WHATS THE MATTER WITH SOLIDS LIQUIDS GASES WHAT






- Slides: 19

WHAT’S THE MATTER WITH SOLIDS, LIQUIDS, & GASES

WHAT IS MATTER Matter- Anything that takes up space and has mass – The substance that an object is made of – Every form of matter has two kinds of properties- physical and chemical

PHYSICAL PROPERTIES OF MATTER • A physical property is a characteristic of a pure substance that can be observed without changing it into another substance -Physical state -Temperature for freezing, boiling, or vaporizing -Ability to dissolve in water -Hardness, texture, color, and flexibility • Can be used to classify matter (ex. Metals are flexible and can conduct heat and electricity)

CHEMICAL PROPERTIES OF MATTER • Chemical properties are characteristics of a pure substance that describe its ability to change into different substances • -Properties can be used to classify substances • -Examples: Ability to burn and/or react with other substances § Only observed when the original substance is changed into a different substance • -New substances may have different properties then the original substances

CHANGES IN MATTER Matter can undergo two basic types of changes- physical changes and chemical changes • Physical changes- alters the form or appearance of matter but does not form any new substances • Chemical changes- changes in matter that produce one or more new substances

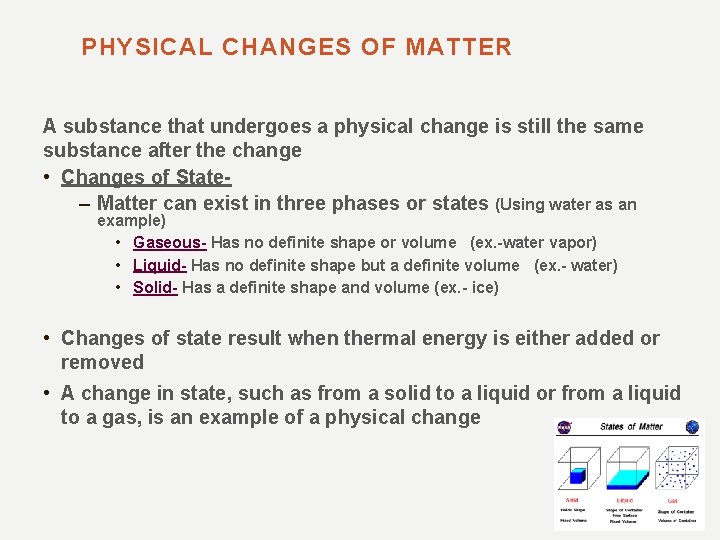
PHYSICAL CHANGES OF MATTER A substance that undergoes a physical change is still the same substance after the change • Changes of State– Matter can exist in three phases or states (Using water as an example) • Gaseous- Has no definite shape or volume (ex. -water vapor) • Liquid- Has no definite shape but a definite volume (ex. - water) • Solid- Has a definite shape and volume (ex. - ice) • Changes of state result when thermal energy is either added or removed • A change in state, such as from a solid to a liquid or from a liquid to a gas, is an example of a physical change

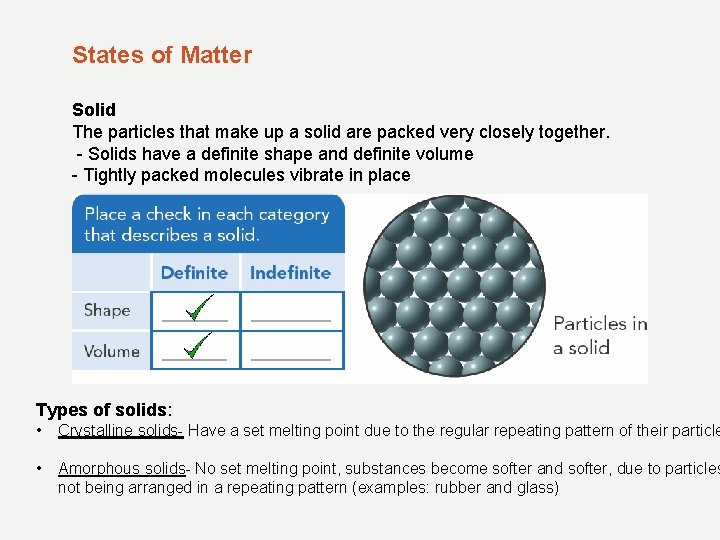
States of Matter Solid The particles that make up a solid are packed very closely together. - Solids have a definite shape and definite volume - Tightly packed molecules vibrate in place Types of solids: • Crystalline solids- Have a set melting point due to the regular repeating pattern of their particle • Amorphous solids- No set melting point, substances become softer and softer, due to particles not being arranged in a repeating pattern (examples: rubber and glass)

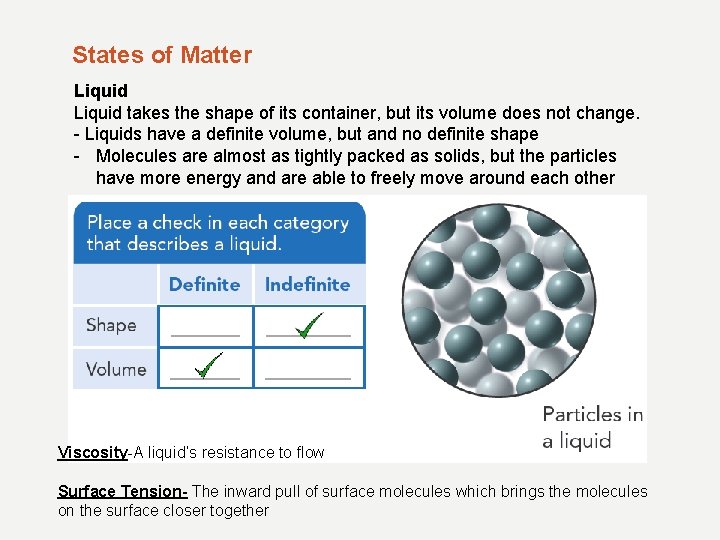
States of Matter Liquid takes the shape of its container, but its volume does not change. - Liquids have a definite volume, but and no definite shape - Molecules are almost as tightly packed as solids, but the particles have more energy and are able to freely move around each other Viscosity-A liquid’s resistance to flow Surface Tension- The inward pull of surface molecules which brings the molecules on the surface closer together

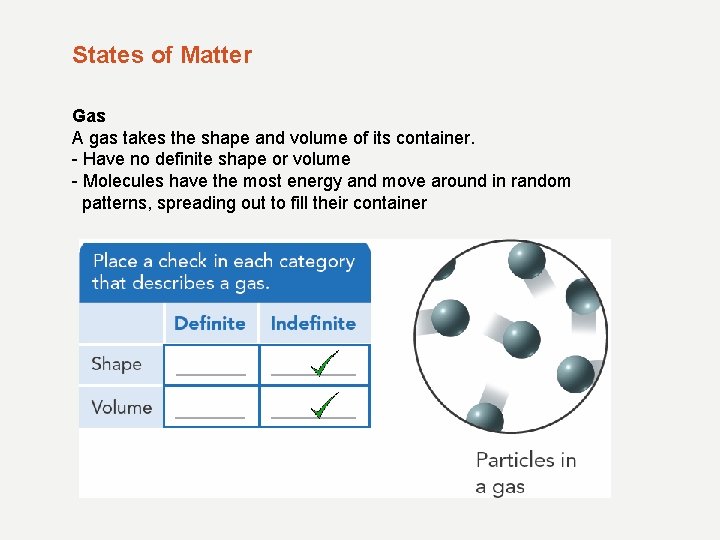
States of Matter Gas A gas takes the shape and volume of its container. - Have no definite shape or volume - Molecules have the most energy and move around in random patterns, spreading out to fill their container

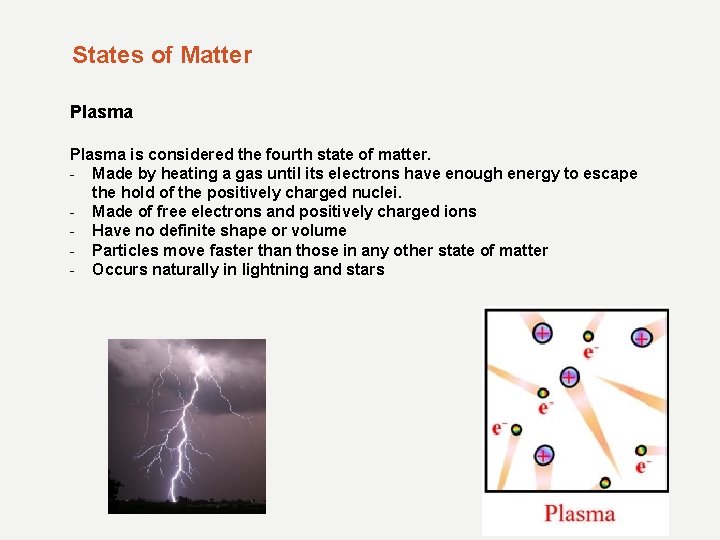
States of Matter Plasma is considered the fourth state of matter. - Made by heating a gas until its electrons have enough energy to escape the hold of the positively charged nuclei. - Made of free electrons and positively charged ions - Have no definite shape or volume - Particles move faster than those in any other state of matter - Occurs naturally in lightning and stars

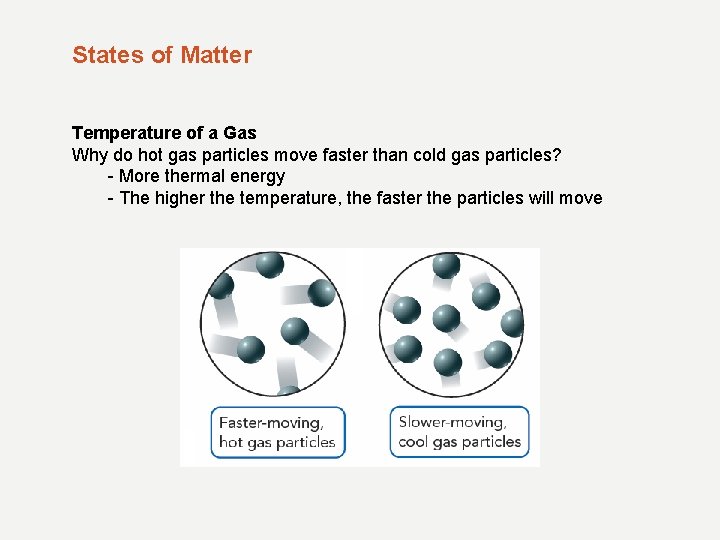
States of Matter Temperature of a Gas Why do hot gas particles move faster than cold gas particles? - More thermal energy - The higher the temperature, the faster the particles will move

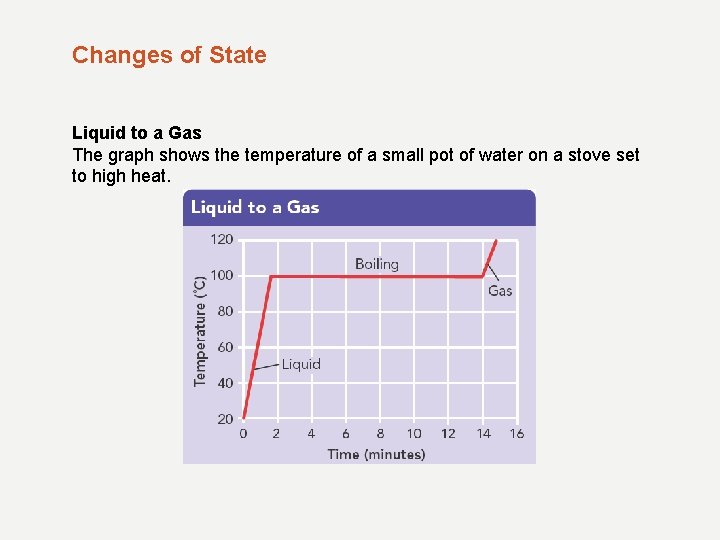
Changes of State Liquid to a Gas The graph shows the temperature of a small pot of water on a stove set to high heat.

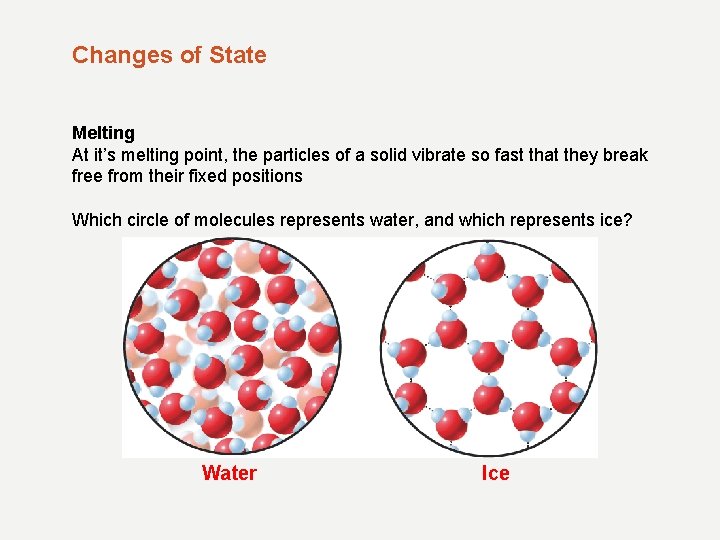
Changes of State Melting At it’s melting point, the particles of a solid vibrate so fast that they break free from their fixed positions Which circle of molecules represents water, and which represents ice? Water Ice

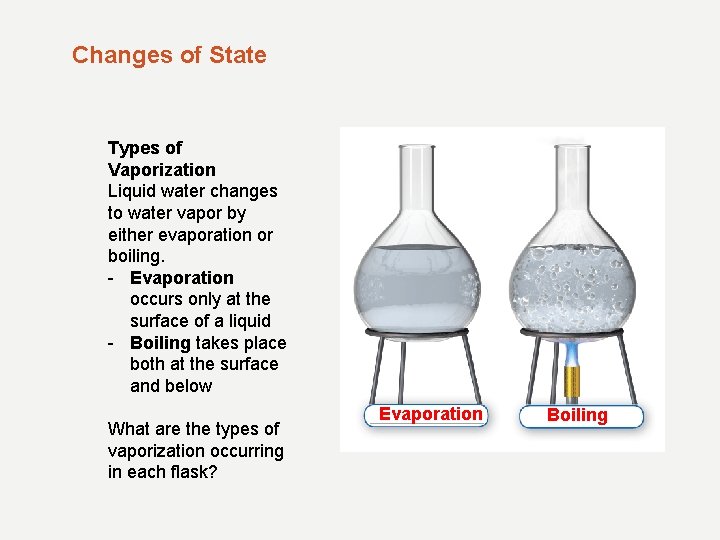
Changes of State Types of Vaporization Liquid water changes to water vapor by either evaporation or boiling. - Evaporation occurs only at the surface of a liquid - Boiling takes place both at the surface and below What are the types of vaporization occurring in each flask? Evaporation Boiling

Changes of State Skipping the middle phase If enough thermal energy is gained or lost, a solid can change directly into a gas or a gas can phase into a solid • Sublimation occurs when a solid gains enough energy to skip the liquid phase and go directly to a gaseous state (ex. dry ice) Sublimation • Deposition takes place when a gas changes directly into a solid due to a loss in thermal energy (ex. The frost found on the grass or a window on a winter morning) Deposition

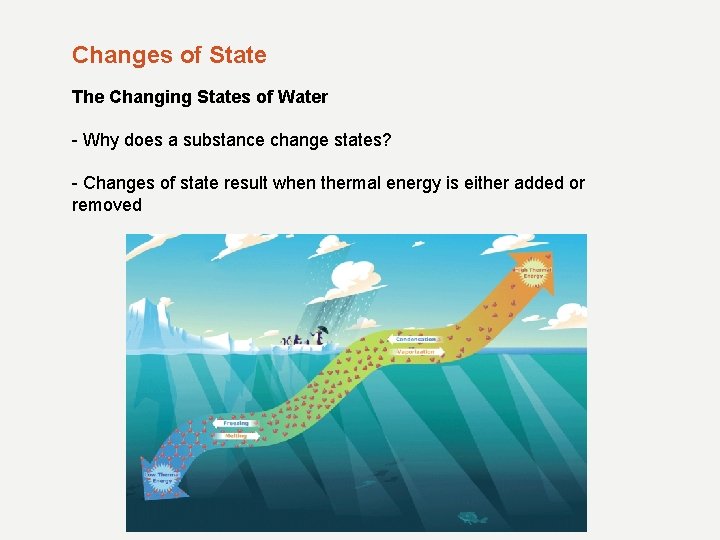
Changes of State The Changing States of Water - Why does a substance change states? - Changes of state result when thermal energy is either added or removed

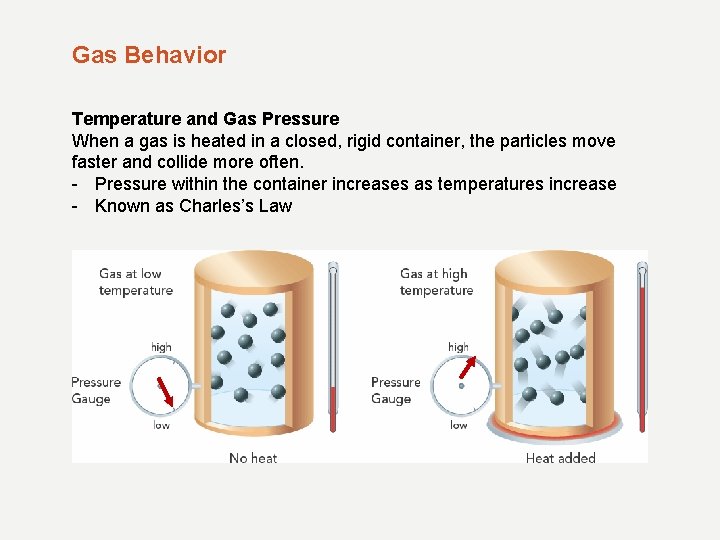
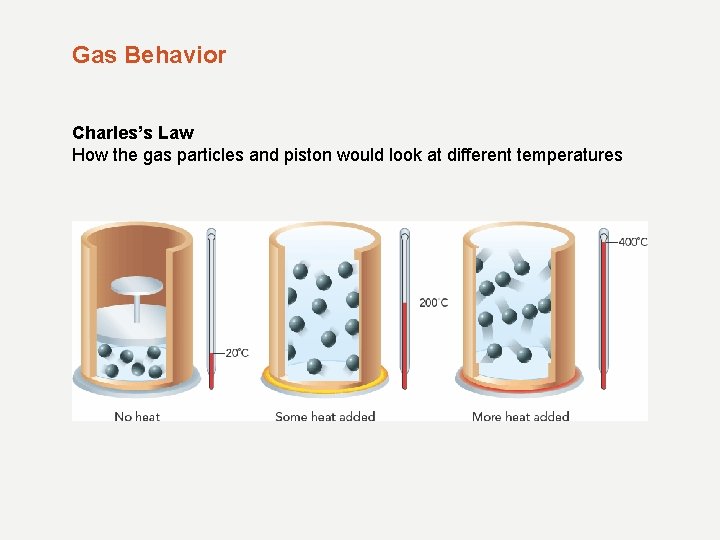
Gas Behavior Temperature and Gas Pressure When a gas is heated in a closed, rigid container, the particles move faster and collide more often. - Pressure within the container increases as temperatures increase - Known as Charles’s Law

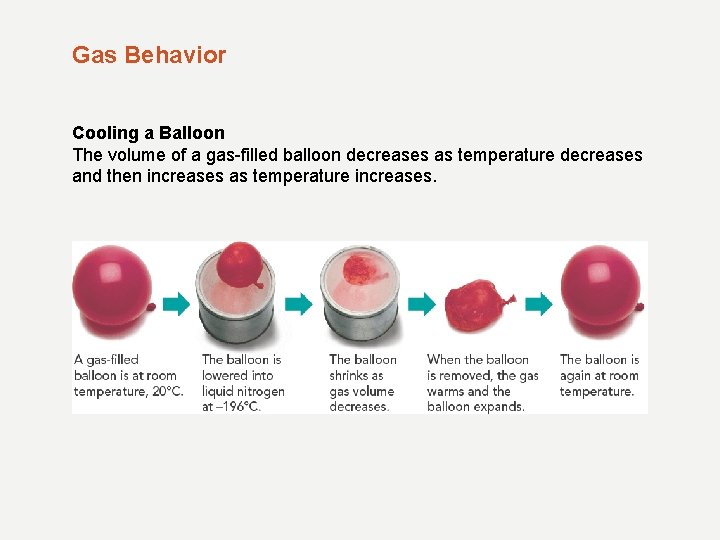
Gas Behavior Cooling a Balloon The volume of a gas-filled balloon decreases as temperature decreases and then increases as temperature increases.

Gas Behavior Charles’s Law How the gas particles and piston would look at different temperatures