Chapter 12 The Gaseous State of Matter The





















- Slides: 51

Chapter 12 The Gaseous State of Matter The air in a hot air balloon expands When it is heated. Some of the air escapes from the top of the balloon, lowering the air density inside the balloon, making the balloon buoyant. Introduction to General, Organic, and Biochemistry 10 e John Wiley & Sons, Inc Morris Hein, Scott Pattison, and Susan Arena

Chapter Outline 12. 1 General Properties 12. 8 Combined Gas Laws 12. 2 The Kinetic-Molecular Theory 12. 9 Dalton’s Law of Partial Pressures 12. 3 Measurement of Pressure 12. 10 Avogadro’s Law 12. 4 Dependence of Pressure on Number of Molecules and Temperature 12. 11 Mole-Mass-Volume Relationships of Gases 12. 5 Boyle’s Law 12. 13 Ideal Gas Law 12. 6 Charles’ Law 12. 14 Gas Stoichiometry 12. 7 Gay-Lussac’s Law 12. 15 Real Gases 12. 12 Density of Gases Copyright 2012 John Wiley & Sons, Inc

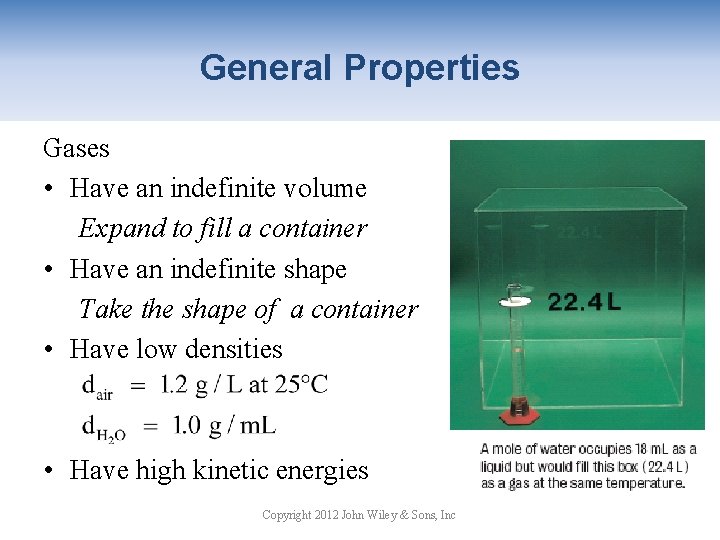
General Properties Gases • Have an indefinite volume Expand to fill a container • Have an indefinite shape Take the shape of a container • Have low densities • Have high kinetic energies Copyright 2012 John Wiley & Sons, Inc

Kinetic Molecular Theory (KMT) Assumptions of the KMT and ideal gases include: 1. Gases consist of tiny particles 2. The distance between particles is large compared with the size of the particles. 3. Gas particles have no attraction for each other 4. Gas particles move in straight lines in all directions, colliding frequently with each other and with the walls of the container. Copyright 2012 John Wiley & Sons, Inc

Kinetic Molecular Theory Assumptions of the KMT (continued): 5. Collisions are perfectly elastic (no energy is lost in the collision). 6. The average kinetic energy for particles is the same for all gases at the same temperature. 7. The average kinetic energy is directly proportional to the Kelvin temperature. Copyright 2012 John Wiley & Sons, Inc

Diffusion Copyright 2012 John Wiley & Sons, Inc

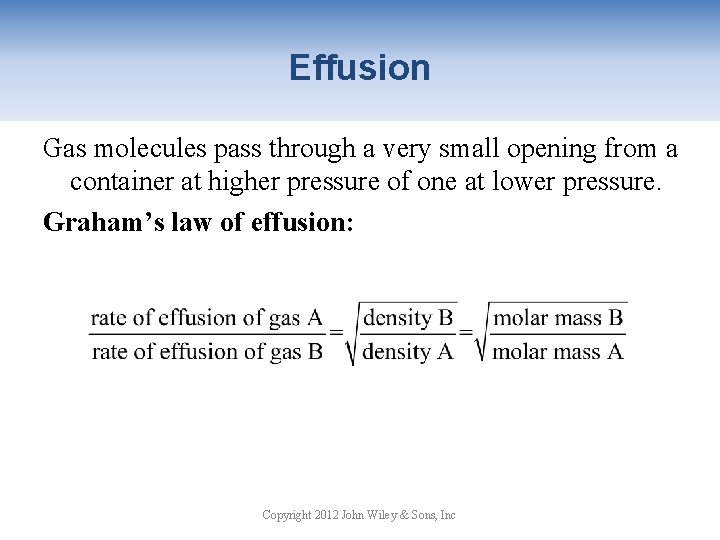
Effusion Gas molecules pass through a very small opening from a container at higher pressure of one at lower pressure. Graham’s law of effusion: Copyright 2012 John Wiley & Sons, Inc

Your Turn! Which gas will diffuse most rapidly? a. He b. Ne c. Ar d. Kr Copyright 2012 John Wiley & Sons, Inc

Measurement of Pressure depends on the • Number of gas molecules • Temperature of the gas • Volume the gas occupies Copyright 2012 John Wiley & Sons, Inc

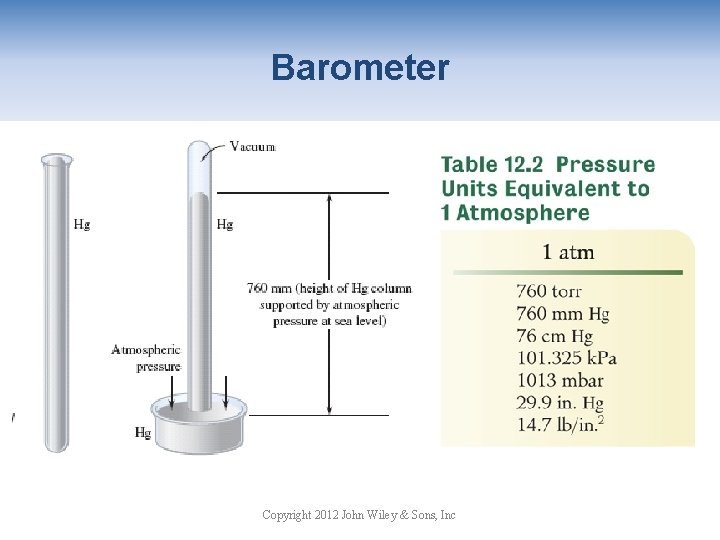
Atmospheric Pressure Atmospheric pressure is due to the mass of the atmospheric gases pressing down on the earth’s surface. Copyright 2012 John Wiley & Sons, Inc

Barometer Copyright 2012 John Wiley & Sons, Inc

Pressure Conversions Convert 675 mm Hg to atm. Note: 760 mm Hg = 1 atm Convert 675 mm Hg to torr. Note: 760 mm Hg = 760 torr. Copyright 2012 John Wiley & Sons, Inc

Your Turn! A pressure of 3. 00 atm is equal to a. 819 torr b. 3000 torr c. 2280 torr d. 253 torr Copyright 2012 John Wiley & Sons, Inc

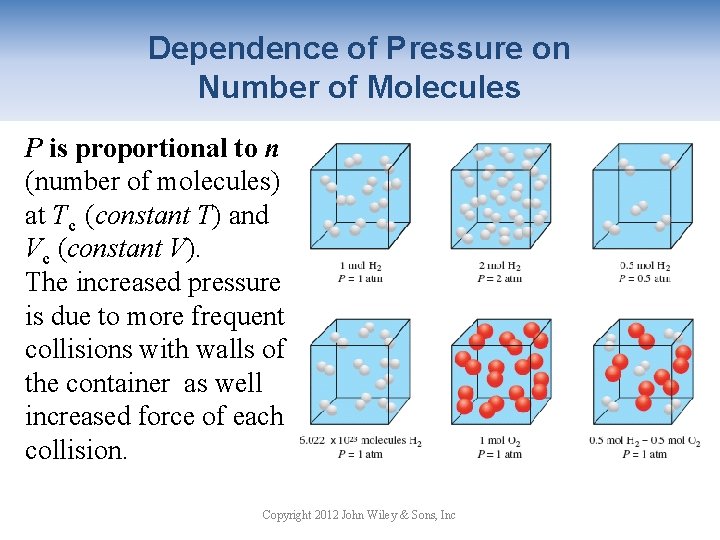
Dependence of Pressure on Number of Molecules P is proportional to n (number of molecules) at Tc (constant T) and Vc (constant V). The increased pressure is due to more frequent collisions with walls of the container as well increased force of each collision. Copyright 2012 John Wiley & Sons, Inc

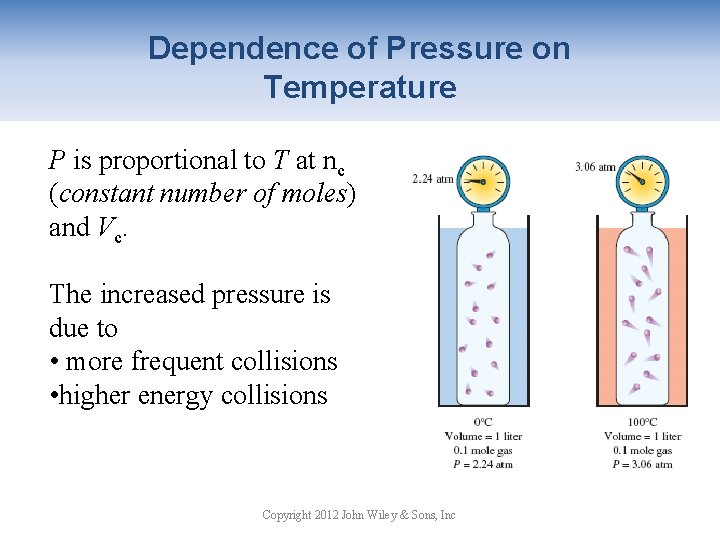
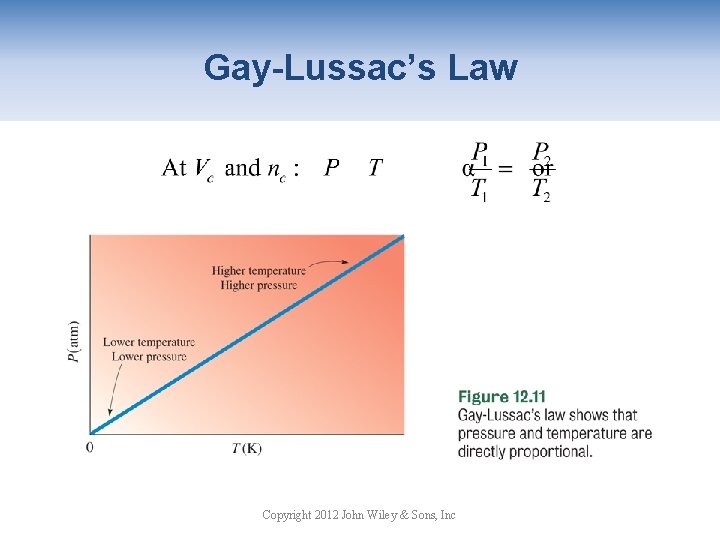
Dependence of Pressure on Temperature P is proportional to T at nc (constant number of moles) and Vc. The increased pressure is due to • more frequent collisions • higher energy collisions Copyright 2012 John Wiley & Sons, Inc

Your Turn! If you change the temperature of a sample of gas from 80°C to 25°C at constant volume, the pressure of the gas a. will increase. b. will decrease. c. will not change Copyright 2012 John Wiley & Sons, Inc

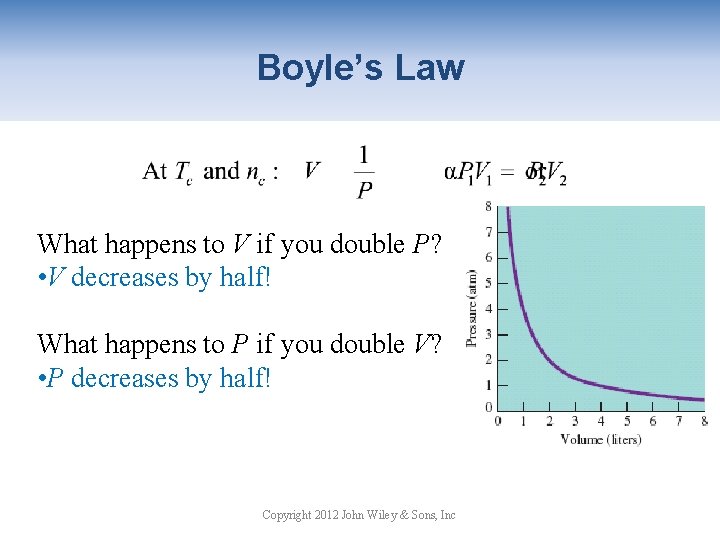
Boyle’s Law What happens to V if you double P? • V decreases by half! What happens to P if you double V? • P decreases by half! Copyright 2012 John Wiley & Sons, Inc

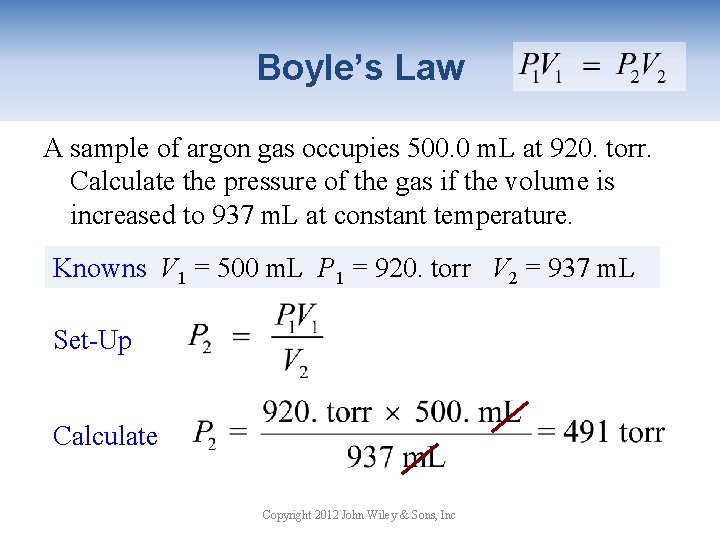
Boyle’s Law A sample of argon gas occupies 500. 0 m. L at 920. torr. Calculate the pressure of the gas if the volume is increased to 937 m. L at constant temperature. Knowns V 1 = 500 m. L P 1 = 920. torr V 2 = 937 m. L Set-Up Calculate Copyright 2012 John Wiley & Sons, Inc

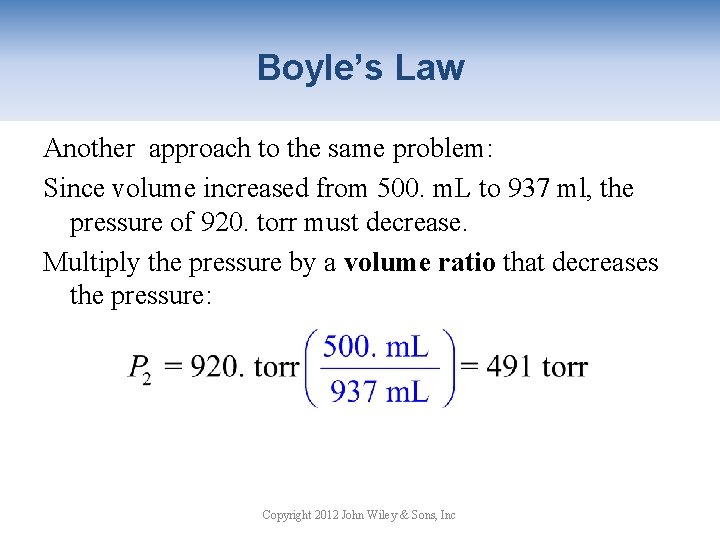
Boyle’s Law Another approach to the same problem: Since volume increased from 500. m. L to 937 ml, the pressure of 920. torr must decrease. Multiply the pressure by a volume ratio that decreases the pressure: Copyright 2012 John Wiley & Sons, Inc

Your Turn! A 6. 00 L sample of a gas at a pressure of 8. 00 atm is compressed to 4. 00 L at a constant temperature. What is the pressure of the gas? a. 4. 00 atm b. 12. 0 atm c. 24. 0 atm d. 48. 0 atm Copyright 2012 John Wiley & Sons, Inc

Your Turn! A 400. m. L sample of a gas is at a pressure of 760. torr. If the temperature remains constant, what will be its volume at 190. torr? A. 100. m. L B. 400. m. L C. 25. 0 m. L D. 1. 60 x 102 m. L Copyright 2012 John Wiley & Sons, Inc

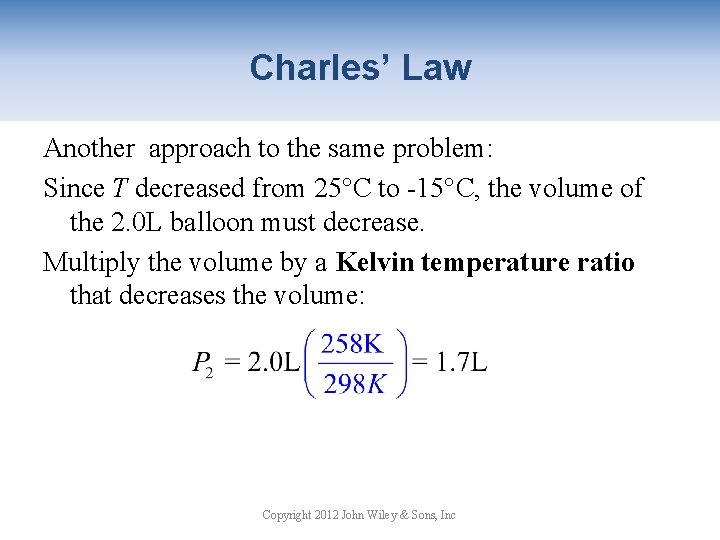
Charles’ Law • The volume of an ideal gas at absolute zero (-273°C) is zero. • Real gases condense at their boiling point so it is not possible to have a gas with zero volume. • The gas laws are based on Kelvin temperature. • All gas law problems must be worked in Kelvin! Copyright 2012 John Wiley & Sons, Inc

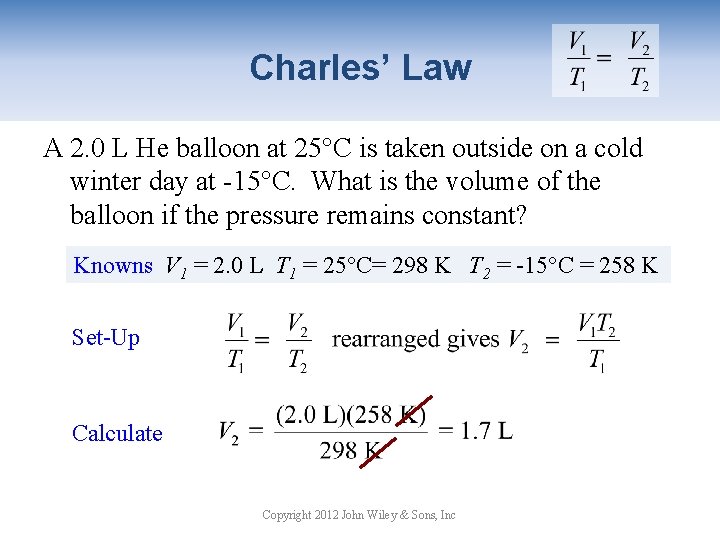
Charles’ Law A 2. 0 L He balloon at 25°C is taken outside on a cold winter day at -15°C. What is the volume of the balloon if the pressure remains constant? Knowns V 1 = 2. 0 L T 1 = 25°C= 298 K T 2 = -15°C = 258 K Set-Up Calculate Copyright 2012 John Wiley & Sons, Inc

Charles’ Law Another approach to the same problem: Since T decreased from 25°C to -15°C, the volume of the 2. 0 L balloon must decrease. Multiply the volume by a Kelvin temperature ratio that decreases the volume: Copyright 2012 John Wiley & Sons, Inc

Your Turn The volume of a gas always increases when a. Temperature increases and pressure decreases b. Temperature increases and pressure increases c. Temperature decreases and pressure increases d. Temperature decreases and pressure decreases Copyright 2012 John Wiley & Sons, Inc

Your Turn! A sample of CO 2 has a volume of 200. m. L at 20. 0 ° C. What will be its volume at 40. 0 °C, assuming that the pressure remains constant? a. 18. 8 m. L b. 100. m. L c. 213 m. L d. 400. m. L Copyright 2012 John Wiley & Sons, Inc

Your Turn! A sample of gas has a volume of 3. 00 L at 10. 0 °C. What will be its temperature in °C if the gas expands to 6. 00 L at constant pressure? a. 20. 0°C b. 293°C c. 566°C d. 142°C Copyright 2012 John Wiley & Sons, Inc

Gay-Lussac’s Law Copyright 2012 John Wiley & Sons, Inc

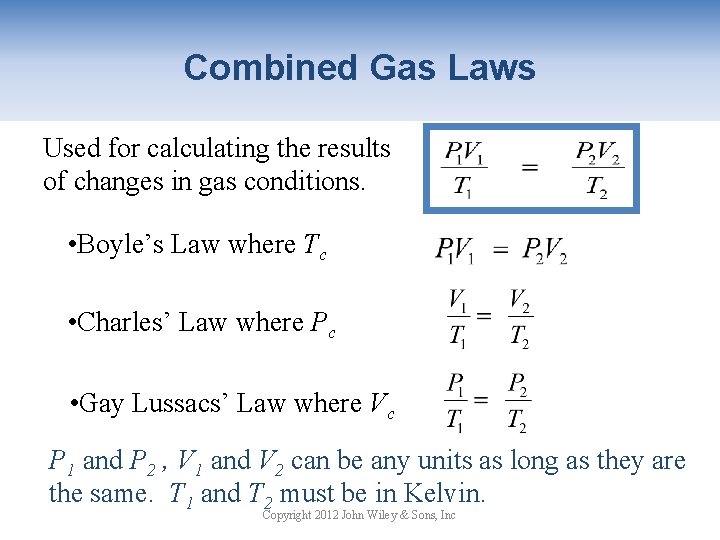
Combined Gas Laws Used for calculating the results of changes in gas conditions. • Boyle’s Law where Tc • Charles’ Law where Pc • Gay Lussacs’ Law where Vc P 1 and P 2 , V 1 and V 2 can be any units as long as they are the same. T 1 and T 2 must be in Kelvin. Copyright 2012 John Wiley & Sons, Inc

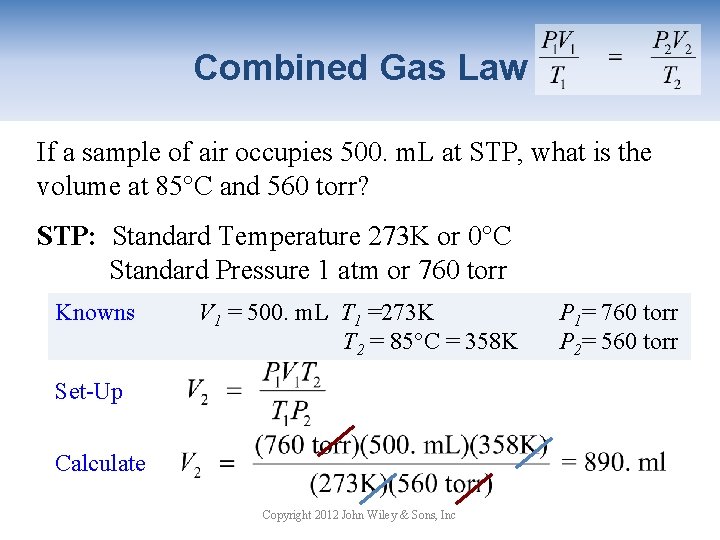
Combined Gas Law If a sample of air occupies 500. m. L at STP, what is the volume at 85°C and 560 torr? STP: Standard Temperature 273 K or 0°C Standard Pressure 1 atm or 760 torr Knowns V 1 = 500. m. L T 1 =273 K T 2 = 85°C = 358 K Set-Up Calculate Copyright 2012 John Wiley & Sons, Inc P 1= 760 torr P 2= 560 torr

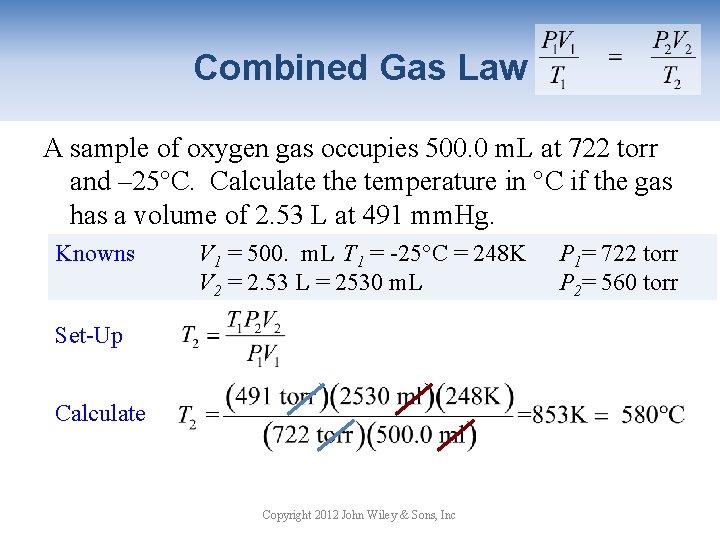
Combined Gas Law A sample of oxygen gas occupies 500. 0 m. L at 722 torr and – 25°C. Calculate the temperature in °C if the gas has a volume of 2. 53 L at 491 mm. Hg. Knowns V 1 = 500. m. L T 1 = -25°C = 248 K P 1= 722 torr V 2 = 2. 53 L = 2530 m. L P 2= 560 torr Set-Up Calculate Copyright 2012 John Wiley & Sons, Inc

Your Turn! A sample of gas has a volume of 8. 00 L at 20. 0 ° C and 700. torr. What will be its volume at STP? a. 1. 20 L b. 9. 32 L c. 53. 2 L d. 6. 87 L Copyright 2012 John Wiley & Sons, Inc

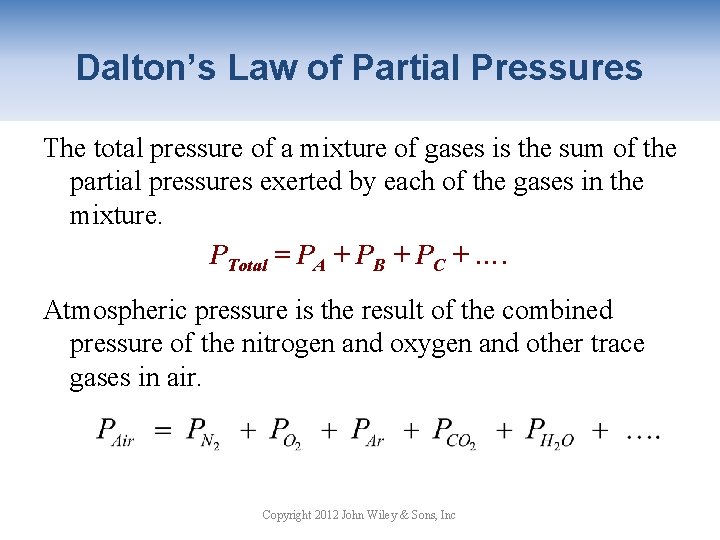
Dalton’s Law of Partial Pressures The total pressure of a mixture of gases is the sum of the partial pressures exerted by each of the gases in the mixture. PTotal = PA + PB + PC + …. Atmospheric pressure is the result of the combined pressure of the nitrogen and oxygen and other trace gases in air. Copyright 2012 John Wiley & Sons, Inc

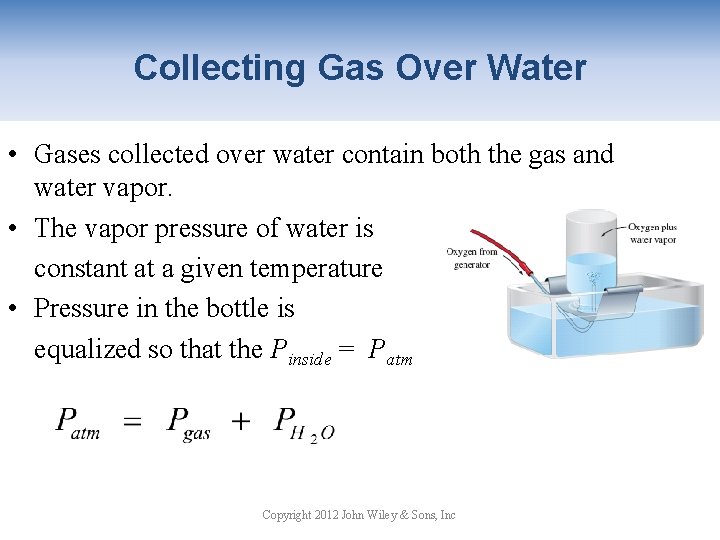
Collecting Gas Over Water • Gases collected over water contain both the gas and water vapor. • The vapor pressure of water is constant at a given temperature • Pressure in the bottle is equalized so that the Pinside = Patm Copyright 2012 John Wiley & Sons, Inc

Your Turn! A sample of oxygen is collected over water at 22 ° C and 762 torr. What is the partial pressure of the dry oxygen? The vapor pressure of water at 22°C is 19. 8 torr. a. 742 torr b. 782 torr c. 784 torr d. 750. torr Copyright 2012 John Wiley & Sons, Inc

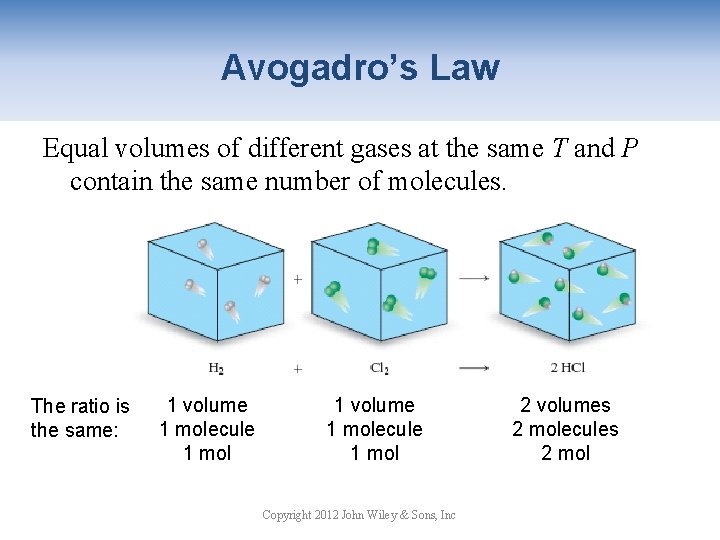
Avogadro’s Law Equal volumes of different gases at the same T and P contain the same number of molecules. The ratio is the same: 1 volume 1 molecule 1 mol Copyright 2012 John Wiley & Sons, Inc 2 volumes 2 molecules 2 mol

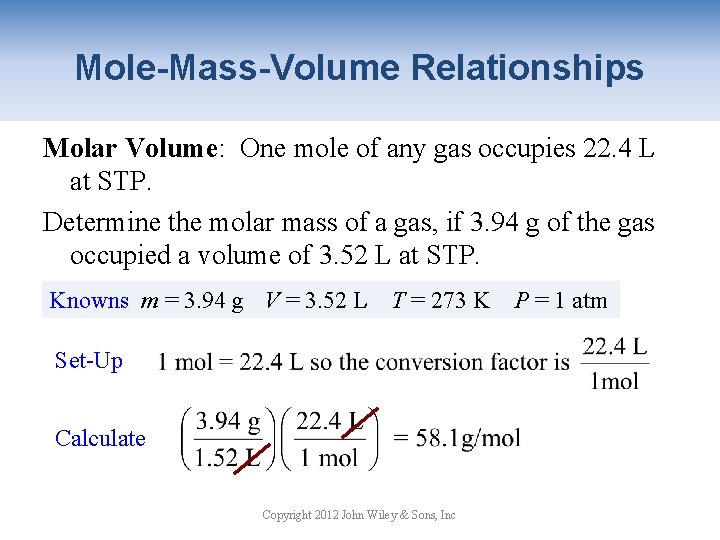
Mole-Mass-Volume Relationships Molar Volume: One mole of any gas occupies 22. 4 L at STP. Determine the molar mass of a gas, if 3. 94 g of the gas occupied a volume of 3. 52 L at STP. Knowns m = 3. 94 g V = 3. 52 L T = 273 K P = 1 atm Set-Up Calculate Copyright 2012 John Wiley & Sons, Inc

Your Turn! What is the molar mass of a gas if 240. m. L of the gas at STP has a mass of 0. 320 grams? a. 8. 57 g b. 22. 4 g c. 16. 8 g d. 29. 9 g Copyright 2012 John Wiley & Sons, Inc

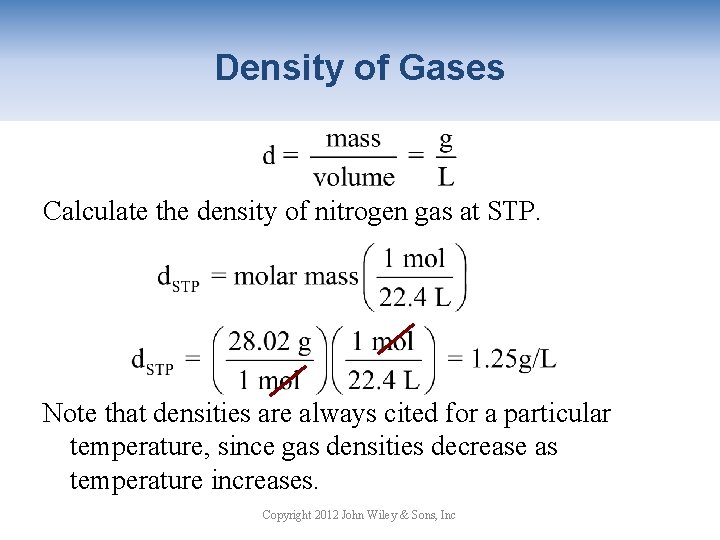
Density of Gases Calculate the density of nitrogen gas at STP. Note that densities are always cited for a particular temperature, since gas densities decrease as temperature increases. Copyright 2012 John Wiley & Sons, Inc

Your Turn! Which of the following gases is the most dense? a. H 2 b. N 2 c. CO 2 d. O 2 Carbon dioxide fire extinguishers can be used to put out fires because CO 2 is more dense than air and can be used to push oxygen away from the fuel source. Copyright 2012 John Wiley & Sons, Inc

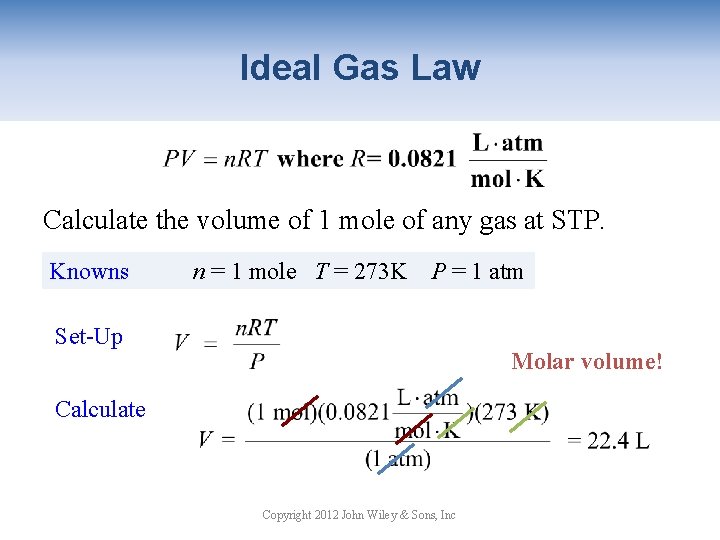
Ideal Gas Law Calculate the volume of 1 mole of any gas at STP. Knowns n = 1 mole T = 273 K P = 1 atm Set-Up Molar volume! Calculate Copyright 2012 John Wiley & Sons, Inc

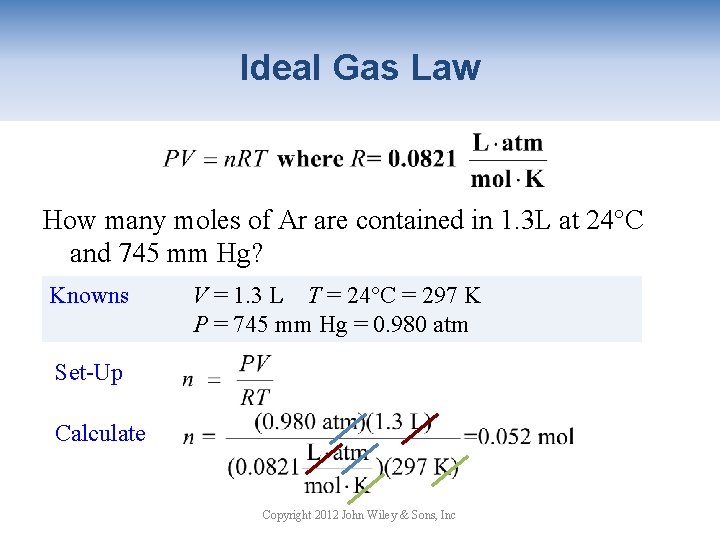
Ideal Gas Law How many moles of Ar are contained in 1. 3 L at 24°C and 745 mm Hg? Knowns V = 1. 3 L T = 24°C = 297 K P = 745 mm Hg = 0. 980 atm Set-Up Calculate Copyright 2012 John Wiley & Sons, Inc

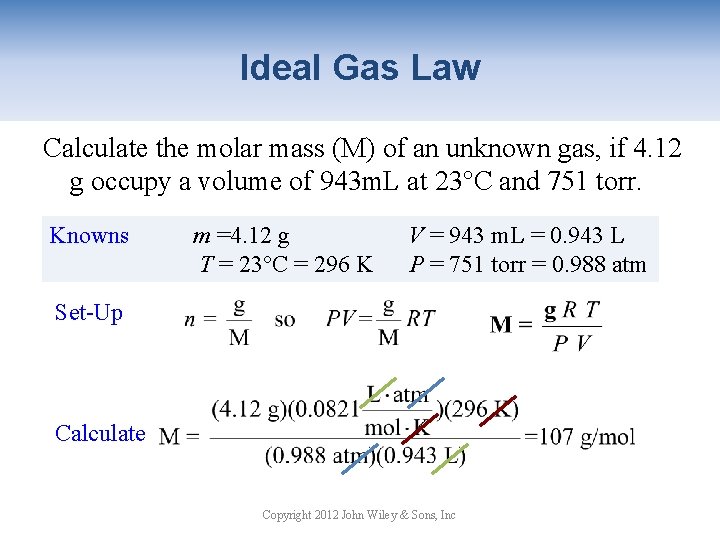
Ideal Gas Law Calculate the molar mass (M) of an unknown gas, if 4. 12 g occupy a volume of 943 m. L at 23°C and 751 torr. Knowns m =4. 12 g T = 23°C = 296 K V = 943 m. L = 0. 943 L P = 751 torr = 0. 988 atm Set-Up Calculate Copyright 2012 John Wiley & Sons, Inc

Your Turn! What is the molar mass of a gas if 40. 0 L of the gas has a mass of 36. 0 g at 740. torr and 30. 0 ° C? a. 33. 1 g b. 23. 0 g c. 56. 0 g d. 333 g Copyright 2012 John Wiley & Sons, Inc

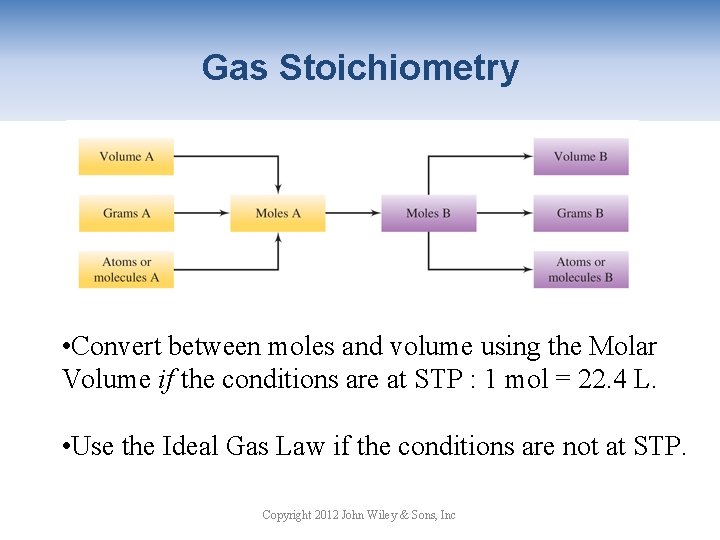
Gas Stoichiometry • Convert between moles and volume using the Molar Volume if the conditions are at STP : 1 mol = 22. 4 L. • Use the Ideal Gas Law if the conditions are not at STP. Copyright 2012 John Wiley & Sons, Inc

Gas Stoichiometry Calculate the number of moles of phosphorus needed to react with 4. 0 L of hydrogen gas at 273 K and 1 atm. P 4(s) + 6 H 2(g) 4 PH 3(g) Knowns Solution Map V = 4. 0 L T = 273 K P = 1 atm L H 2 mol P 4 Calculate Copyright 2012 John Wiley & Sons, Inc

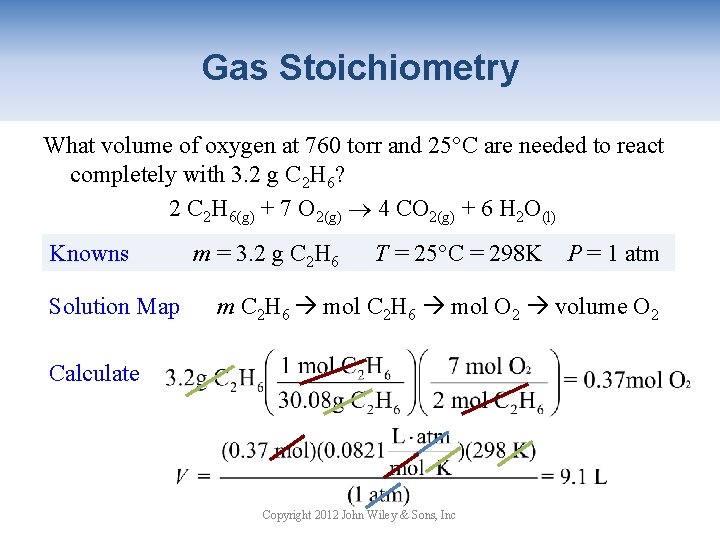
Gas Stoichiometry What volume of oxygen at 760 torr and 25°C are needed to react completely with 3. 2 g C 2 H 6? 2 C 2 H 6(g) + 7 O 2(g) 4 CO 2(g) + 6 H 2 O(l) Knowns Solution Map m = 3. 2 g C 2 H 6 T = 25°C = 298 K P = 1 atm m C 2 H 6 mol O 2 volume O 2 Calculate Copyright 2012 John Wiley & Sons, Inc

Your Turn! How many moles of oxygen gas are used up during the reaction with 18. 0 L of CH 4 gas measured at STP? CH 4(g) + 2 O 2(g) CO 2(g) + 2 H 2 O(l) a. 1. 61 moles b. 2. 49 moles c. 18. 0 moles d. 36. 0 moles Copyright 2012 John Wiley & Sons, Inc

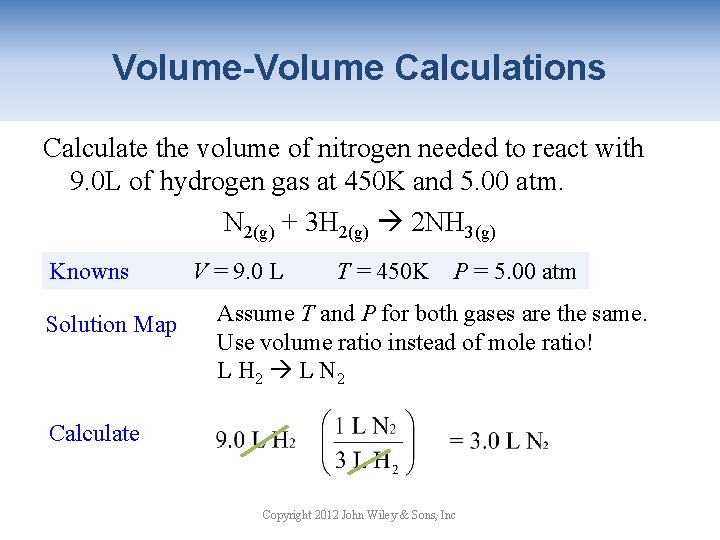
Volume-Volume Calculations Calculate the volume of nitrogen needed to react with 9. 0 L of hydrogen gas at 450 K and 5. 00 atm. N 2(g) + 3 H 2(g) 2 NH 3(g) Knowns Solution Map V = 9. 0 L T = 450 K P = 5. 00 atm Assume T and P for both gases are the same. Use volume ratio instead of mole ratio! L H 2 L N 2 Calculate Copyright 2012 John Wiley & Sons, Inc

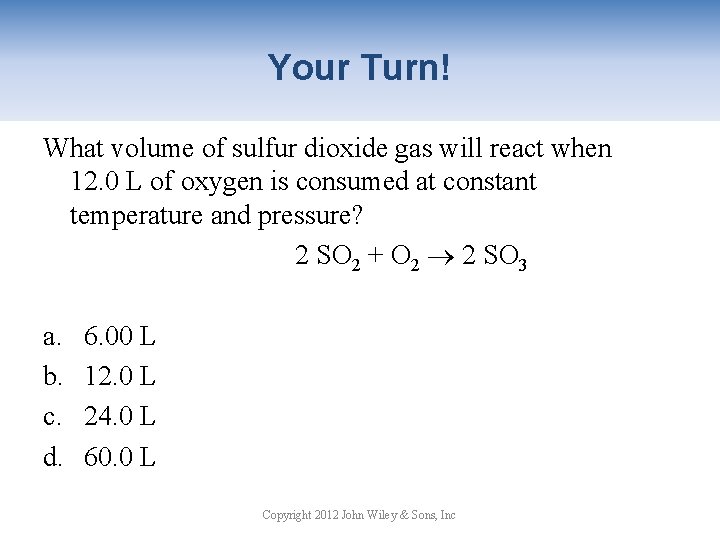
Your Turn! What volume of sulfur dioxide gas will react when 12. 0 L of oxygen is consumed at constant temperature and pressure? 2 SO 2 + O 2 2 SO 3 a. 6. 00 L b. 12. 0 L c. 24. 0 L d. 60. 0 L Copyright 2012 John Wiley & Sons, Inc

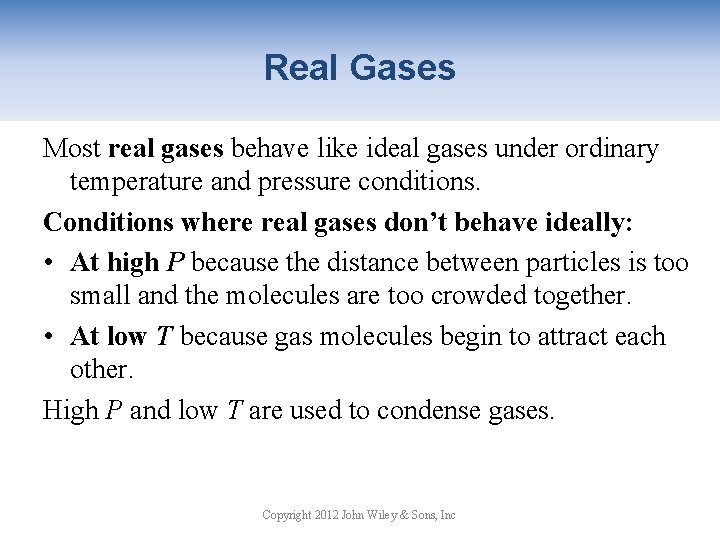
Real Gases Most real gases behave like ideal gases under ordinary temperature and pressure conditions. Conditions where real gases don’t behave ideally: • At high P because the distance between particles is too small and the molecules are too crowded together. • At low T because gas molecules begin to attract each other. High P and low T are used to condense gases. Copyright 2012 John Wiley & Sons, Inc
 Gaseous state chapter
Gaseous state chapter Chapter 2 section 1 classifying matter answers
Chapter 2 section 1 classifying matter answers My very excited mother just
My very excited mother just Inorganic gaseous pollutants of air
Inorganic gaseous pollutants of air Gaseous exchange in protozoa
Gaseous exchange in protozoa Inorganic gases
Inorganic gases Skin gas exchange
Skin gas exchange Contraction porosity denture
Contraction porosity denture Gaseous exchange in animals
Gaseous exchange in animals Gaseous dosage forms
Gaseous dosage forms Gaseous equilibrium
Gaseous equilibrium A thin gaseous layer that envelopes the earth
A thin gaseous layer that envelopes the earth Gaseous exchange practical grade 11
Gaseous exchange practical grade 11 The earthworm
The earthworm Gaseous solution
Gaseous solution Khbo2
Khbo2 What is dosage form
What is dosage form At 500 k one mole of gaseous oncl
At 500 k one mole of gaseous oncl Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter White matter
White matter Composition of matter section 1
Composition of matter section 1 Label the cranial dura septa and associated sinuses.
Label the cranial dura septa and associated sinuses. Composition of matter section 1
Composition of matter section 1 Gray matter and white matter
Gray matter and white matter Ncl. caudatus
Ncl. caudatus Energy naturally flows from warmer matter to cooler matter
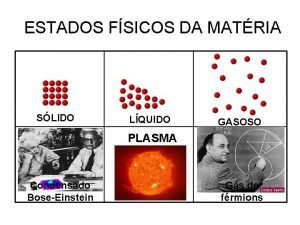
Energy naturally flows from warmer matter to cooler matter Plasma and bose-einstein condensate
Plasma and bose-einstein condensate Do
Do States of matter thermal energy
States of matter thermal energy Sixth state of matter
Sixth state of matter Which is a gas
Which is a gas Pepsi and mentos
Pepsi and mentos Physical state of matter
Physical state of matter Change in state of matter
Change in state of matter Vaporization matter
Vaporization matter Diagram states of matter
Diagram states of matter Colloidal suspension meaning
Colloidal suspension meaning State of matter in chemical equations
State of matter in chemical equations Anisotropy
Anisotropy Compressible state of matter
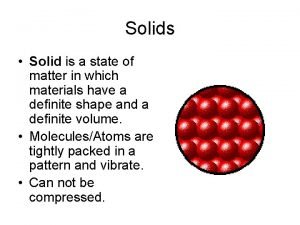
Compressible state of matter State of matter
State of matter State of matter
State of matter 6th state of matter
6th state of matter State of matter particle diagram
State of matter particle diagram Concept map of matter solid liquid and gas
Concept map of matter solid liquid and gas State to state regionalism
State to state regionalism Present state next state table
Present state next state table Software implementation of state graph
Software implementation of state graph T and r state of hemoglobin
T and r state of hemoglobin Orbit orbital shell subshell
Orbit orbital shell subshell R state t state hemoglobin
R state t state hemoglobin Absorptive state and postabsorptive state
Absorptive state and postabsorptive state