12 The Gaseous State of Matter Air in





- Slides: 55

12 The Gaseous State of Matter Air in a hot air balloon expands upon heating. Some air escapes from the top, lowering the air density, making the balloon buoyant. Foundations of College Chemistry, 14 th Ed. Morris Hein and Susan Arena Copyright © 2014 John Wiley & Sons, Inc. All rights reserved.

Chapter Outline 12. 1 Properties of Gases A. Measuring the Pressure of a Gas B. Pressure Dependence: Number of Molecules and Temperature 12. 2 Boyle’s Law 12. 3 Charles’ Law 12. 4 Avogadro’s Law A. Mole-Mass-Volume Calculations 12. 5 Combined Gas Laws 12. 6 Ideal Gas Law A. Kinetic-Molecular Theory B. Real Gases 12. 7 Dalton’s Law of Partial Pressures 12. 8 Density of Gases 12. 9 Gas Stoichiometry © 2014 John Wiley & Sons, Inc. All rights reserved.

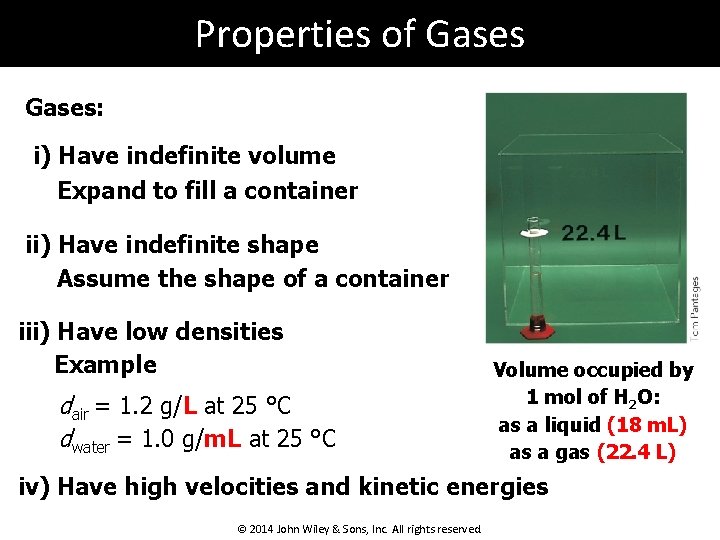
Properties of Gases: i) Have indefinite volume Expand to fill a container ii) Have indefinite shape Assume the shape of a container iii) Have low densities Example dair = 1. 2 g/L at 25 °C dwater = 1. 0 g/m. L at 25 °C Volume occupied by 1 mol of H 2 O: as a liquid (18 m. L) as a gas (22. 4 L) iv) Have high velocities and kinetic energies © 2014 John Wiley & Sons, Inc. All rights reserved.

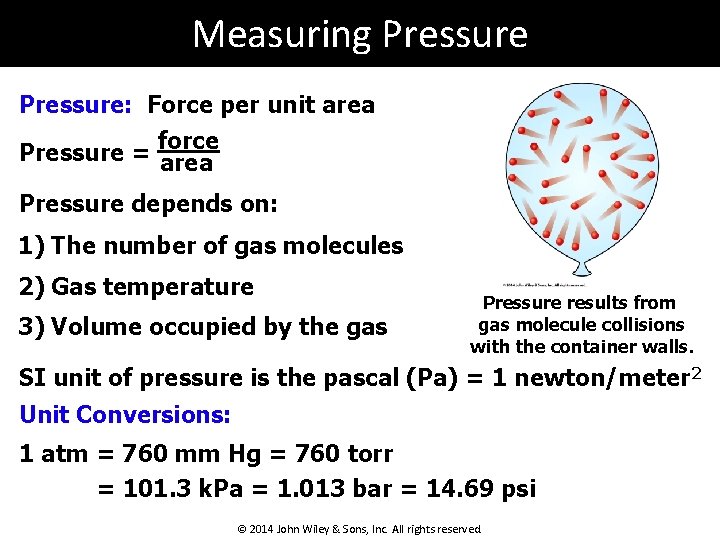
Measuring Pressure: Force per unit area force Pressure = area Pressure depends on: 1) The number of gas molecules 2) Gas temperature 3) Volume occupied by the gas Pressure results from gas molecule collisions with the container walls. SI unit of pressure is the pascal (Pa) = 1 newton/meter 2 Unit Conversions: 1 atm = 760 mm Hg = 760 torr = 101. 3 k. Pa = 1. 013 bar = 14. 69 psi © 2014 John Wiley & Sons, Inc. All rights reserved.

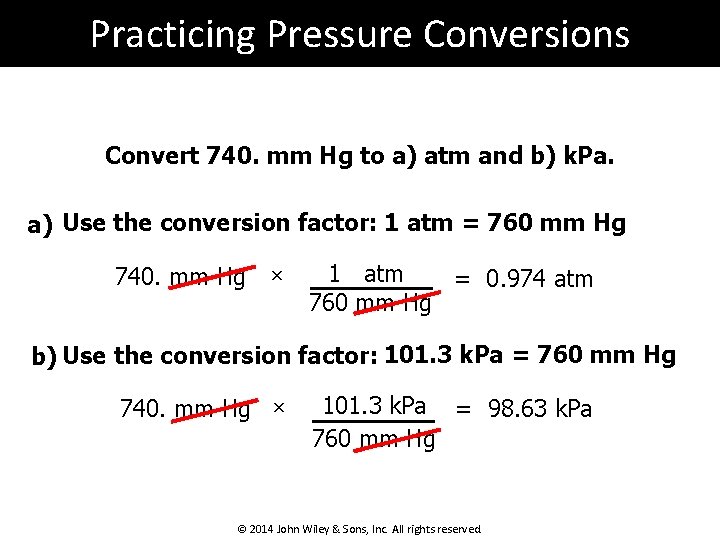
Practicing Pressure Conversions Convert 740. mm Hg to a) atm and b) k. Pa. a) Use the conversion factor: 1 atm = 760 mm Hg 740. mm Hg × 1 atm = 0. 974 atm 760 mm Hg b) Use the conversion factor: 101. 3 k. Pa = 760 mm Hg 740. mm Hg × 101. 3 k. Pa = 98. 63 k. Pa 760 mm Hg © 2014 John Wiley & Sons, Inc. All rights reserved.

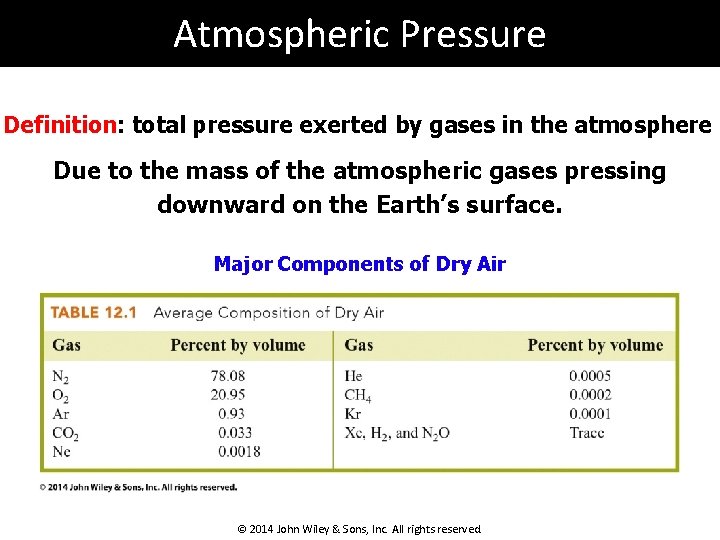
Atmospheric Pressure Definition: total pressure exerted by gases in the atmosphere Due to the mass of the atmospheric gases pressing downward on the Earth’s surface. Major Components of Dry Air © 2014 John Wiley & Sons, Inc. All rights reserved.

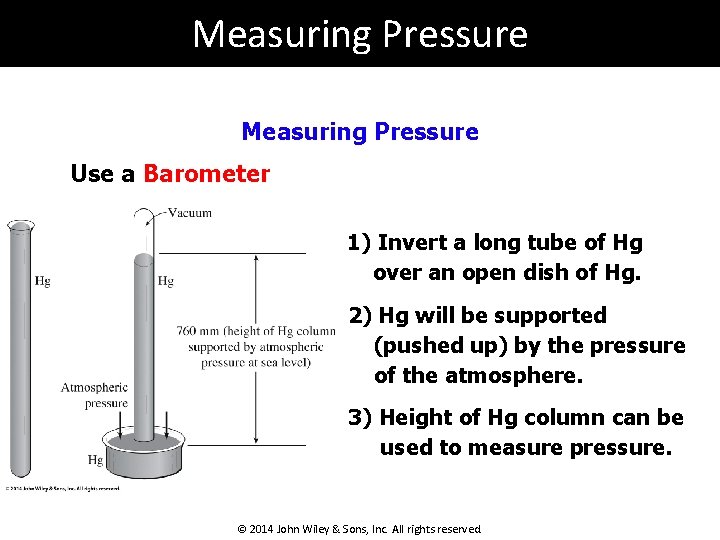
Measuring Pressure Use a Barometer 1) Invert a long tube of Hg over an open dish of Hg. 2) Hg will be supported (pushed up) by the pressure of the atmosphere. 3) Height of Hg column can be used to measure pressure. © 2014 John Wiley & Sons, Inc. All rights reserved.

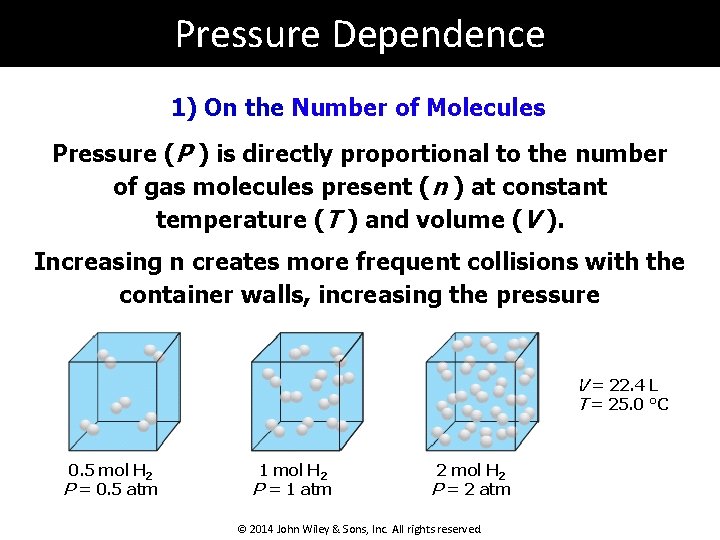
Pressure Dependence 1) On the Number of Molecules Pressure (P ) is directly proportional to the number of gas molecules present (n ) at constant temperature (T ) and volume (V ). Increasing n creates more frequent collisions with the container walls, increasing the pressure V = 22. 4 L T = 25. 0 °C 0. 5 mol H 2 P = 0. 5 atm 1 mol H 2 P = 1 atm 2 mol H 2 P = 2 atm © 2014 John Wiley & Sons, Inc. All rights reserved.

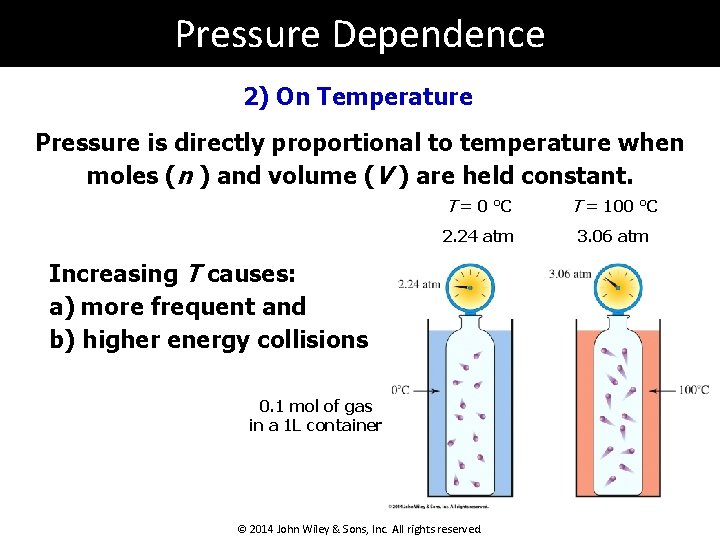
Pressure Dependence 2) On Temperature Pressure is directly proportional to temperature when moles (n ) and volume (V ) are held constant. T = 0 °C T = 100 °C 2. 24 atm 3. 06 atm Increasing T causes: a) more frequent and b) higher energy collisions 0. 1 mol of gas in a 1 L container © 2014 John Wiley & Sons, Inc. All rights reserved.

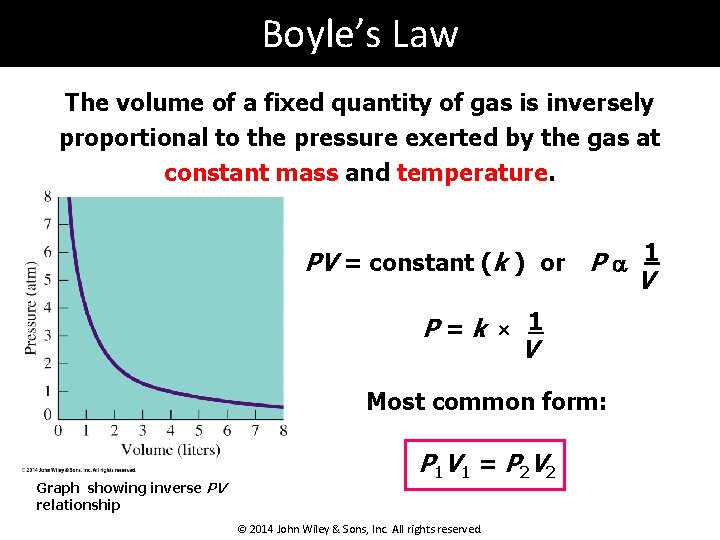
Boyle’s Law The volume of a fixed quantity of gas is inversely proportional to the pressure exerted by the gas at constant mass and temperature. PV = constant (k ) or P a 1 V P=k × 1 V Most common form: Graph showing inverse PV relationship P 1 V 1 = P 2 V 2 © 2014 John Wiley & Sons, Inc. All rights reserved.

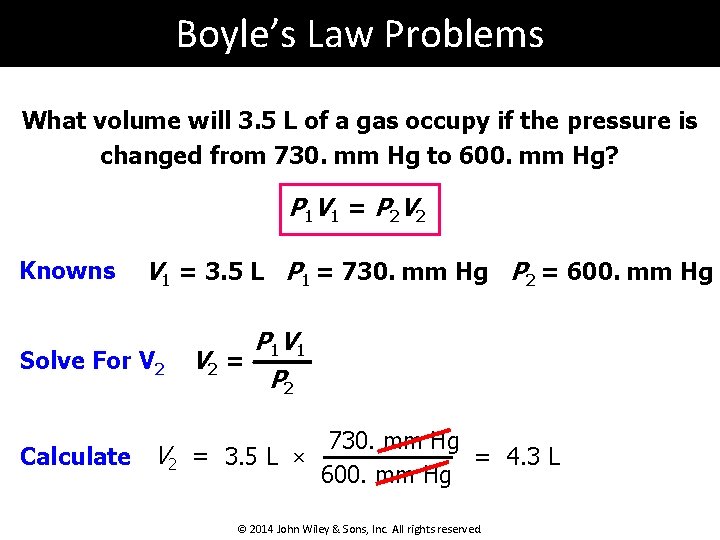
Boyle’s Law Problems What volume will 3. 5 L of a gas occupy if the pressure is changed from 730. mm Hg to 600. mm Hg? P 1 V 1 = P 2 V 2 Knowns V 1 = 3. 5 L P 1 = 730. mm Hg P 2 = 600. mm Hg Solve For V 2 P 1 V 2 = P 2 Calculate V 2 = 3. 5 L × 730. mm Hg = 4. 3 L 600. mm Hg © 2014 John Wiley & Sons, Inc. All rights reserved.

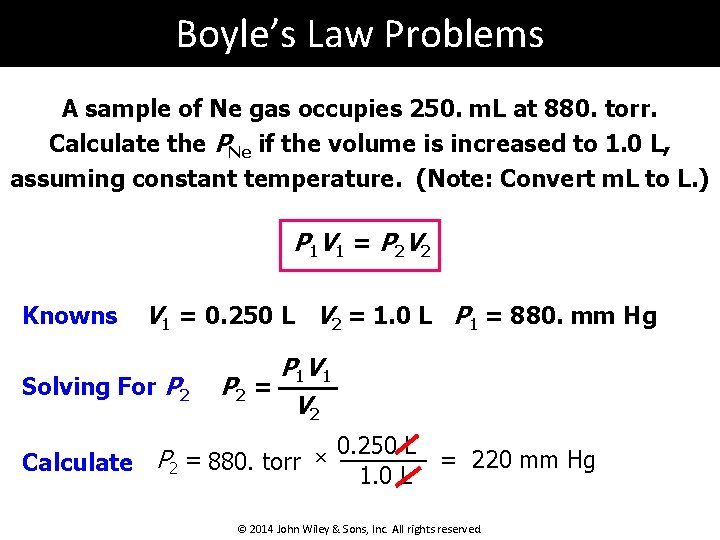
Boyle’s Law Problems A sample of Ne gas occupies 250. m. L at 880. torr. Calculate the PNe if the volume is increased to 1. 0 L, assuming constant temperature. (Note: Convert m. L to L. ) P 1 V 1 = P 2 V 2 Knowns V 1 = 0. 250 L V 2 = 1. 0 L P 1 = 880. mm Hg Solving For P 2 P 1 V 1 P 2 = V 2 0. 250 L × = 220 mm Hg Calculate P 2 = 880. torr 1. 0 L © 2014 John Wiley & Sons, Inc. All rights reserved.

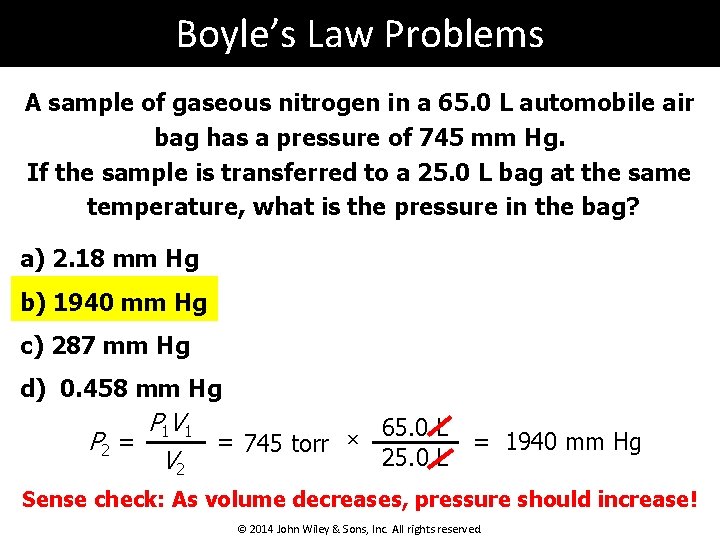
Boyle’s Law Problems A sample of gaseous nitrogen in a 65. 0 L automobile air bag has a pressure of 745 mm Hg. If the sample is transferred to a 25. 0 L bag at the same temperature, what is the pressure in the bag? a) 2. 18 mm Hg b) 1940 mm Hg c) 287 mm Hg d) 0. 458 mm Hg P 1 V 1 65. 0 L P 2 = = 745 torr × = 1940 mm Hg 25. 0 L V 2 Sense check: As volume decreases, pressure should increase! © 2014 John Wiley & Sons, Inc. All rights reserved.

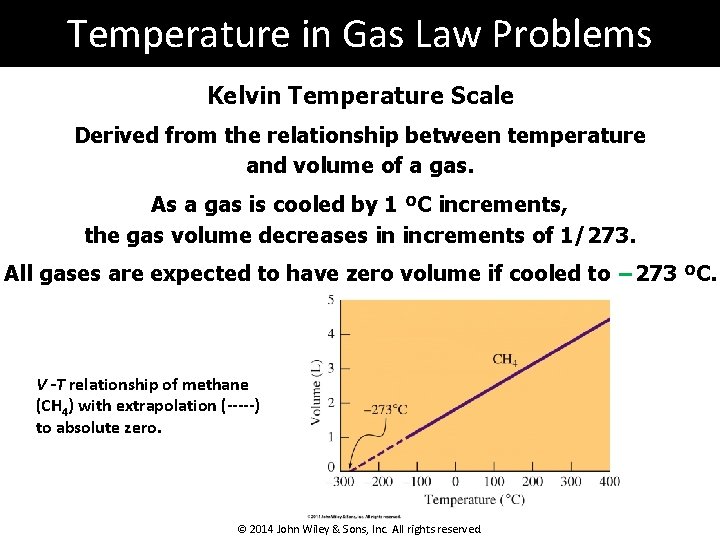
Temperature in Gas Law Problems Kelvin Temperature Scale Derived from the relationship between temperature and volume of a gas. As a gas is cooled by 1 ºC increments, the gas volume decreases in increments of 1/273. All gases are expected to have zero volume if cooled to − 273 ºC. V -T relationship of methane (CH 4) with extrapolation (-----) to absolute zero. © 2014 John Wiley & Sons, Inc. All rights reserved.

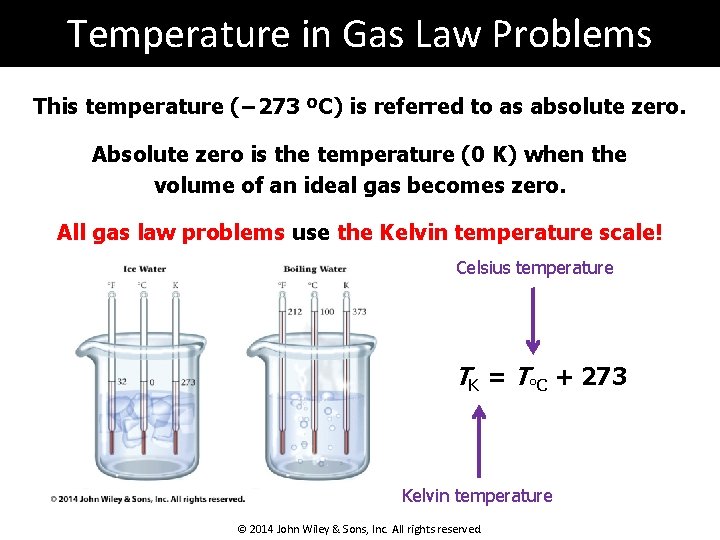
Temperature in Gas Law Problems This temperature (− 273 ºC) is referred to as absolute zero. Absolute zero is the temperature (0 K) when the volume of an ideal gas becomes zero. All gas law problems use the Kelvin temperature scale! Celsius temperature TK = T°C + 273 Kelvin temperature © 2014 John Wiley & Sons, Inc. All rights reserved.

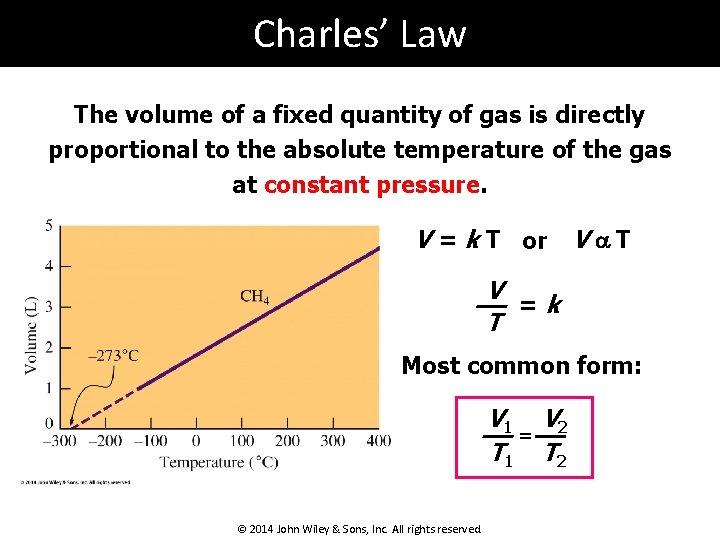
Charles’ Law The volume of a fixed quantity of gas is directly proportional to the absolute temperature of the gas at constant pressure. V = k T or V a T V =k T Most common form: V 1 V 2 = T 1 T 2 © 2014 John Wiley & Sons, Inc. All rights reserved.

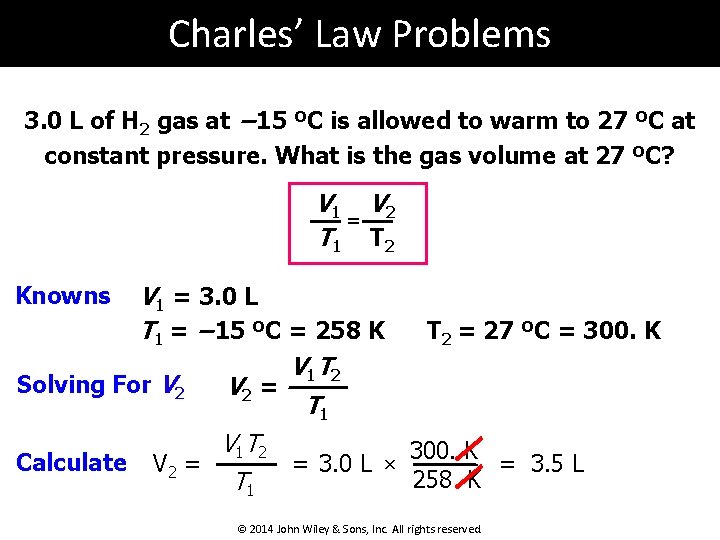
Charles’ Law Problems 3. 0 L of H 2 gas at − 15 ºC is allowed to warm to 27 ºC at constant pressure. What is the gas volume at 27 ºC? V 1 V 2 = T 1 T 2 V 1 = 3. 0 L T 1 = − 15 ºC = 258 K V 1 T 2 Solving For V 2 = T 1 Knowns Calculate V 1 T 2 V 2 = T 1 = 3. 0 L × T 2 = 27 ºC = 300. K = 3. 5 L 258 K © 2014 John Wiley & Sons, Inc. All rights reserved.

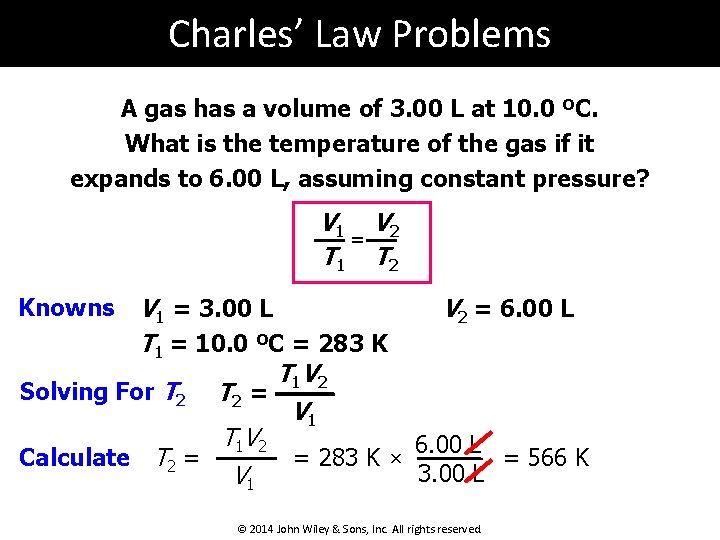
Charles’ Law Problems A gas has a volume of 3. 00 L at 10. 0 ºC. What is the temperature of the gas if it expands to 6. 00 L, assuming constant pressure? V 1 V 2 = T 1 T 2 V 1 = 3. 00 L V 2 = 6. 00 L T 1 = 10. 0 ºC = 283 K T 1 V 2 Solving For T 2 = V 1 T 1 V 2 6. 00 L = 566 K Calculate T 2 = = 283 K × 3. 00 L V 1 Knowns © 2014 John Wiley & Sons, Inc. All rights reserved.

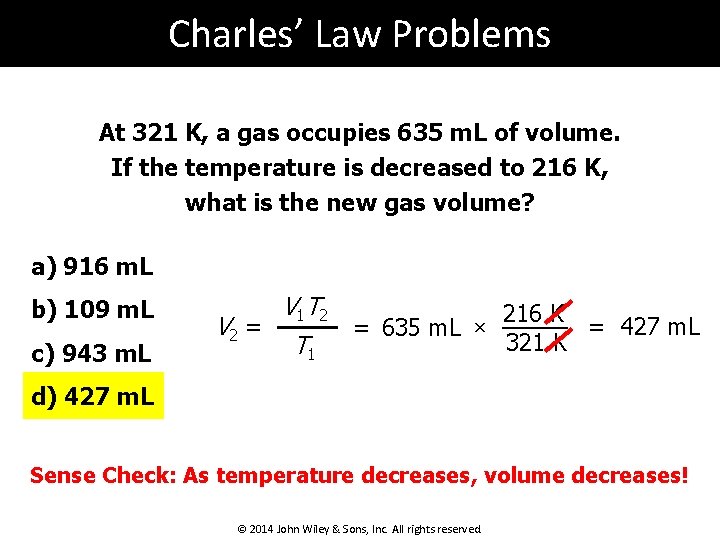
Charles’ Law Problems At 321 K, a gas occupies 635 m. L of volume. If the temperature is decreased to 216 K, what is the new gas volume? a) 916 m. L b) 109 m. L c) 943 m. L V 1 T 2 216 K V 2 = = 427 m. L = 635 m. L × 321 K T 1 d) 427 m. L Sense Check: As temperature decreases, volume decreases! © 2014 John Wiley & Sons, Inc. All rights reserved.

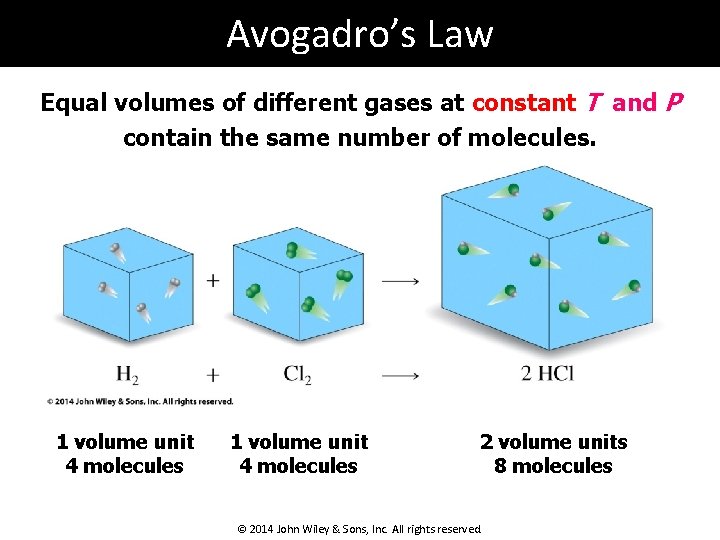
Avogadro’s Law Equal volumes of different gases at constant T and P contain the same number of molecules. 1 volume unit 4 molecules 2 volume units 8 molecules © 2014 John Wiley & Sons, Inc. All rights reserved.

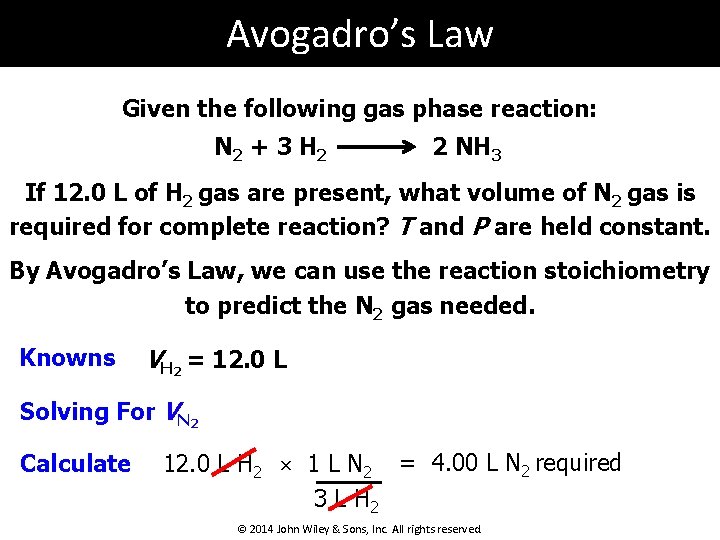
Avogadro’s Law Given the following gas phase reaction: N 2 + 3 H 2 2 NH 3 If 12. 0 L of H 2 gas are present, what volume of N 2 gas is required for complete reaction? T and P are held constant. By Avogadro’s Law, we can use the reaction stoichiometry to predict the N 2 gas needed. Knowns VH 2 = 12. 0 L Solving For VN 2 Calculate 12. 0 L H 2 × 1 L N 2 = 4. 00 L N 2 required 3 L H 2 © 2014 John Wiley & Sons, Inc. All rights reserved.

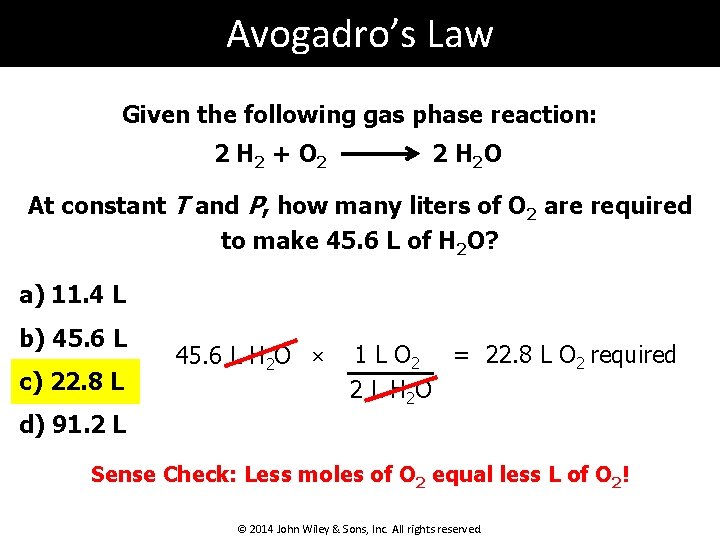
Avogadro’s Law Given the following gas phase reaction: 2 H 2 + O 2 2 H 2 O At constant T and P, how many liters of O 2 are required to make 45. 6 L of H 2 O? a) 11. 4 L b) 45. 6 L c) 22. 8 L 45. 6 L H 2 O × 1 L O 2 = 22. 8 L O 2 required 2 L H 2 O d) 91. 2 L Sense Check: Less moles of O 2 equal less L of O 2! © 2014 John Wiley & Sons, Inc. All rights reserved.

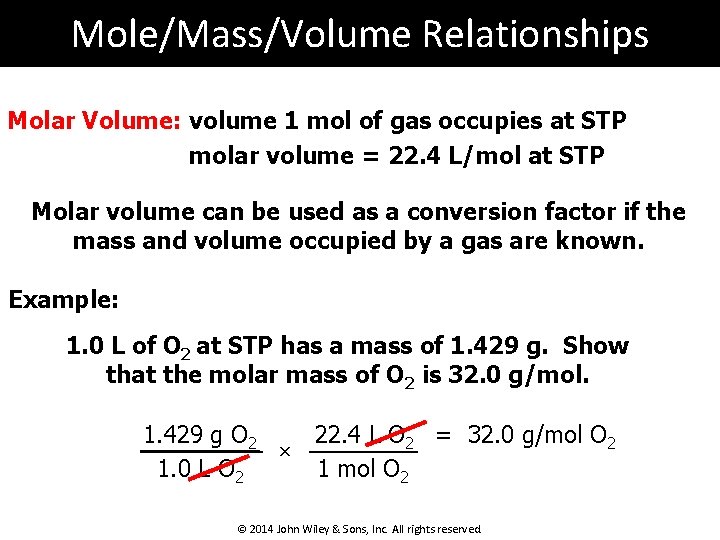
Mole/Mass/Volume Relationships Molar Volume: volume 1 mol of gas occupies at STP molar volume = 22. 4 L/mol at STP Molar volume can be used as a conversion factor if the mass and volume occupied by a gas are known. Example: 1. 0 L of O 2 at STP has a mass of 1. 429 g. Show that the molar mass of O 2 is 32. 0 g/mol. 22. 4 L O 2 = 32. 0 g/mol O 2 1. 429 g O 2 × 1. 0 L O 2 1 mol O 2 © 2014 John Wiley & Sons, Inc. All rights reserved.

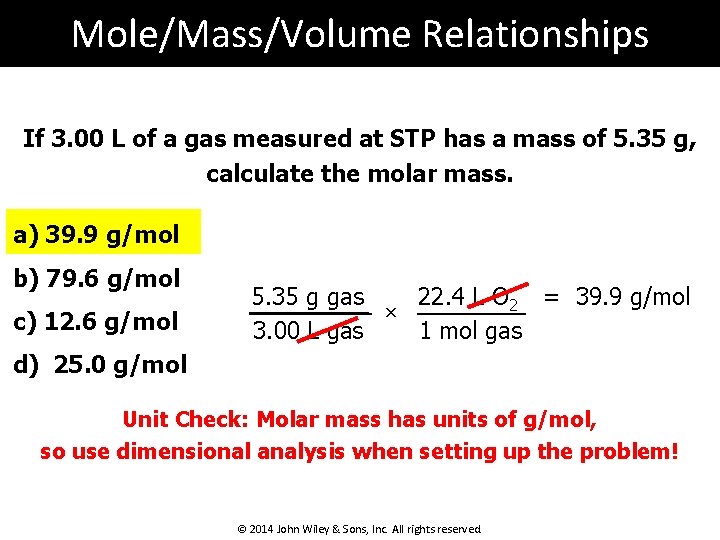
Mole/Mass/Volume Relationships If 3. 00 L of a gas measured at STP has a mass of 5. 35 g, calculate the molar mass. a) 39. 9 g/mol b) 79. 6 g/mol c) 12. 6 g/mol 22. 4 L O 2 = 39. 9 g/mol 5. 35 g gas × 1 mol gas 3. 00 L gas d) 25. 0 g/mol Unit Check: Molar mass has units of g/mol, so use dimensional analysis when setting up the problem! © 2014 John Wiley & Sons, Inc. All rights reserved.

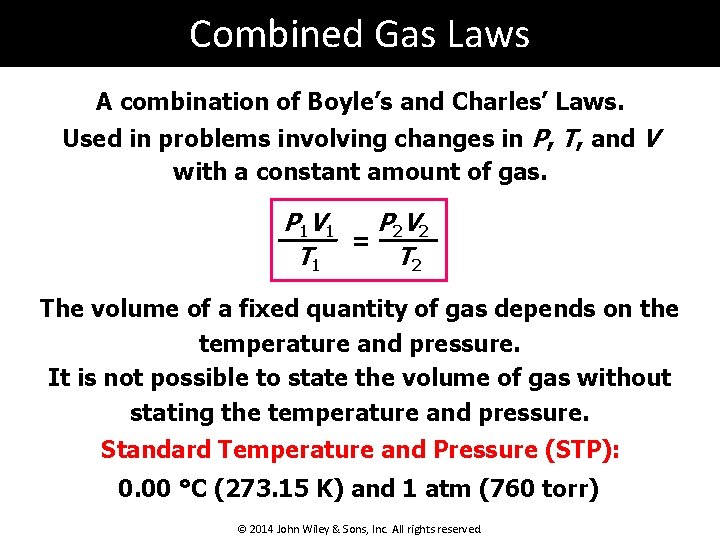
Combined Gas Laws A combination of Boyle’s and Charles’ Laws. Used in problems involving changes in P, T, and V with a constant amount of gas. P 1 V 1 P 2 V 2 = T 1 T 2 The volume of a fixed quantity of gas depends on the temperature and pressure. It is not possible to state the volume of gas without stating the temperature and pressure. Standard Temperature and Pressure (STP): 0. 00 °C (273. 15 K) and 1 atm (760 torr) © 2014 John Wiley & Sons, Inc. All rights reserved.

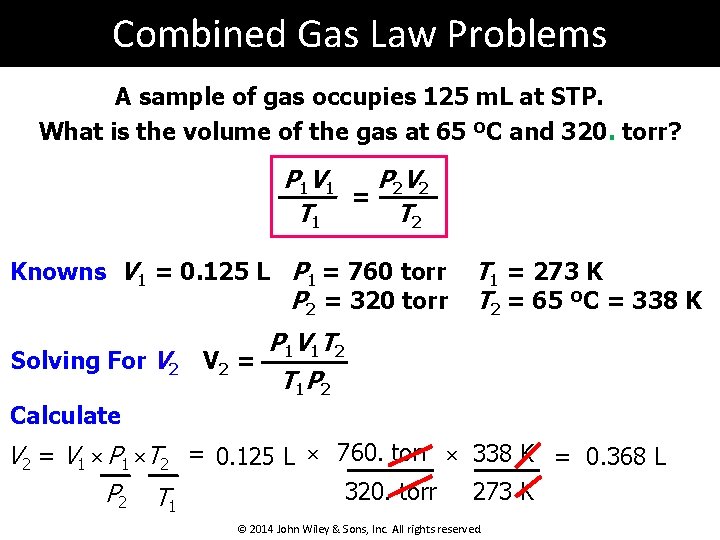
Combined Gas Law Problems A sample of gas occupies 125 m. L at STP. What is the volume of the gas at 65 ºC and 320. torr? P 1 V 1 P 2 V 2 = T 1 T 2 Knowns V 1 = 0. 125 L P 1 = 760 torr P 2 = 320 torr Solving For V 2 T 1 = 273 K T 2 = 65 ºC = 338 K P 1 V 1 T 2 V 2 = T 1 P 2 Calculate V 2 = V 1 × P 1 ×T 2 = 0. 125 L × 760. torr × 338 K = 0. 368 L 320. torr 273 K P 2 T 1 © 2014 John Wiley & Sons, Inc. All rights reserved.

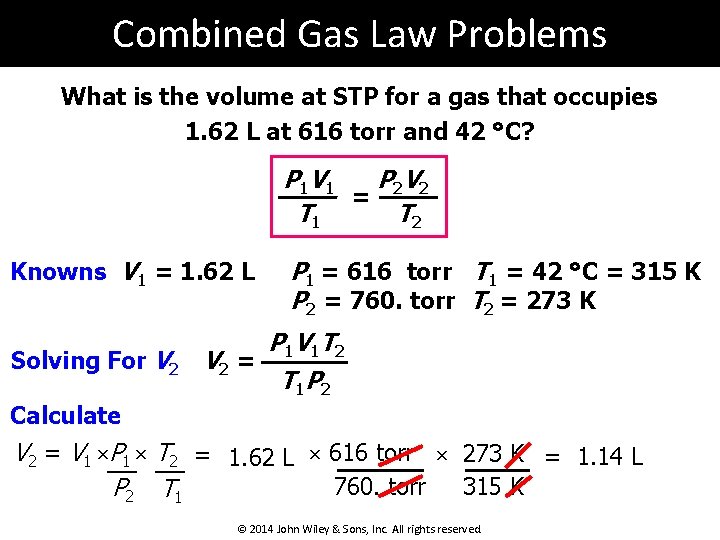
Combined Gas Law Problems What is the volume at STP for a gas that occupies 1. 62 L at 616 torr and 42 °C? P 1 V 1 P 2 V 2 = T 1 T 2 Knowns V 1 = 1. 62 L Solving For V 2 P 1 = 616 torr T 1 = 42 °C = 315 K P 2 = 760. torr T 2 = 273 K P 1 V 1 T 2 V 2 = T 1 P 2 Calculate V 2 = V 1 ×P 1 × T 2 = 1. 62 L × 616 torr × 273 K = 1. 14 L 760. torr 315 K P 2 T 1 © 2014 John Wiley & Sons, Inc. All rights reserved.

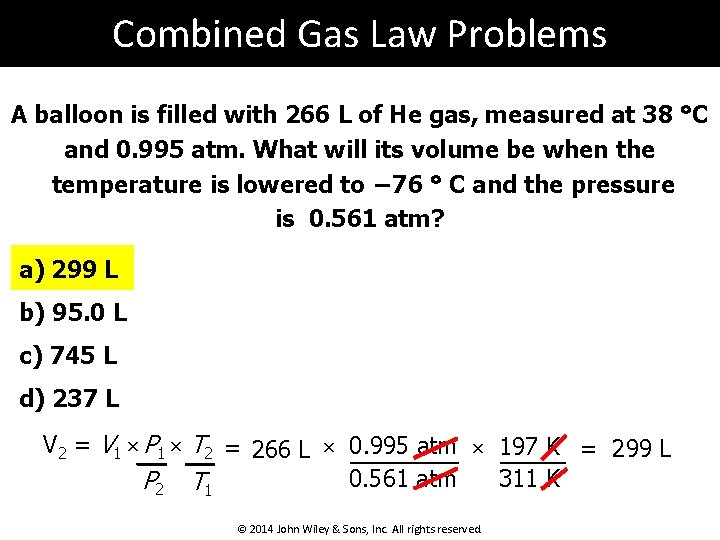
Combined Gas Law Problems A balloon is filled with 266 L of He gas, measured at 38 °C and 0. 995 atm. What will its volume be when the temperature is lowered to − 76 ° C and the pressure is 0. 561 atm? a) 299 L b) 95. 0 L c) 745 L d) 237 L V 2 = V 1 × P 1 × T 2 = 266 L × 0. 995 atm × 197 K = 299 L 0. 561 atm 311 K P 2 T 1 © 2014 John Wiley & Sons, Inc. All rights reserved.

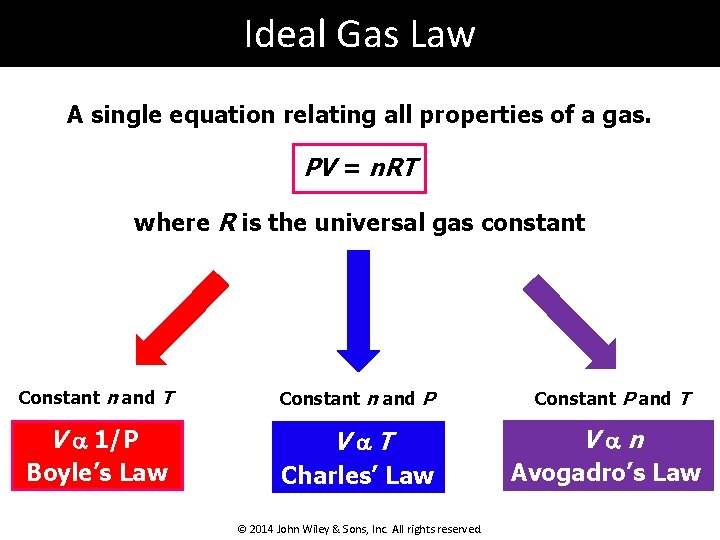
Ideal Gas Law A single equation relating all properties of a gas. PV = n. RT where R is the universal gas constant Constant n and T Constant n and P Constant P and T V a 1/P Va. T Van Boyle’s Law Charles’ Law Avogadro’s Law © 2014 John Wiley & Sons, Inc. All rights reserved.

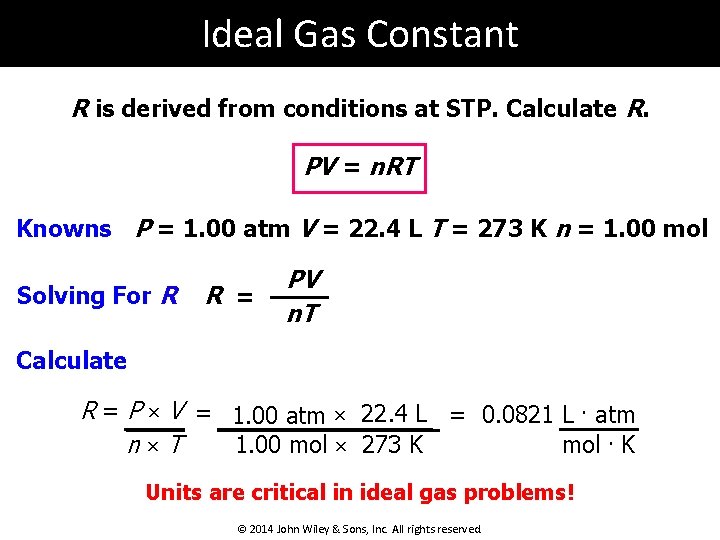
Ideal Gas Constant R is derived from conditions at STP. Calculate R. PV = n. RT Knowns P = 1. 00 atm V = 22. 4 L T = 273 K n = 1. 00 mol Solving For R R = PV n. T Calculate R = P × V = 1. 00 atm × 22. 4 L = 0. 0821 L. atm 1. 00 mol × 273 K n×T mol. K Units are critical in ideal gas problems! © 2014 John Wiley & Sons, Inc. All rights reserved.

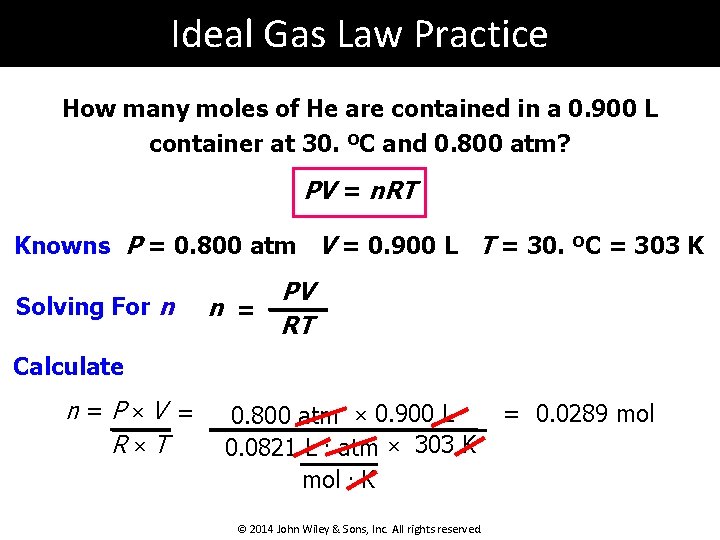
Ideal Gas Law Practice How many moles of He are contained in a 0. 900 L container at 30. ºC and 0. 800 atm? PV = n. RT Knowns P = 0. 800 atm V = 0. 900 L T = 30. ºC = 303 K Solving For n n = PV RT Calculate n=P×V = R×T 0. 800 atm × 0. 900 L 0. 0821 L. atm × 303 K mol. K © 2014 John Wiley & Sons, Inc. All rights reserved. = 0. 0289 mol

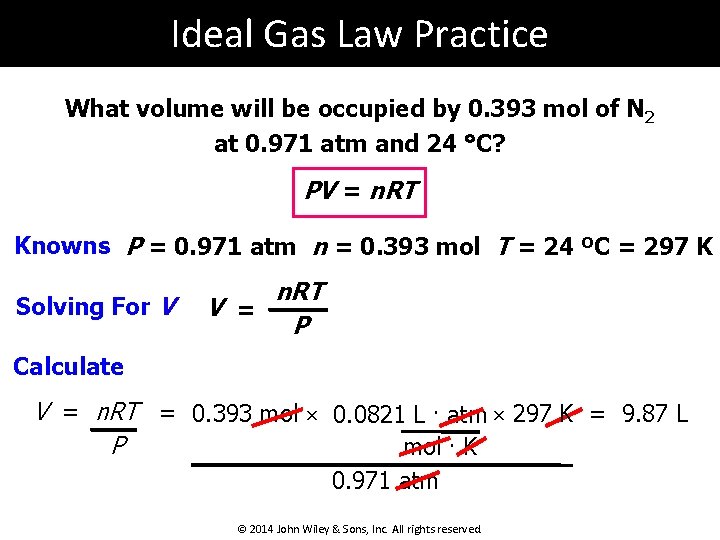
Ideal Gas Law Practice What volume will be occupied by 0. 393 mol of N 2 at 0. 971 atm and 24 °C? PV = n. RT Knowns P = 0. 971 atm n = 0. 393 mol T = 24 ºC = 297 K Solving For V V = n. RT P Calculate V = n. RT = 0. 393 mol × 0. 0821 L. atm × 297 K = 9. 87 L P mol. K 0. 971 atm © 2014 John Wiley & Sons, Inc. All rights reserved.

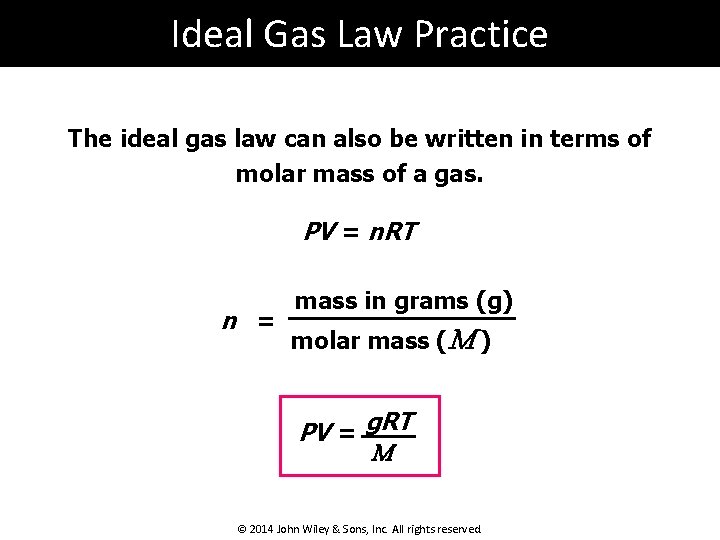
Ideal Gas Law Practice The ideal gas law can also be written in terms of molar mass of a gas. PV = n. RT n = mass in grams (g) molar mass (M ) PV = g. RT M © 2014 John Wiley & Sons, Inc. All rights reserved.

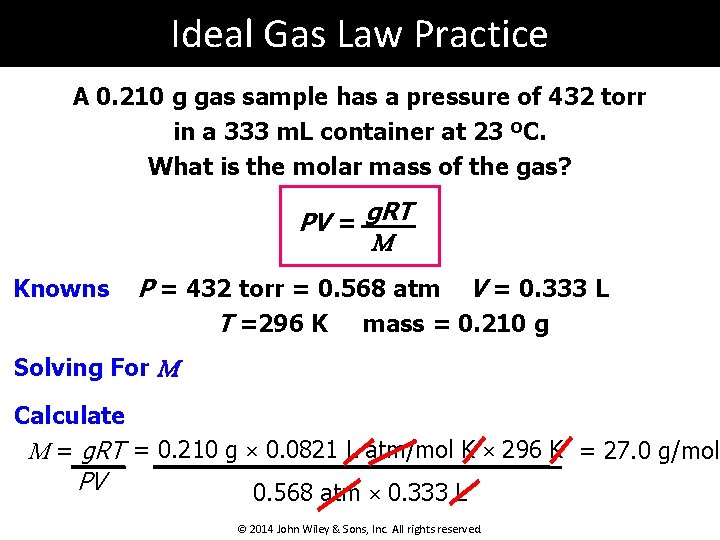
Ideal Gas Law Practice A 0. 210 g gas sample has a pressure of 432 torr in a 333 m. L container at 23 ºC. What is the molar mass of the gas? PV = g. RT M Knowns P = 432 torr = 0. 568 atm V = 0. 333 L T =296 K mass = 0. 210 g Solving For M Calculate M = g. RT = 0. 210 g × 0. 0821 L atm/mol K × 296 K = 27. 0 g/mol PV 0. 568 atm × 0. 333 L © 2014 John Wiley & Sons, Inc. All rights reserved.

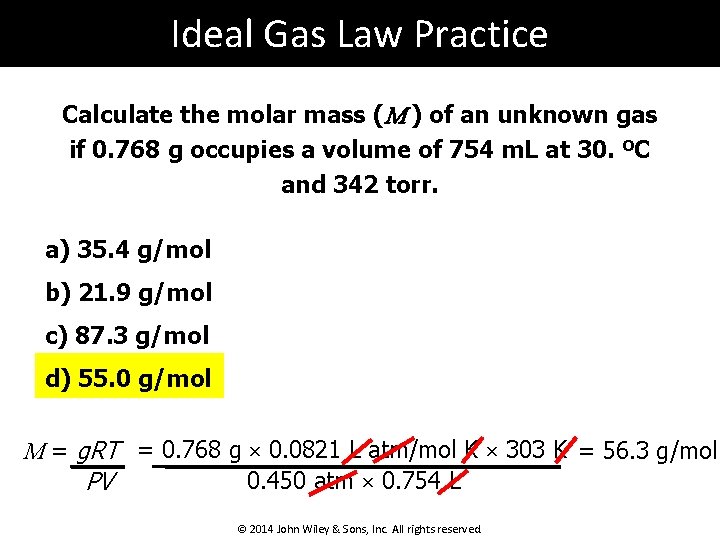
Ideal Gas Law Practice Calculate the molar mass (M ) of an unknown gas if 0. 768 g occupies a volume of 754 m. L at 30. ºC and 342 torr. a) 35. 4 g/mol b) 21. 9 g/mol c) 87. 3 g/mol d) 55. 0 g/mol M = g. RT = 0. 768 g × 0. 0821 L atm/mol K × 303 K = 56. 3 g/mol 0. 450 atm × 0. 754 L PV © 2014 John Wiley & Sons, Inc. All rights reserved.

Kinetic Molecular Theory A general theory developed to explain the behavior and theory of gases, based on the motion of particles. Assumptions of Kinetic Molecular Theory (KMT): 1) Gases consist of tiny particles. 2) The distance between particles is large when compared to particle size. The volume occupied by a gas is mostly empty space. 3) Gas particles have no attraction for one another. 4) Gas particles move linearly in all directions, frequently colliding with the container walls or other particles. © 2014 John Wiley & Sons, Inc. All rights reserved.

Kinetic Molecular Theory Assumptions of KMT (continued): 5) Collisions are perfectly elastic. No energy is lost during collisions. 6) The average kinetic energy for particles is the same for all gases (regardless of molar mass) at the same temperature. KE = 1/2 mv 2 where m is the mass and v is the velocity of the particle The average kinetic energy is directly proportional to temperature (in K). Gases which behave under these assumptions are know as ideal gases. © 2014 John Wiley & Sons, Inc. All rights reserved.

Real Gases Real gases typically behave like ideal gases over a fairly wide range of temperatures and pressures. Conditions where real gases deviate from ideal gases: 1) At high pressure (small volumes) Distance between particles is small and the particles do not behave independently. 2) At low temperature Particles experience intermolecular interactions. © 2014 John Wiley & Sons, Inc. All rights reserved.

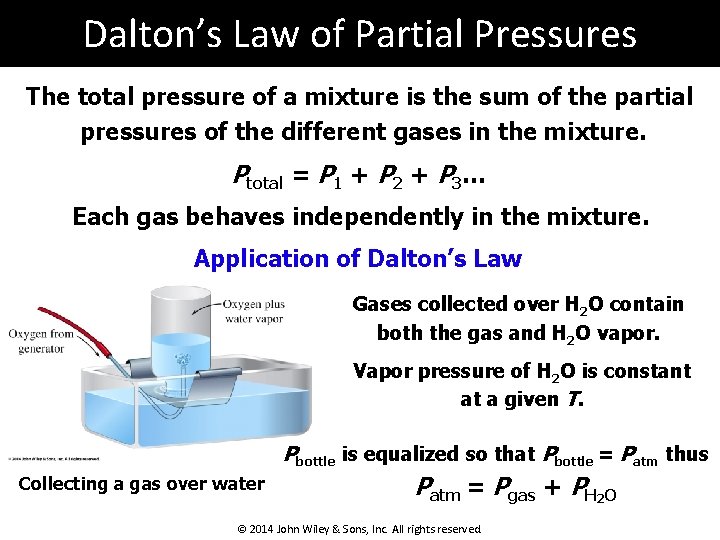
Dalton’s Law of Partial Pressures The total pressure of a mixture is the sum of the partial pressures of the different gases in the mixture. Ptotal = P 1 + P 2 + P 3… Each gas behaves independently in the mixture. Application of Dalton’s Law Gases collected over H 2 O contain both the gas and H 2 O vapor. Vapor pressure of H 2 O is constant at a given T. Pbottle is equalized so that Pbottle = Patm thus Collecting a gas over water Patm = Pgas + PH 2 O © 2014 John Wiley & Sons, Inc. All rights reserved.

Partial Pressures Problems A sample of O 2 gas is collected over water at 22 ºC and 662 torr. What is the partial pressure of O 2 gas? The vapor pressure of water is 19. 8 torr at 22 ºC. Knowns Patm = 662 torr Solving For PO 2 Calculate PH 2 O = 19. 8 torr PO 2 = Patm – PH 2 O PO 2 = 662 torr – 19. 8 torr = 642 torr © 2014 John Wiley & Sons, Inc. All rights reserved.

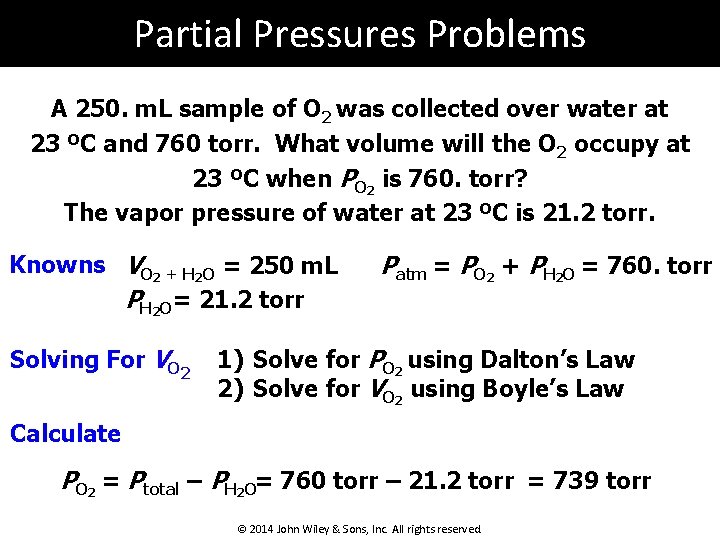
Partial Pressures Problems A 250. m. L sample of O 2 was collected over water at 23 ºC and 760 torr. What volume will the O 2 occupy at 23 ºC when PO 2 is 760. torr? The vapor pressure of water at 23 ºC is 21. 2 torr. Knowns VO 2 + H 2 O = 250 m. L PH 2 O= 21. 2 torr Solving For VO 2 Patm = PO 2 + PH 2 O = 760. torr 1) Solve for PO 2 using Dalton’s Law 2) Solve for VO 2 using Boyle’s Law Calculate PO 2 = Ptotal – PH 2 O= 760 torr – 21. 2 torr = 739 torr © 2014 John Wiley & Sons, Inc. All rights reserved.

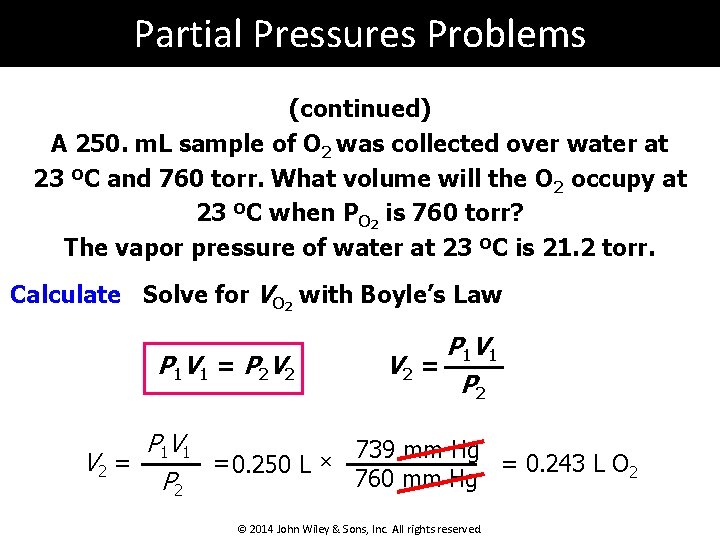
Partial Pressures Problems (continued) A 250. m. L sample of O 2 was collected over water at 23 ºC and 760 torr. What volume will the O 2 occupy at 23 ºC when PO 2 is 760 torr? The vapor pressure of water at 23 ºC is 21. 2 torr. Calculate Solve for VO 2 with Boyle’s Law P 1 V 1 = P 2 V 2 P 1 V 2 = P 2 P 1 V 1 739 mm Hg V 2 = = 0. 250 L × = 0. 243 L O 2 760 mm Hg P 2 © 2014 John Wiley & Sons, Inc. All rights reserved.

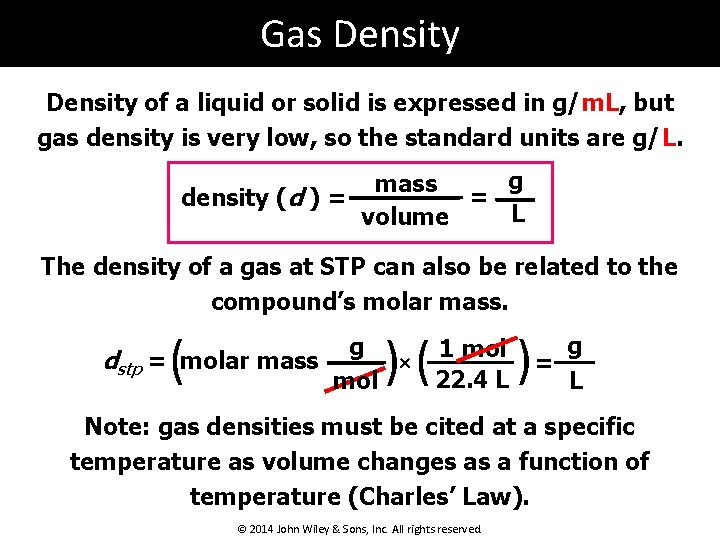
Gas Density of a liquid or solid is expressed in g/m. L, but gas density is very low, so the standard units are g/L. g mass = density (d ) = L volume The density of a gas at STP can also be related to the compound’s molar mass. ( )( g dstp = molar mass × mol 1 mol 22. 4 L ) g = L Note: gas densities must be cited at a specific temperature as volume changes as a function of temperature (Charles’ Law). © 2014 John Wiley & Sons, Inc. All rights reserved.

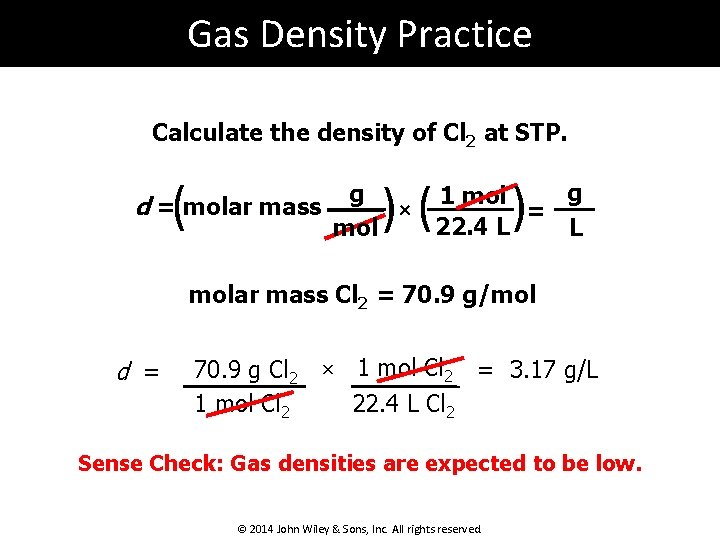
Gas Density Practice Calculate the density of Cl 2 at STP. ( d = molar mass )( g × mol ) g 1 mol = 22. 4 L L molar mass Cl 2 = 70. 9 g/mol d = 70. 9 g Cl 2 1 mol Cl 2 × 1 mol Cl 2 = 3. 17 g/L 22. 4 L Cl 2 Sense Check: Gas densities are expected to be low. © 2014 John Wiley & Sons, Inc. All rights reserved.

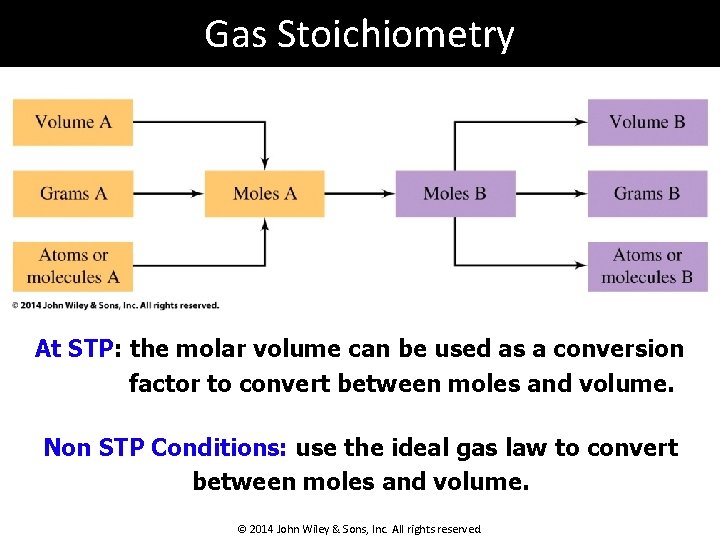
Gas Stoichiometry At STP: the molar volume can be used as a conversion factor to convert between moles and volume. Non STP Conditions: use the ideal gas law to convert between moles and volume. © 2014 John Wiley & Sons, Inc. All rights reserved.

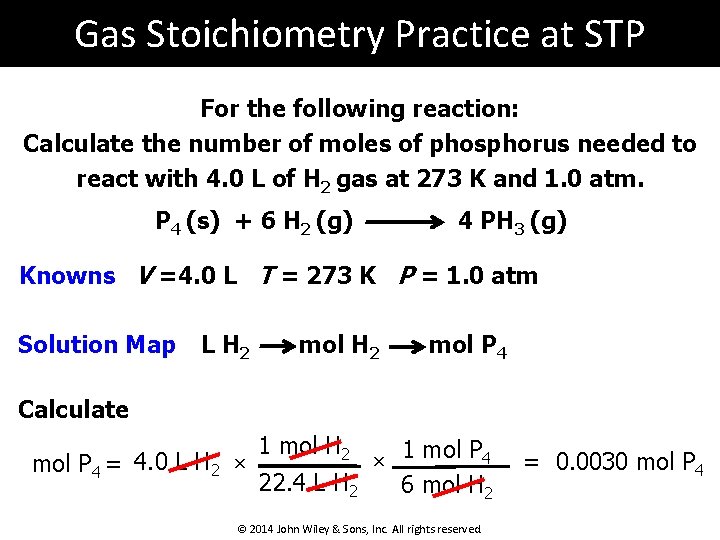
Gas Stoichiometry Practice at STP For the following reaction: Calculate the number of moles of phosphorus needed to react with 4. 0 L of H 2 gas at 273 K and 1. 0 atm. P 4 (s) + 6 H 2 (g) 4 PH 3 (g) Knowns V =4. 0 L T = 273 K P = 1. 0 atm Solution Map L H 2 mol P 4 Calculate mol P 4 = 4. 0 L H 2 × 1 mol H 2 22. 4 L H 2 × 1 mol P 4 6 mol H 2 © 2014 John Wiley & Sons, Inc. All rights reserved. = 0. 0030 mol P 4

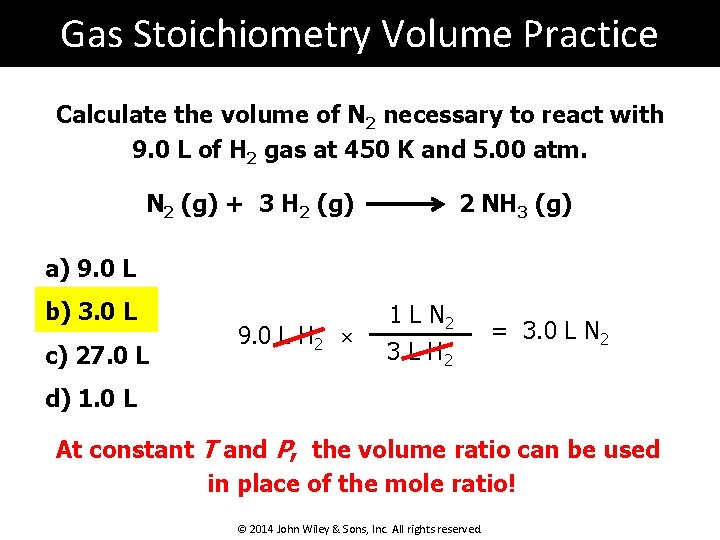
Gas Stoichiometry Volume Practice Calculate the volume of N 2 necessary to react with 9. 0 L of H 2 gas at 450 K and 5. 00 atm. N 2 (g) + 3 H 2 (g) 2 NH 3 (g) a) 9. 0 L b) 3. 0 L c) 27. 0 L 9. 0 L H 2 × 1 L N 2 3 L H 2 = 3. 0 L N 2 d) 1. 0 L At constant T and P, the volume ratio can be used in place of the mole ratio! © 2014 John Wiley & Sons, Inc. All rights reserved.

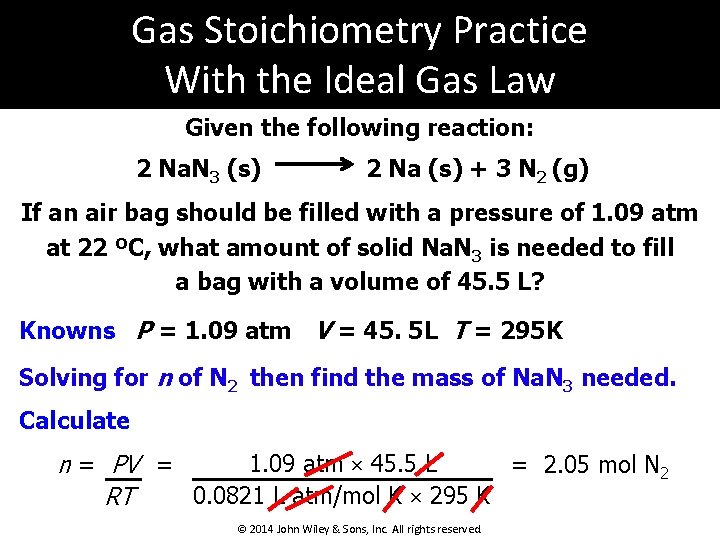
Gas Stoichiometry Practice With the Ideal Gas Law Given the following reaction: 2 Na. N 3 (s) 2 Na (s) + 3 N 2 (g) If an air bag should be filled with a pressure of 1. 09 atm at 22 ºC, what amount of solid Na. N 3 is needed to fill a bag with a volume of 45. 5 L? Knowns P = 1. 09 atm V = 45. 5 L T = 295 K Solving for n of N 2 then find the mass of Na. N 3 needed. Calculate 1. 09 atm × 45. 5 L = 2. 05 mol N 2 n = PV = 0. 0821 L atm/mol K × 295 K RT © 2014 John Wiley & Sons, Inc. All rights reserved.

Gas Stoichiometry Practice With the Ideal Gas Law (continued) Given the following reaction: 2 Na. N 3 (s) 2 Na (s) + 3 N 2 (g) If an air bag should be filled with a pressure of 1. 09 atm at 22. 0 ºC, what amount of solid Na. N 3 is needed to fill a bag with a volume of 45. 5 L? Calculate Use the reaction stoichiometry! 2. 05 mol N 2 × 2 mol Na. N 3 3 mol N 2 × 64. 99 g Na. N 3 = 88. 8 g Na. N 3 1 mol Na. N 3 © 2014 John Wiley & Sons, Inc. All rights reserved.

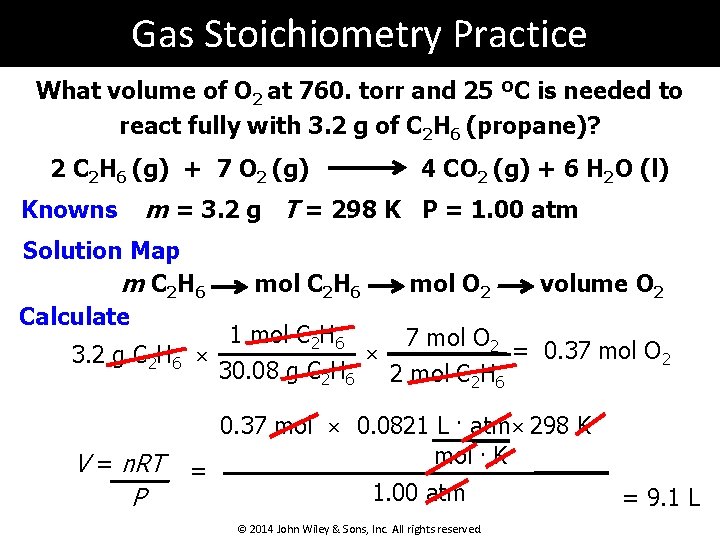
Gas Stoichiometry Practice What volume of O 2 at 760. torr and 25 ºC is needed to react fully with 3. 2 g of C 2 H 6 (propane)? 2 C 2 H 6 (g) + 7 O 2 (g) Knowns 4 CO 2 (g) + 6 H 2 O (l) m = 3. 2 g T = 298 K P = 1. 00 atm Solution Map m C 2 H 6 Calculate mol C 2 H 6 mol O 2 volume O 2 1 mol C 2 H 6 7 mol O 2 = 0. 37 mol O 2 × 3. 2 g C 2 H 6 × 30. 08 g C 2 H 6 2 mol C 2 H 6 V = n. RT P = 0. 37 mol × 0. 0821 L. atm × 298 K mol. K 1. 00 atm © 2014 John Wiley & Sons, Inc. All rights reserved. = 9. 1 L

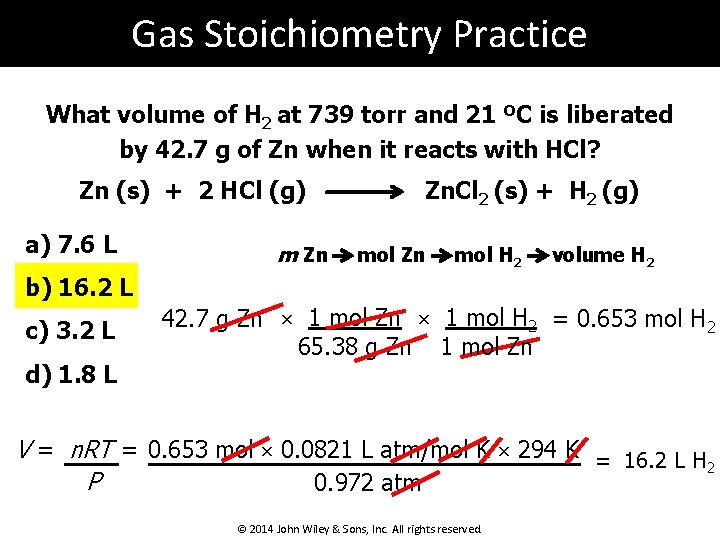
Gas Stoichiometry Practice What volume of H 2 at 739 torr and 21 ºC is liberated by 42. 7 g of Zn when it reacts with HCl? Zn (s) + 2 HCl (g) a) 7. 6 L m Zn Zn. Cl 2 (s) + H 2 (g) mol Zn mol H 2 volume H 2 b) 16. 2 L c) 3. 2 L d) 1. 8 L 42. 7 g Zn × 1 mol H 2 = 0. 653 mol H 2 65. 38 g Zn 1 mol Zn V = n. RT = 0. 653 mol × 0. 0821 L atm/mol K × 294 K = 16. 2 L H 2 P 0. 972 atm © 2014 John Wiley & Sons, Inc. All rights reserved.

Chemistry in Action What the Nose Knows Dogs use smell to detect many drugs, explosives, etc. based on trace amounts of chemical compounds in the air. Sensing low concentrations of chemicals is useful! Better Coffee Better Science Artificial noses could sniff out cancer or explosives! For more information, see: http: //www. scs. illinois. edu/suslick/smell_seeing. html © 2014 John Wiley & Sons, Inc. All rights reserved.

Learning Objectives 12. 1 Properties of Gases 1) Explain atmospheric pressure and how it is measured. 2) Be able to convert between the various units of pressure. 12. 2 Boyle’s Law 3) Use Boyle’s Law to calculate changes in pressure or volume of a gas at constant temperature. 12. 3 Charles’ Law 4) Use Charles’ Law to calculate changes in temperature or volume of a gas at constant pressure. © 2014 John Wiley & Sons, Inc. All rights reserved.

Learning Objectives 12. 4 Avogadro’s Law 5) Solve problems using the relationships between moles, mass, and volume of gases. 12. 5 Combined Gas Law 6) Use the combined gas law to calculate changes in pressure, volume, or temperature of a gas sample. 12. 6 Ideal Gas Law 7) Use the ideal gas law to solve problems involving pressure, volume, temperature, and moles of a gas. © 2014 John Wiley & Sons, Inc. All rights reserved.

Learning Objectives 12. 7 Dalton’s Law of Partial Pressures 8) Use Dalton’s Law of Partial Pressures to calculate the total pressure for a mixture of gases or the pressure of a single gas in a mixture of gases. 12. 8 Density of Gases 9) Calculate the density of a gas. (Pay attention to units!) 12. 9 Gas Stoichiometry 10) Solve stoichiometry problems involving gases. (Pay attention to the states of matter and use gas laws only for gases!) © 2014 John Wiley & Sons, Inc. All rights reserved.
 Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Inorganic gases
Inorganic gases Gaseous state chapter
Gaseous state chapter Air higroskopis air kapiler dan air gravitasi
Air higroskopis air kapiler dan air gravitasi Is venus terrestrial or gaseous
Is venus terrestrial or gaseous Xeromorphic plants
Xeromorphic plants Gas exchange in worms
Gas exchange in worms Gaseous porosity denture
Gaseous porosity denture Gaseous exchange in animals
Gaseous exchange in animals Inhalanda
Inhalanda Gaseous equilibrium
Gaseous equilibrium Gaseous envelope of the sun
Gaseous envelope of the sun Grade 11 practical gaseous exchange
Grade 11 practical gaseous exchange The earthworm
The earthworm Gaseous solution
Gaseous solution Non gaseous alkalosis
Non gaseous alkalosis Dosage forms list
Dosage forms list At 500 k one mole of gaseous oncl
At 500 k one mole of gaseous oncl Section 1 composition of matter
Section 1 composition of matter Grey vs white matter
Grey vs white matter Section 1 composition of matter
Section 1 composition of matter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers Arbor vitae
Arbor vitae Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Gray matter and white matter
Gray matter and white matter Grey matter and white matter in brain
Grey matter and white matter in brain Energy naturally flows from warmer matter to cooler matter
Energy naturally flows from warmer matter to cooler matter State of matter
State of matter Properties of liquids
Properties of liquids States of matter thermal energy
States of matter thermal energy Sixth state of matter
Sixth state of matter øis
øis Pepsi mentos
Pepsi mentos Physical state of matter
Physical state of matter Change in state of matter
Change in state of matter Whats the fourth state of matter
Whats the fourth state of matter Matter venn diagram
Matter venn diagram Colloidal state
Colloidal state Chemical equation with states of matter
Chemical equation with states of matter Law of constancy of interfacial angle
Law of constancy of interfacial angle Compressible state of matter
Compressible state of matter State of matter
State of matter Volume solid liquid gas
Volume solid liquid gas 6th state of matter
6th state of matter Particle diagram of a solid
Particle diagram of a solid Phases of matter concept map
Phases of matter concept map Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Chó sói
Chó sói Thang điểm glasgow
Thang điểm glasgow Chúa yêu trần thế alleluia
Chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới