1 Chapter 4 Compounds Safer Materials for a




























- Slides: 57

1 Chapter 4 Compounds: Safer Materials for a Safer World Green Chemistry and the Ten Commandments of Sustainability, 3 rd Edition Stanley E. Manahan Chem. Char 2011 For questions, contact: Stanley E. Manahan manahans@missouri. edu

2 4. 1. Chemical Bonds and Compound Formation Chemical compounds consist of molecules or aggregates of ions consisting of two or more elements held together by chemical bonds • H 2 O • NH 3 • Na. Cl Bonds holding chemical compounds together • Covalent bonds composed of shared electrons • Ionic bonds consisting of positively charged cations and negatively charged anions

Importance of Bond Strengths 3 • Chlorofluorocarbons, such as Cl 2 CF 2, have very strong C-Cl bonds and C-F bonds and persist into the stratosphere where they cause ozone destruction • These are not green chemicals because the practice of green chemistry requires that substances that get released to the environment break down readily • Bonds that break very readily are characteristic of reactive compounds, such as explosives, that may be hazardous

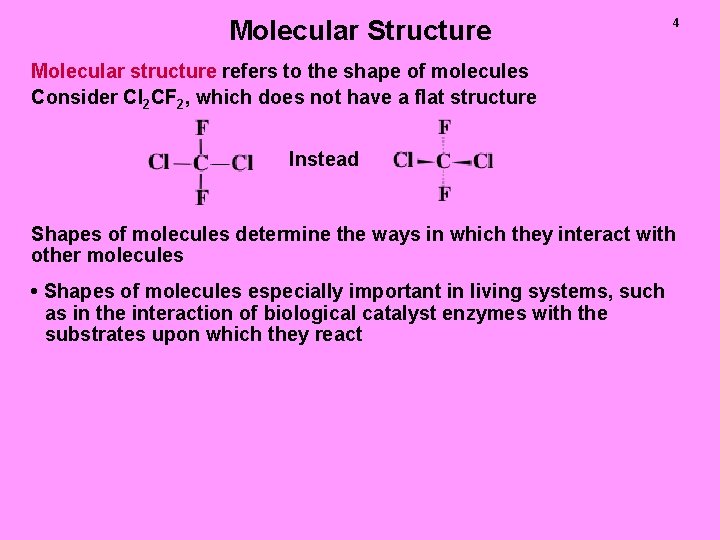
Molecular Structure 4 Molecular structure refers to the shape of molecules Consider Cl 2 CF 2, which does not have a flat structure Instead Shapes of molecules determine the ways in which they interact with other molecules • Shapes of molecules especially important in living systems, such as in the interaction of biological catalyst enzymes with the substrates upon which they react

What Are Green Chemical Compounds? 5 Dichlorodifluoromethane, Cl 2 CF 2, a chlorofluorocarbon (Freon) compound, would not be regarded as a green material because it is so stable and persistent in the atmosphere and causes stratospheric ozone destruction • Green replacement hydrofluorocarbons and hydrochlorofluorocarbons are much more green because they do not last long in the atmosphere and the hydrofluorocarbons do not contain ozonedestroying chlorine

Characteristics of Green Compounds 6 • Preparation from renewable or readily available resources by environmentally friendly processes • Low tendency to undergo sudden, violent, unpredictable reactions, such as explosions that may cause damage, injure personnel, or cause release of chemicals and byproducts to the environment • Nonflammable or poorly flammable • Low toxicity • Absence of toxic or environmentally dangerous constituents, particularly heavy metals • Facile degradability, especially biodegradability, in the environment • Low tendency to undergo bioaccumulation in environmental food chains

Sodium Stearate, Hand Soap, is Green 7 • Prepared by reacting byproduct animal fat with sodium hydroxide, which is prepared by passing an electrical current through saltwater • Flushed down the drain, sodium stearate reacts with calcium in water to form solid calcium stearate that biodegrades readily

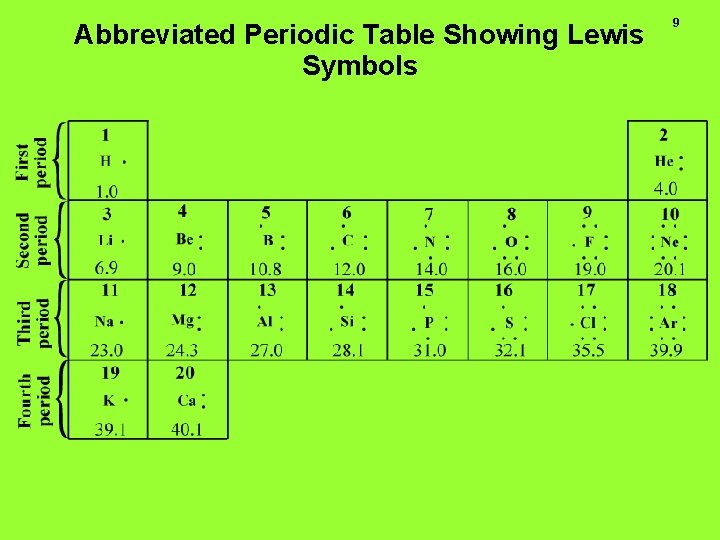
8 4. 2. Electrons Involved in Chemical Bonds and Octets of Electrons Valence electrons are the ones in the outermost shell of atoms that can become involved in chemical bonds Refer to the Lewis symbols in the periodic table (next slide)

Abbreviated Periodic Table Showing Lewis Symbols 9

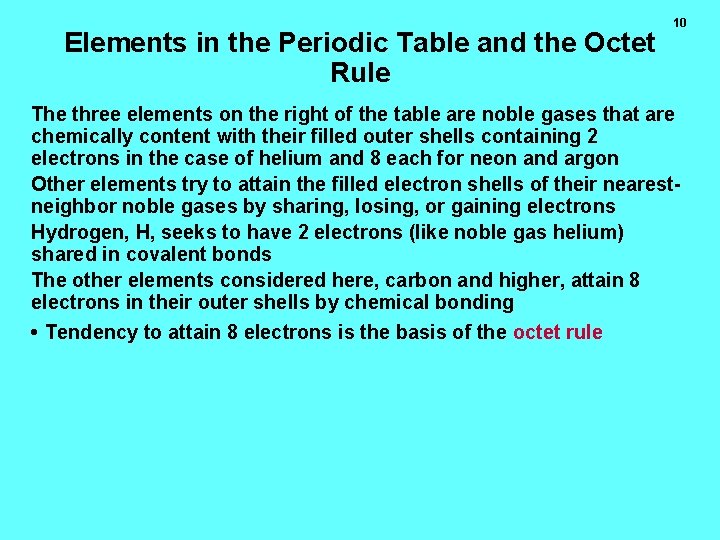
Elements in the Periodic Table and the Octet Rule 10 The three elements on the right of the table are noble gases that are chemically content with their filled outer shells containing 2 electrons in the case of helium and 8 each for neon and argon Other elements try to attain the filled electron shells of their nearestneighbor noble gases by sharing, losing, or gaining electrons Hydrogen, H, seeks to have 2 electrons (like noble gas helium) shared in covalent bonds The other elements considered here, carbon and higher, attain 8 electrons in their outer shells by chemical bonding • Tendency to attain 8 electrons is the basis of the octet rule

Examples of the Octet Rule 11

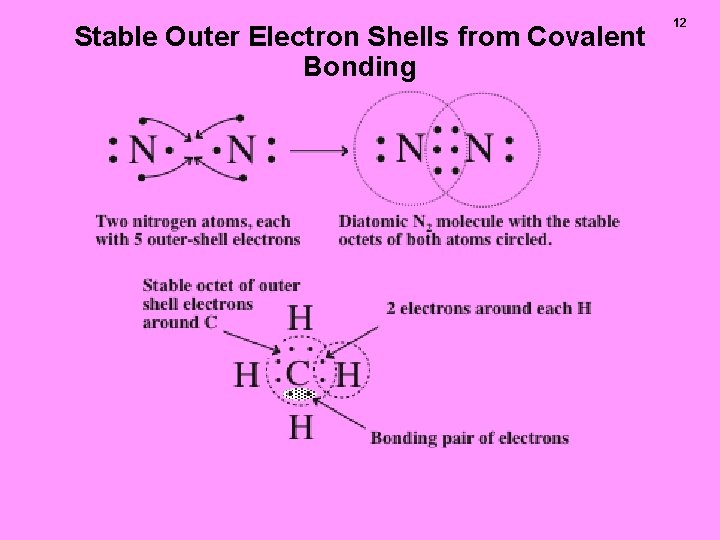
Stable Outer Electron Shells from Covalent Bonding 12

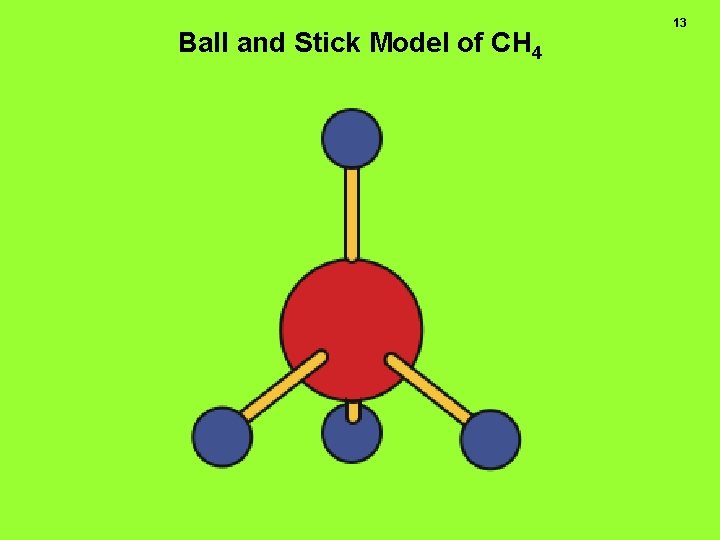
Ball and Stick Model of CH 4 13

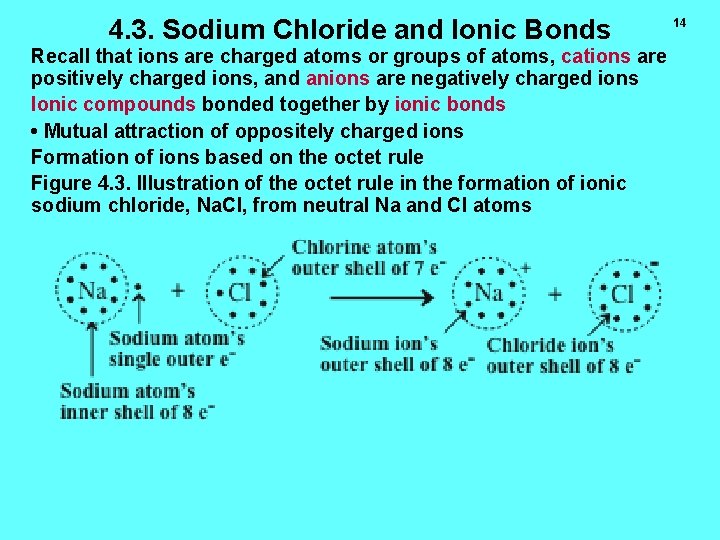
4. 3. Sodium Chloride and Ionic Bonds Recall that ions are charged atoms or groups of atoms, cations are positively charged ions, and anions are negatively charged ions Ionic compounds bonded together by ionic bonds • Mutual attraction of oppositely charged ions Formation of ions based on the octet rule Figure 4. 3. Illustration of the octet rule in the formation of ionic sodium chloride, Na. Cl, from neutral Na and Cl atoms 14

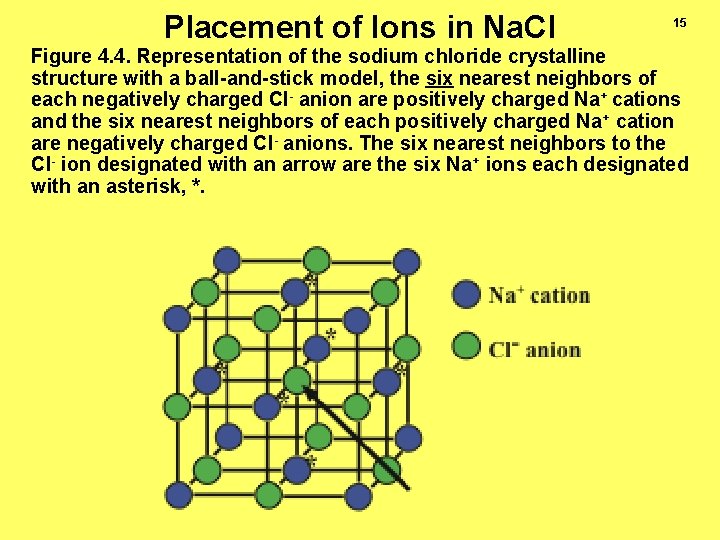
Placement of Ions in Na. Cl 15 Figure 4. 4. Representation of the sodium chloride crystalline structure with a ball-and-stick model, the six nearest neighbors of each negatively charged Cl- anion are positively charged Na+ cations and the six nearest neighbors of each positively charged Na+ cation are negatively charged Cl- anions. The six nearest neighbors to the Cl- ion designated with an arrow are the six Na+ ions each designated with an asterisk, *.

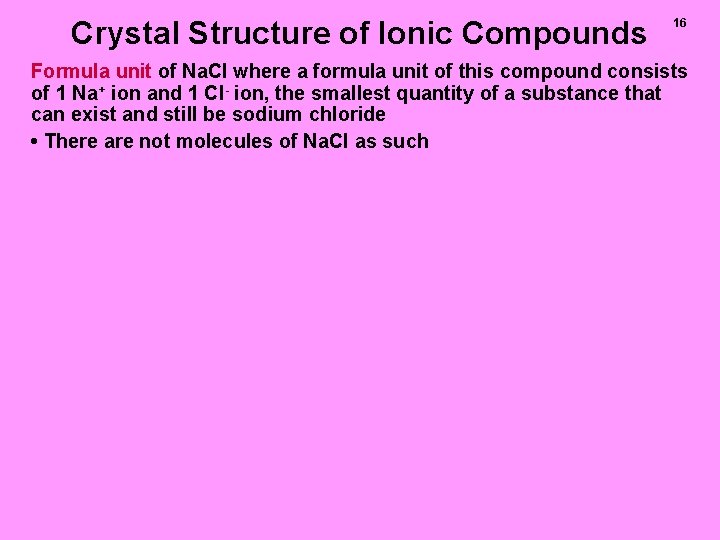
Crystal Structure of Ionic Compounds 16 Formula unit of Na. Cl where a formula unit of this compound consists of 1 Na+ ion and 1 Cl- ion, the smallest quantity of a substance that can exist and still be sodium chloride • There are not molecules of Na. Cl as such

Reaction to Form Na. Cl 17 2 Na(solid) + Cl 2(gas) 2 Na. Cl(solid) This reaction can be broken down into the following steps: 1. The atoms in solid Na are taken apart, which requires energy 2. Each molecule of Cl 2 is split into Cl atoms, which requires energy 3. An electron is taken from each Na atom to produce Na+ ion, which requires energy 4. An electron is added to each Cl atom to produce a Cl- ion, which releases energy 5. All the Na+ cations and 1 Cl- anion are assembled in a 1/1 ratio in a crystal lattice to produce Na. Cl, which releases a very large quantity of lattice energy

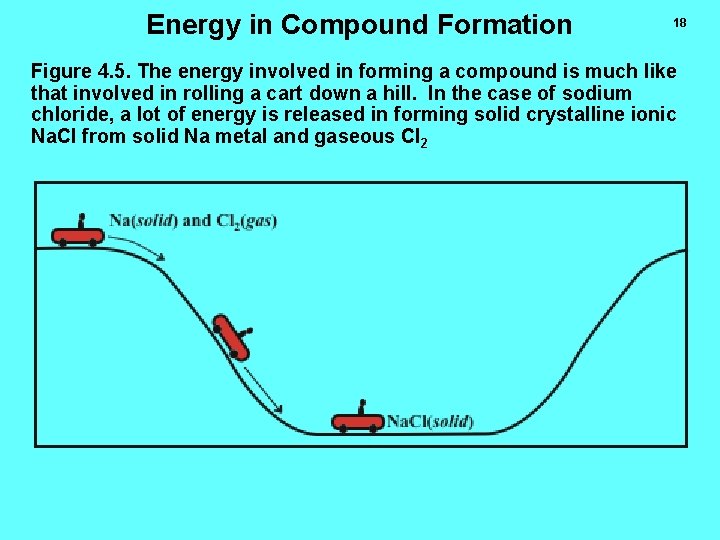
Energy in Compound Formation 18 Figure 4. 5. The energy involved in forming a compound is much like that involved in rolling a cart down a hill. In the case of sodium chloride, a lot of energy is released in forming solid crystalline ionic Na. Cl from solid Na metal and gaseous Cl 2

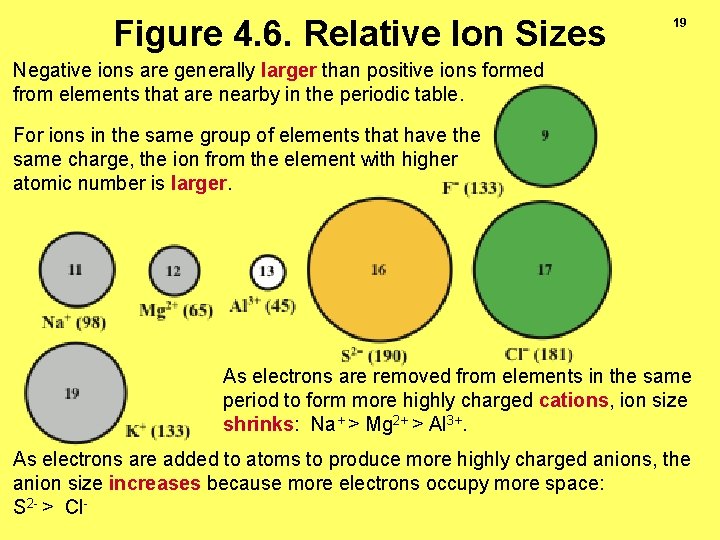
Figure 4. 6. Relative Ion Sizes 19 Negative ions are generally larger than positive ions formed from elements that are nearby in the periodic table. For ions in the same group of elements that have the same charge, the ion from the element with higher atomic number is larger. As electrons are removed from elements in the same period to form more highly charged cations, ion size shrinks: Na+ > Mg 2+ > Al 3+. As electrons are added to atoms to produce more highly charged anions, the anion size increases because more electrons occupy more space: S 2 - > Cl-

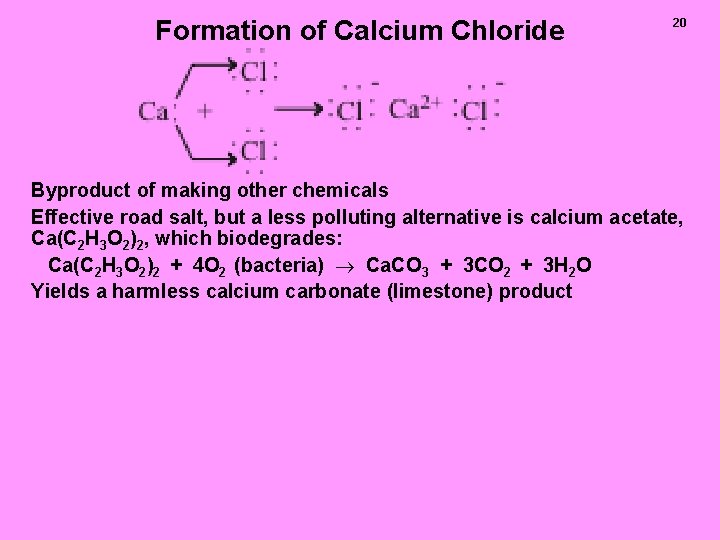
Formation of Calcium Chloride 20 Byproduct of making other chemicals Effective road salt, but a less polluting alternative is calcium acetate, Ca(C 2 H 3 O 2)2, which biodegrades: Ca(C 2 H 3 O 2)2 + 4 O 2 (bacteria) Ca. CO 3 + 3 CO 2 + 3 H 2 O Yields a harmless calcium carbonate (limestone) product

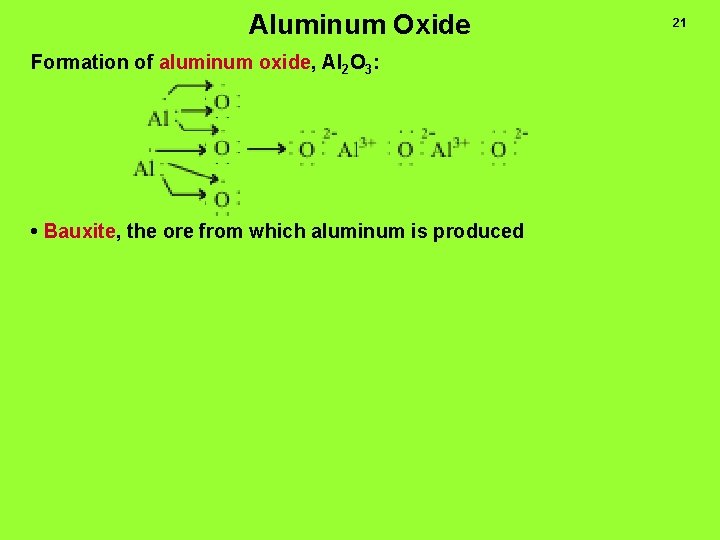
Aluminum Oxide Formation of aluminum oxide, Al 2 O 3: • Bauxite, the ore from which aluminum is produced 21

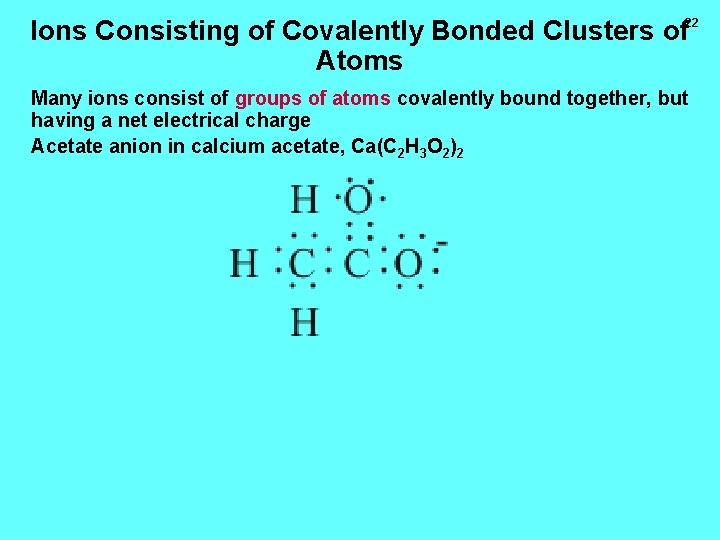
Ions Consisting of Covalently Bonded Clusters of Atoms 22 Many ions consist of groups of atoms covalently bound together, but having a net electrical charge Acetate anion in calcium acetate, Ca(C 2 H 3 O 2)2

Ionic Liquids and Green Chemistry Composed of large ions Unlike most ionic compounds, which are solids, ionic liquids are liquids under normal conditions May substitute for organic solvents in some applications 23

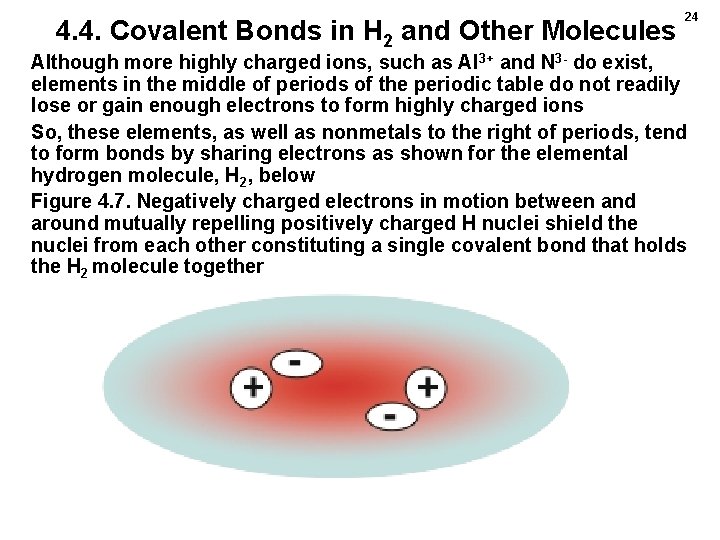
4. 4. Covalent Bonds in H 2 and Other Molecules 24 Although more highly charged ions, such as Al 3+ and N 3 - do exist, elements in the middle of periods of the periodic table do not readily lose or gain enough electrons to form highly charged ions So, these elements, as well as nonmetals to the right of periods, tend to form bonds by sharing electrons as shown for the elemental hydrogen molecule, H 2, below Figure 4. 7. Negatively charged electrons in motion between and around mutually repelling positively charged H nuclei shield the nuclei from each other constituting a single covalent bond that holds the H 2 molecule together

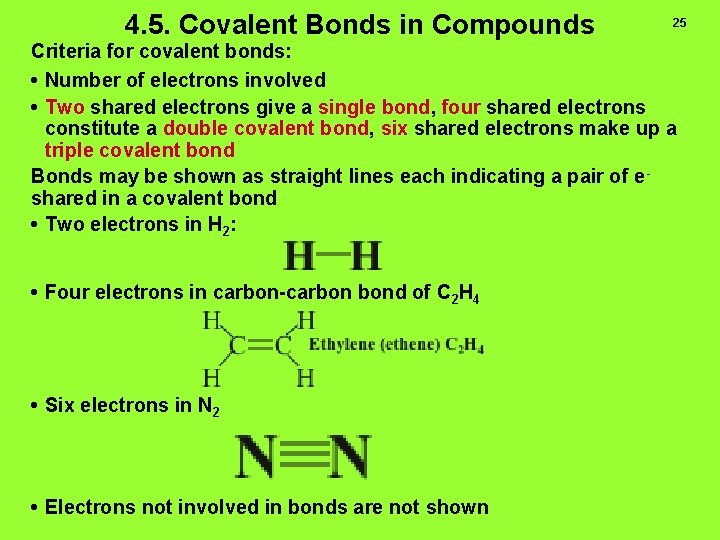
4. 5. Covalent Bonds in Compounds 25 Criteria for covalent bonds: • Number of electrons involved • Two shared electrons give a single bond, four shared electrons constitute a double covalent bond, six shared electrons make up a triple covalent bond Bonds may be shown as straight lines each indicating a pair of e shared in a covalent bond • Two electrons in H 2: • Four electrons in carbon-carbon bond of C 2 H 4 • Six electrons in N 2 • Electrons not involved in bonds are not shown

Covalent Bond Length Covalent bonds have a characteristic bond length • About the same lengths as sizes of atoms • Expressed in picometers (pm) • The H-H bond in H 2 is 75 pm long 26

Bond Energy 27 A third important characteristic of covalent bonds is bond energy • Expressed in kilojoules (k. J) required to break a mole (6. 02 x 10 23) of bonds • The bond energy of the H-H bond in H 2 is equal to 435 k. J/mole • Therefore, 435 k. J of energy is required to break all the H-H bonds in a mole of H 2 (2. 0 g, 6. 02 x 1023 molecules)

4. 6. Covalent Bonds and Green Chemistry 28 • High-energy bonds in raw materials require a lot of energy and severe conditions, such as those of temperature, pressure, and radiation, to take apart in synthesizing chemicals. The practice of green chemistry tries to avoid such conditions. • Especially stable bonds may make substances unduly persistent in the environment • Relatively weak bonds may allow molecules to come apart too readily, and compounds with such bonds are often reactive species in the atmosphere or in biological systems • Unstable bonds or arrangements of bonds may lead to excessive reactivity in chemicals making them prone to explosions and other hazards • Some arrangements of bonds contribute to chemical toxicity.

N 2 as an Energy-Intensive Raw Material 29 The high bond stability of N 2 makes it an energy-intensive source of raw material • Large amounts of energy and severe conditions are required to take the N 2 molecule apart in the synthesis of ammonia, NH 3, the compound that is the basis for the synthesis of most synthetic nitrogen compounds • Rhizobium bacteria on the roots of leguminous plants produce some chemically combined nitrogen from elemental N 2.

Strong Bonds in Chlorofluorocarbons and Ozone Depletion Dichlorodifluoromethane, Cl 2 CF 2, implicated in stratospheric ozone depletion • Especially stable bonds contribute to persistence and ultimate environmental harm • Chlorofluorocarbons penetrate to the stratosphere before contacting electromagnetic radiation energetic enough to break the molecules of these compounds apart • Cl atoms split off from chlorofluorocarbons in the stratosphere attack ozone resulting in destruction of protective stratospheric ozone 30

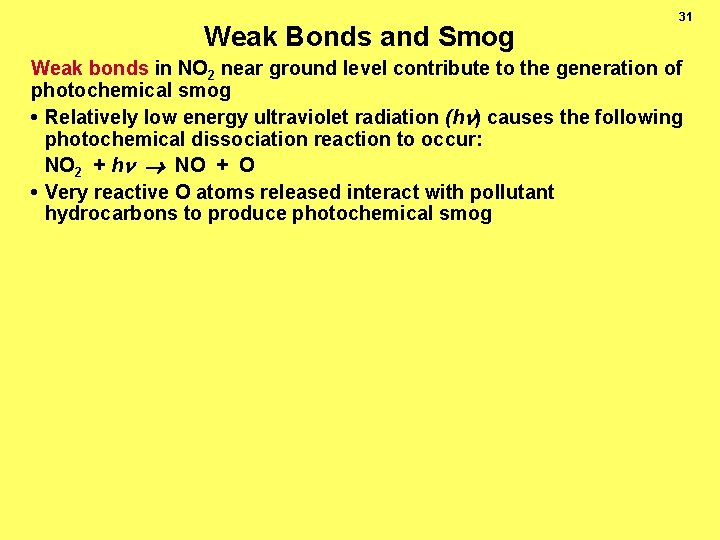
Weak Bonds and Smog 31 Weak bonds in NO 2 near ground level contribute to the generation of photochemical smog • Relatively low energy ultraviolet radiation (h ) causes the following photochemical dissociation reaction to occur: NO 2 + h NO + O • Very reactive O atoms released interact with pollutant hydrocarbons to produce photochemical smog

Instability and Toxicity Some bonding arrangements are noted for instability • Bonds in which two N atoms are adjacent or very close in a molecule and are bonded with double bonds • Arrangements in which N and O atoms are adjacent and double bonds are present The presence of some kinds of bonds in molecules can contribute to their biochemical reactivity and, therefore, to their toxicity • An organic compound with one or more C=C double bonds in the molecule is often more toxic than a similar molecule without such bonds 32

Bonds and Green Chemistry 33 Green chemistry seeks to avoid generation, use, or release to the environment of compounds with the kinds of bonds likely to cause problems Green chemistry seeks to avoid having to protect bonding groups by attaching protective chemical groups • Protecting groups consume chemicals • Protecting groups generate byproducts

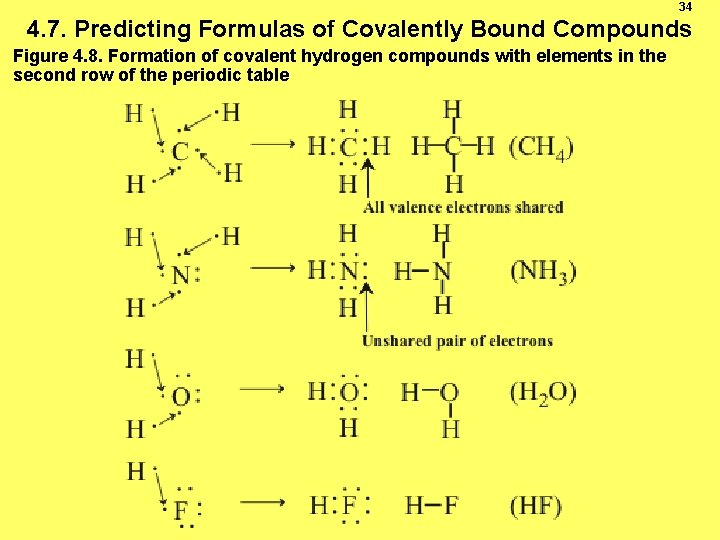
34 4. 7. Predicting Formulas of Covalently Bound Compounds Figure 4. 8. Formation of covalent hydrogen compounds with elements in the second row of the periodic table

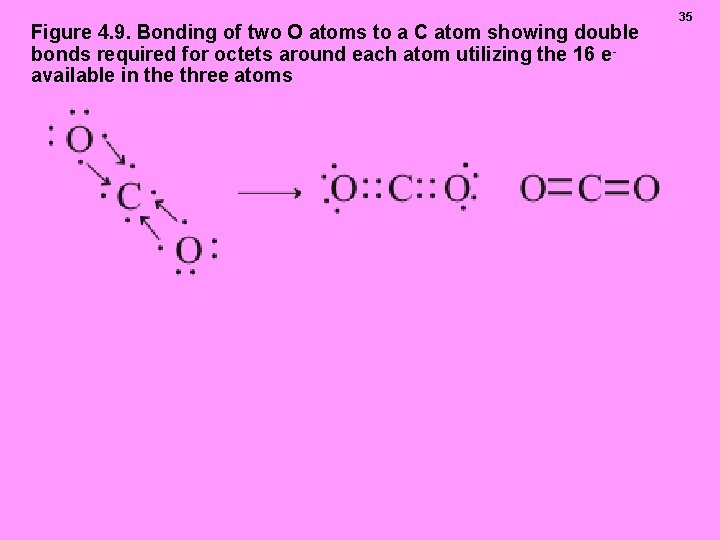
Figure 4. 9. Bonding of two O atoms to a C atom showing double bonds required for octets around each atom utilizing the 16 e available in the three atoms 35

36 Hydrogen Peroxide, a Reactive Green Compound Hydrogen peroxide: Hydrogen peroxide is unstable and decomposes to release oxygen: 2 H 2 O 2(liquid) O 2(gas) + H 2 O(liquid) Dilute hydrogen peroxide makes an effective and safe bleaching agent • Much safer to handle than elemental chlorine • Does not generate the potentially toxic byproducts that chlorine produces Can be pumped underground to serve as an oxidant for acclimated bacteria to use in attacking wastes

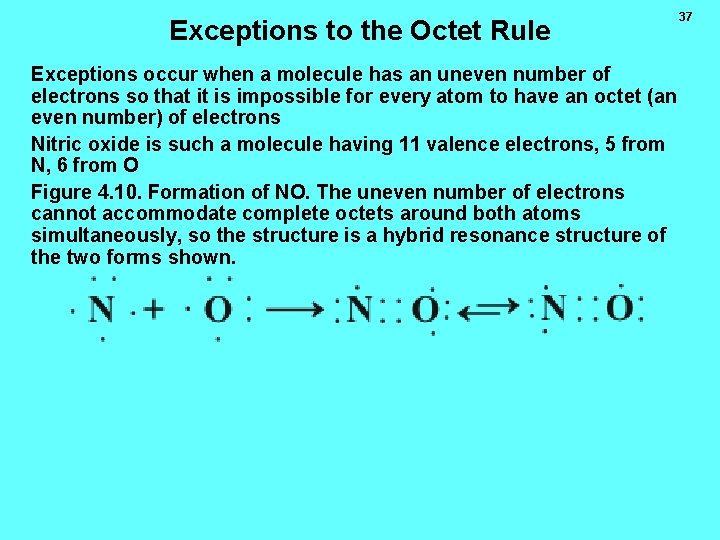
Exceptions to the Octet Rule Exceptions occur when a molecule has an uneven number of electrons so that it is impossible for every atom to have an octet (an even number) of electrons Nitric oxide is such a molecule having 11 valence electrons, 5 from N, 6 from O Figure 4. 10. Formation of NO. The uneven number of electrons cannot accommodate complete octets around both atoms simultaneously, so the structure is a hybrid resonance structure of the two forms shown. 37

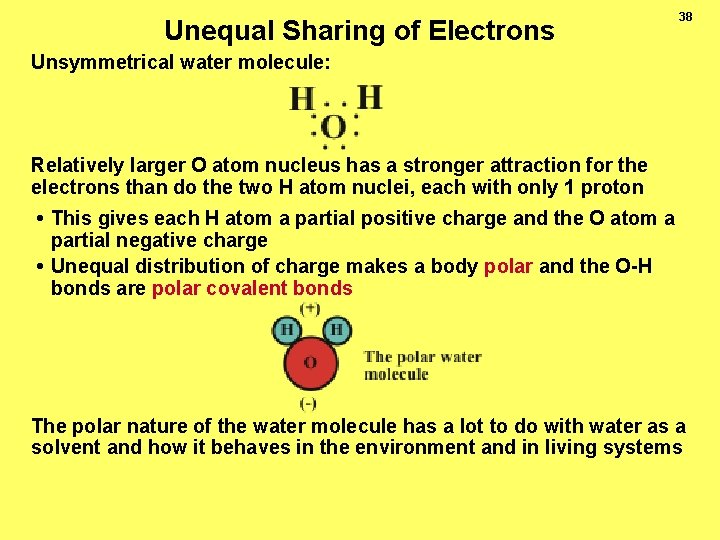
Unequal Sharing of Electrons 38 Unsymmetrical water molecule: Relatively larger O atom nucleus has a stronger attraction for the electrons than do the two H atom nuclei, each with only 1 proton • This gives each H atom a partial positive charge and the O atom a partial negative charge • Unequal distribution of charge makes a body polar and the O-H bonds are polar covalent bonds The polar nature of the water molecule has a lot to do with water as a solvent and how it behaves in the environment and in living systems

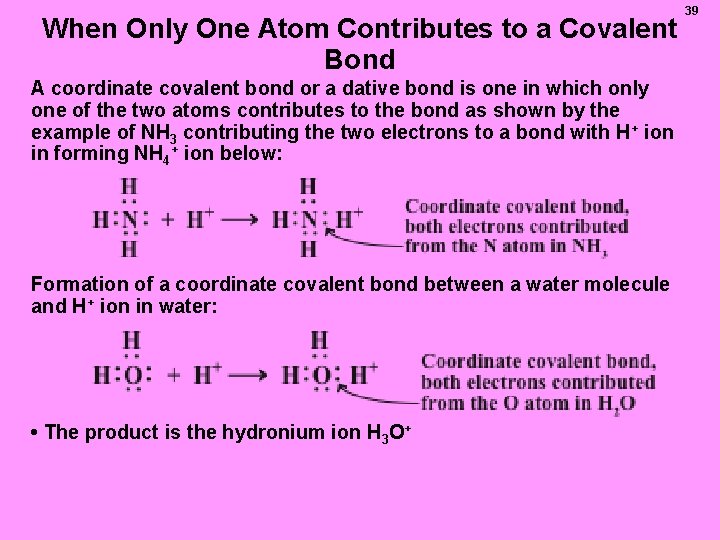
When Only One Atom Contributes to a Covalent Bond A coordinate covalent bond or a dative bond is one in which only one of the two atoms contributes to the bond as shown by the example of NH 3 contributing the two electrons to a bond with H+ ion in forming NH 4+ ion below: Formation of a coordinate covalent bond between a water molecule and H+ ion in water: • The product is the hydronium ion H 3 O+ 39

4. 8. Chemical Formulas, the Mole, and Percentage Composition 40 Chemical formulas are the words of chemical language and include • The elements that compose the compound • The relative numbers of each kind of atom in the compound • How the atoms are grouped, such as in ions (for example, SO 42 -) present in the compound • With a knowledge of atomic masses, the molar mass of the compound can be calculated • With a knowledge of atomic masses, the percentage composition of the compound can be calculated

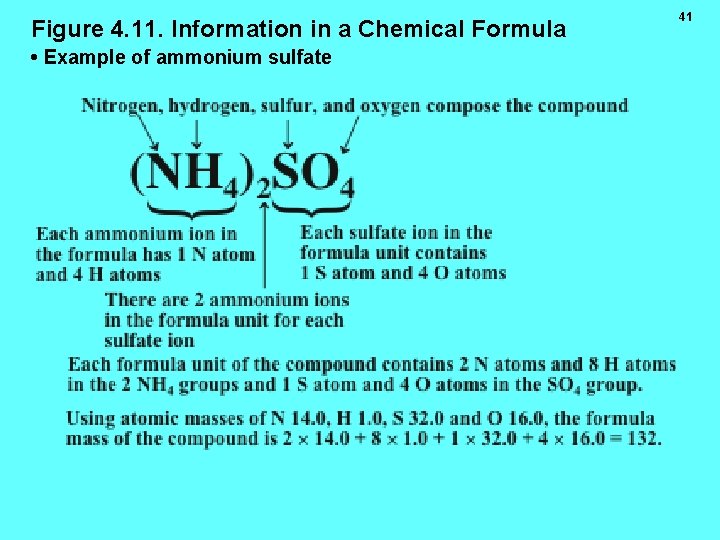
Figure 4. 11. Information in a Chemical Formula • Example of ammonium sulfate 41

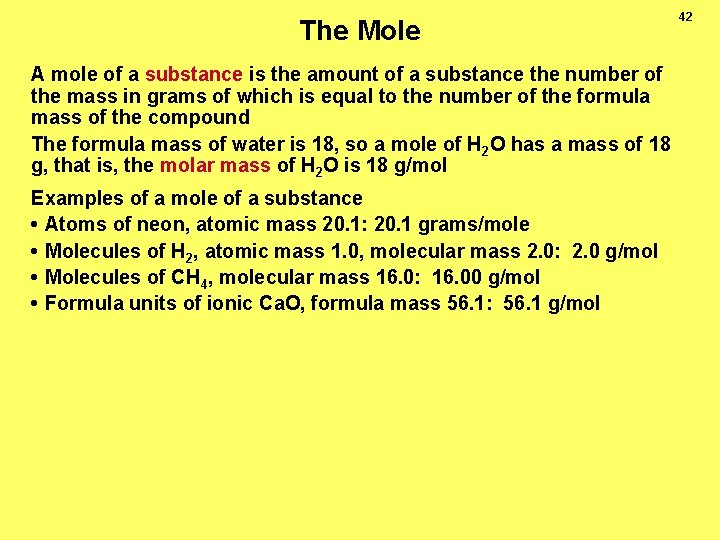
The Mole A mole of a substance is the amount of a substance the number of the mass in grams of which is equal to the number of the formula mass of the compound The formula mass of water is 18, so a mole of H 2 O has a mass of 18 g, that is, the molar mass of H 2 O is 18 g/mol Examples of a mole of a substance • Atoms of neon, atomic mass 20. 1: 20. 1 grams/mole • Molecules of H 2, atomic mass 1. 0, molecular mass 2. 0: 2. 0 g/mol • Molecules of CH 4, molecular mass 16. 0: 16. 00 g/mol • Formula units of ionic Ca. O, formula mass 56. 1: 56. 1 g/mol 42

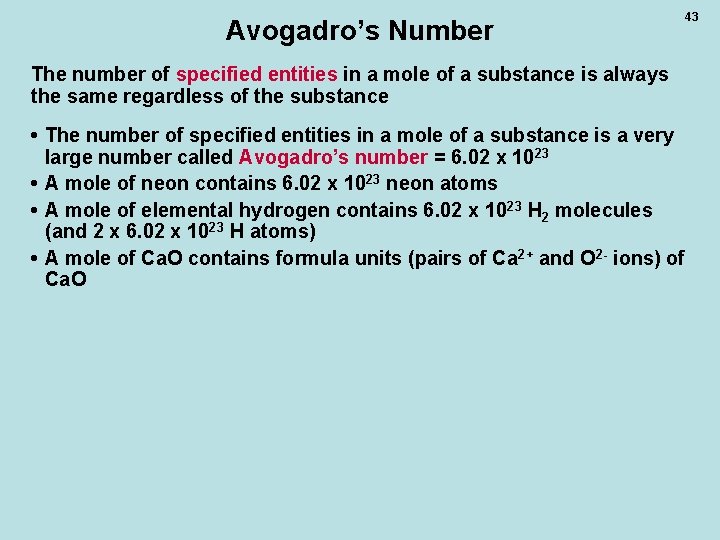
Avogadro’s Number The number of specified entities in a mole of a substance is always the same regardless of the substance • The number of specified entities in a mole of a substance is a very large number called Avogadro’s number = 6. 02 x 1023 • A mole of neon contains 6. 02 x 1023 neon atoms • A mole of elemental hydrogen contains 6. 02 x 1023 H 2 molecules (and 2 x 6. 02 x 1023 H atoms) • A mole of Ca. O contains formula units (pairs of Ca 2+ and O 2 - ions) of Ca. O 43

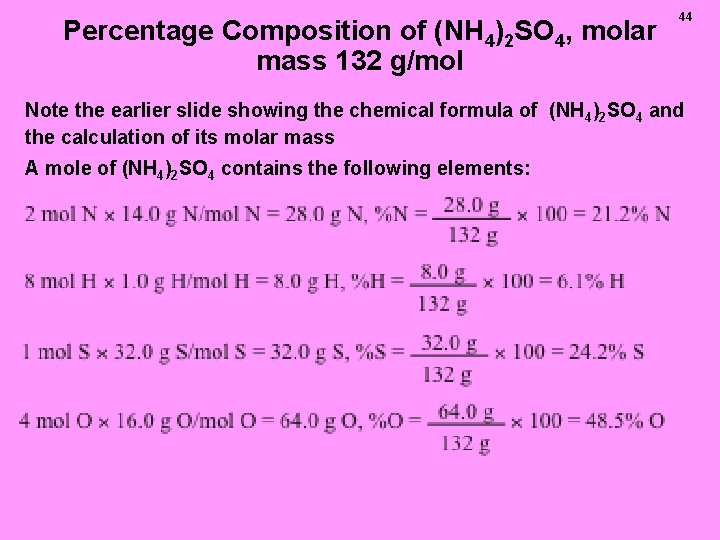
Percentage Composition of (NH 4)2 SO 4, molar mass 132 g/mol 44 Note the earlier slide showing the chemical formula of (NH 4)2 SO 4 and the calculation of its molar mass A mole of (NH 4)2 SO 4 contains the following elements:

45 4. 9. What Are Chemical Compounds called? Prefixes: 1 -mono, 2 -di, 3 -tri, 4 -tetra, 5 -penta, 6 -hexa, 7 -hepta, 8 -octa, 9 -nona, 10 -deca Binary molecular compounds • The first part of the name is that of the first element in the compound formula • The second part of the name is that of the second element in the compound formula modified to have the ending -ide • Prefixes are added to indicate how many of each kind of atoms are present in the molecule, for example, N 2 O 5 is called dinitrogen pentoxide • Si. Cl 4, silicon tetrachloride • Si 2 F 6, disilicon hexafluoride • PCl 5, phosphorus pentachloride • SCl 2, sulfur dichloride

Ionic Compounds Names Ionic compounds are referred to as formula units (rather than molecules) equal to the smallest aggregate of ions that can compose the compound • For example, a formula unit of sodium sulfate consists of 2 Na + ions and 1 SO 42 - ion composing a unit of Na 2 SO 4 Every ionic compound must be electrically neutral with the same number of positive as negative charges • The requirement for electrical neutrality fixes the formula of an ionic compound, so it is usually not necessary to use prefixes to denote relative numbers of ions in the compound formula • For example, we do not call Na 2 SO 4 disodium monosulfate, but call it simply sodium sulfate 46

Naming Ionic Compounds Exercise: Give the formulas and names of compounds formed from each cation on the left, below, with each anion on the right. 1. NH 4+ (A) Cl 2. Ca 2+ (B) SO 423. Al 3+ (C) PO 43 Answers: 1(A) NH 4 Cl, ammonium chloride 1(B) (NH 4)2 SO 4 ammonium sulfate 1(C) (NH 4)3 PO 4, ammonium phosphate 2(A) Ca. Cl 2, calcium chloride 2(B) Ca. SO 4, calcium sulfate 2(C) Ca 3(PO 4)2, calcium phosphate 3(A) Al. Cl 3, aluminum chloride 3(B) Al 2(SO 4)3, aluminum sulfate 3(B) Al. PO 4 aluminum phosphate 47

Ionic Compounds With More Than One Cation or Anion Prefixes are used in naming ionic compounds where more than 1 cation or more than 1 anion are present in the formula unit • Na 2 HPO 4 in which each formula unit is composed of 2 Na+ ions, 1 H+ ion, and 1 PO 43 - ion is called disodium monohydrogen phosphate • KH 2 PO 4 in which each formula unit is composed of 1 K+ ion, 2 H+ ions, and 1 PO 43 - ion is called monopotassium dihydrogen phosphate 48

4. 10. Acids, Bases, and Salts Other than covalently bound binary compounds, most inorganic compounds can be classified as acids, bases, or salts Acids are characterized by H+ ion in water H+ ion dissolved in water makes the water acidic An acid either contains H+ ion or produces it when it dissolves in water Sulfuric acid, H 2 SO 4, contains 2 H+ ions per molecule 49

Carbon Dioxide As Acid Carbon dioxide produces H+ ion by reacting with water • CO 2 + H 2 O H+ + HCO 3 • Only a small fraction of the CO 2 molecules dissolved in water undergo the above reaction to produce H+ ion, so water solutions of CO 2 are weakly acidic • Carbon dioxide is classified as a weak acid • Rainfall is weakly acidic because of dissolved CO 2 from air 50

Strong Acids such as hydrochloric acid, HCl, are completely dissociated in water to H+ and an anion. • Such acids are strong acids • HCl H+ + Cl- (Reaction to 100% complete dissociation) • HNO 3 H+ + NO 3 - (Complete dissociation) 51

Acids With Different Numbers of Oxygen Atoms • An acid that contains only H and one other element is a “hydro-ic” acid, such as HCl, which is called hydrochloric acid • Different rules apply when an acid contains oxygen • With acids containing oxygen, the one with more oxygen, such as H 2 SO 4, is sulfuric acid, the one with less oxygen, H 2 SO 3, is sulfurous acid • For greater or lesser amounts of oxygen consider the following example: HCl. O 4, perchloric acid HCl. O 3, chloric acid HCl. O 2, chlorous acid HCl. O, hypochlorous acid 52

Uses and Occurrence of Acids 53 Sulfuric acid is the top chemical produced at about 40 million metric tons (40 billion kilograms) annually in the United States • Greatest use is to treat phosphate minerals to produce phosphate crop fertilizers • Other uses include removal of corrosion from steel (steel pickling), detergent synthesis, petroleum refining, lead storage battery manufacture, and alcohol synthesis About 7 -9 metric million tons of nitric acid, HNO 3, are produced in the U. S. each year ranking it 10 th in chemical manufacture Hydrochloric acid ranks about 25 th, with annual production around 3 million metric tons

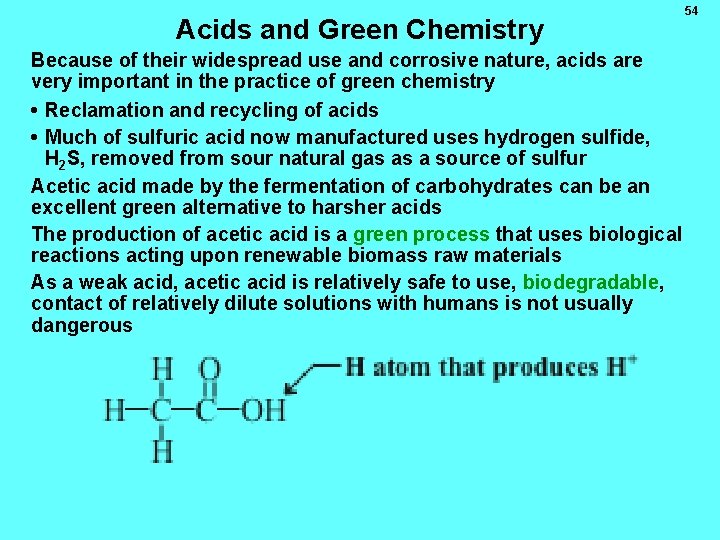
Acids and Green Chemistry Because of their widespread use and corrosive nature, acids are very important in the practice of green chemistry • Reclamation and recycling of acids • Much of sulfuric acid now manufactured uses hydrogen sulfide, H 2 S, removed from sour natural gas as a source of sulfur Acetic acid made by the fermentation of carbohydrates can be an excellent green alternative to harsher acids The production of acetic acid is a green process that uses biological reactions acting upon renewable biomass raw materials As a weak acid, acetic acid is relatively safe to use, biodegradable, contact of relatively dilute solutions with humans is not usually dangerous 54

Bases A base either contains hydroxide ion, OH-, or reacts with water to produce hydroxide Most bases that contain hydroxide are metal hydroxides: Sodium hydroxide, Na. OH, and calcium hydroxide, Ca(OH)2, are examples. The most common basic substance that does not contain, but produces, hydroxide ion in water is ammonia, NH 3: NH 3 + H 2 O NH 4+ + OH- 55

Weak and Strong Bases The reaction of ammonia with H 2 O to produce OH- ion is a reversible reaction that lies far to the left: NH 3 + H 2 O NH 4+ + OHOnly a small fraction of the ammonia reacts • Therefore, ammonia is a weak base The metal hydroxides, such as KOH, that completely dissociate in water are strong bases Metal hydroxides are named by the metal followed by “hydroxide” Mg(OH)2 is a weak base because of low solubility (ingredient of milk of magnesia) 56

Salts An acid and a base react to form a salt, an ionic compound that has a cation other than H+ and an anion other than OH • Always produces water and is known as a neutralization reaction Na. OH + HCl Na. Cl + H 2 O base acid salt (sodium chloride) Some example salts: • Na. Cl, sodium chloride • Ca. Cl 2, calcium chloride, used to melt ice • Na. CO 3, sodium carbonate, used to make glass, treat water, other Salts with only one cation and one anion are named with just the name of the cation followed by the name of the anion Salts with more than one cation or anion are named to denote the number of each: KH 2 PO 4 is potassium dihydrogen phosphate 57