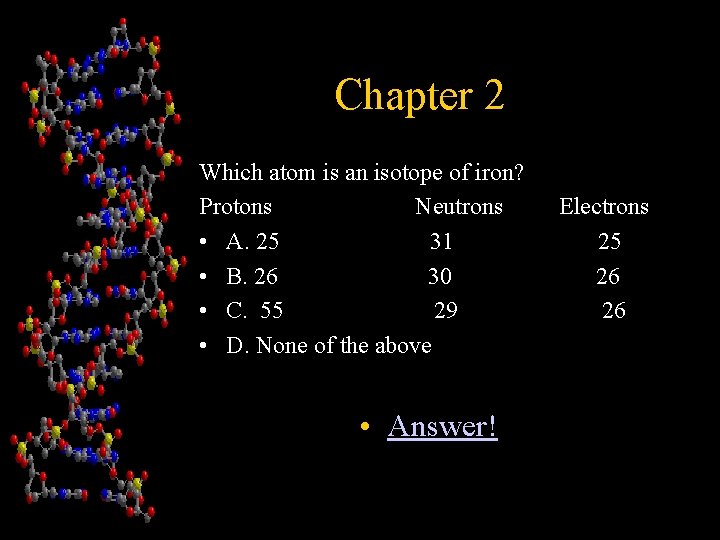
Chapter 2 Which atom is an isotope of iron? Protons Neutrons • A. 25 31 • B. 26 30 • C. 55 29 • D. None of the above • Answer! Electrons 25 26 26

Answer! • Back

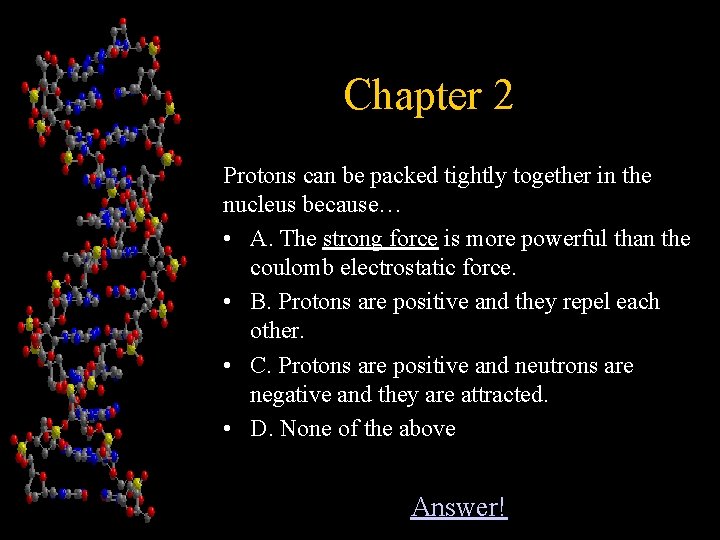
Chapter 2 Protons can be packed tightly together in the nucleus because… • A. The strong force is more powerful than the coulomb electrostatic force. • B. Protons are positive and they repel each other. • C. Protons are positive and neutrons are negative and they are attracted. • D. None of the above Answer!

Answer! • A • Back

Chapter 2 • The alkaline earth metals have how many valence electrons? • A. 8 B. 3 C. 2 D. 1 • Answer!

Answer! • C • Back

Chapter 2 Draw the Lewis Structure for dinitrogen tetrahydride Answer!

Answer! • Nitrogen atom single bonded to another nitrogen atom. Both nitrogen atoms are bonded to two hydrogen atoms. (Nitrogen atoms make 3 bonds, and Hydrogen makes 1 bond) • Back

Chapter 4 • Predict whether or not the following reactions will occur. • • Use the activity series: Li > Na > Mg > Al > Zn > Fe > Sn > Pb > H > Cu > Hg > Ag > Pt > Au • Circle yes or no. • • (a) Pb(s) + Mg. Cl 2(aq) • • (b) Ag. Cl (aq) + Na(s) yes no • Answer!

Answer! • A) no • B) yes • Back

Chapter 4 What is entropy? • Answer!

Answer! • Entropy is the measure of disorder of particles in a substance. • Back

Chapter 4 • How many moles of Na. Cl are there in 5. 34 x 1024 formula units of Na. Cl? • Answer!

Answer! • 8. 87 mol Na. Cl • Back

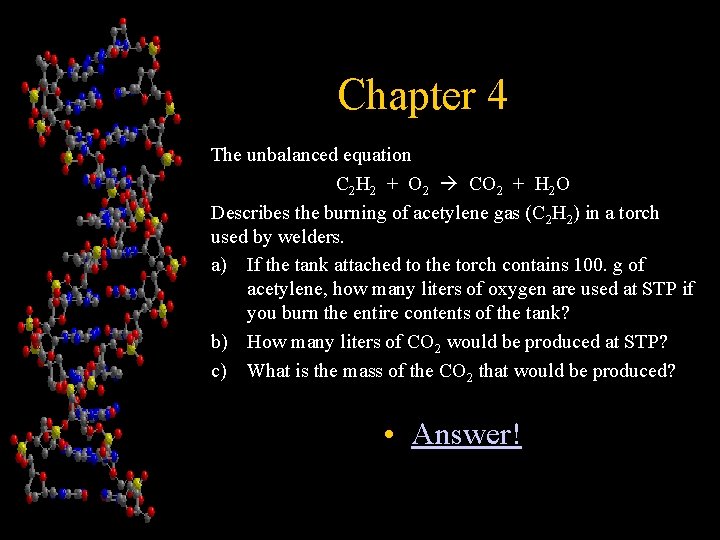
Chapter 4 The unbalanced equation C 2 H 2 + O 2 CO 2 + H 2 O Describes the burning of acetylene gas (C 2 H 2) in a torch used by welders. a) If the tank attached to the torch contains 100. g of acetylene, how many liters of oxygen are used at STP if you burn the entire contents of the tank? b) How many liters of CO 2 would be produced at STP? c) What is the mass of the CO 2 that would be produced? • Answer!
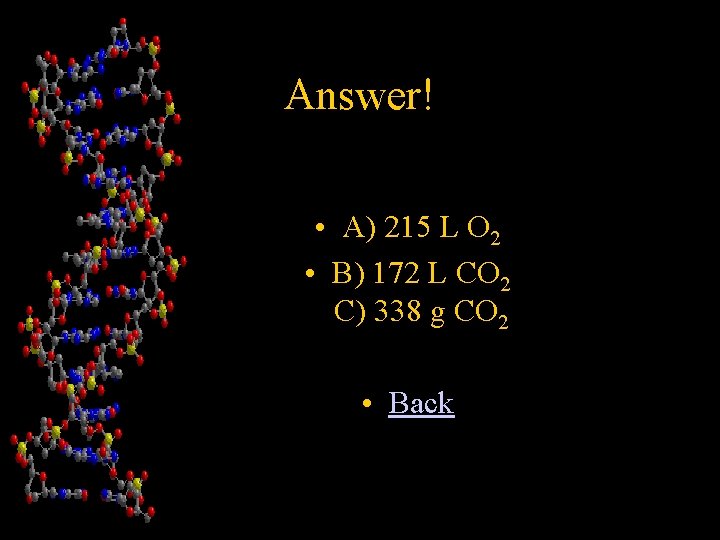
Answer! • A) 215 L O 2 • B) 172 L CO 2 C) 338 g CO 2 • Back

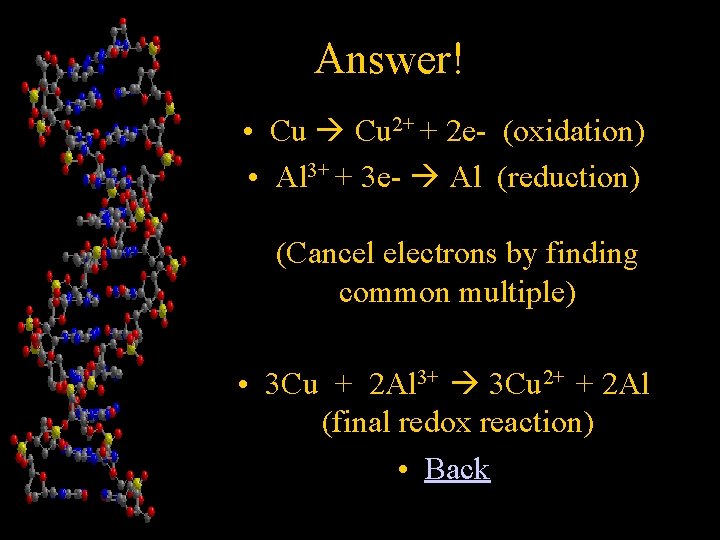
Chapter 4 • Write the redox reaction that occurs between Calcium metal and an aqueous solution of aluminum nitrate • Answer!

Answer! • Cu 2+ + 2 e- (oxidation) • Al 3+ + 3 e- Al (reduction) (Cancel electrons by finding common multiple) • 3 Cu + 2 Al 3+ 3 Cu 2+ + 2 Al (final redox reaction) • Back

Chapter 4 • Calculate the frequency and the energy of light that has a wavelength of 450 nm. • Answer!

Answer! • c = frequency x wavelength Frequency = c/wavelength F = 6. 67 x 1014 Hz • E=hf E= 4. 4 x 10 -19 J • Back

Chapter 4 • Which color of visible light has a longer wavelength, yellow or green? • Answer!

Answer! • Yellow light (Look at electromagnetic Spectrum p. 326 -327) • Back

Chapter 4 • Molten iron and carbon monoxide are produced in a blast furnace by the reaction of iron(III) oxide and pure carbon. 25. 0 kilograms of pure Fe 2 O 3 are reacted with 45. 0 kilograms of carbon. The reaction is: Fe 2 O 3 + 3 C 2 Fe + 3 CO • a) What is limiting reactant? b) How many kilograms of iron can be produced? c) How much of the excess reactant remains? • Answer!

Answer! • A) • B) • C) • Back