Where Have all the Trials Gone and Ethical





















































































- Slides: 105

Where Have all the Trials Gone? . . . and Ethical Aspects of the Shift Overseas Judy Stone, MD www. conductingclinicalresearch. com © Creative Commons Attribution. Noncommercial-Share Alike 1

ARS Background: What % of Sites are Outside the US? a) 10 % b) 20 % c) 40 % d) 60 % 2

Background: What % of Sites are Outside the US? a) 10 % b) 20 % c) 40 % d) ~60 % 3

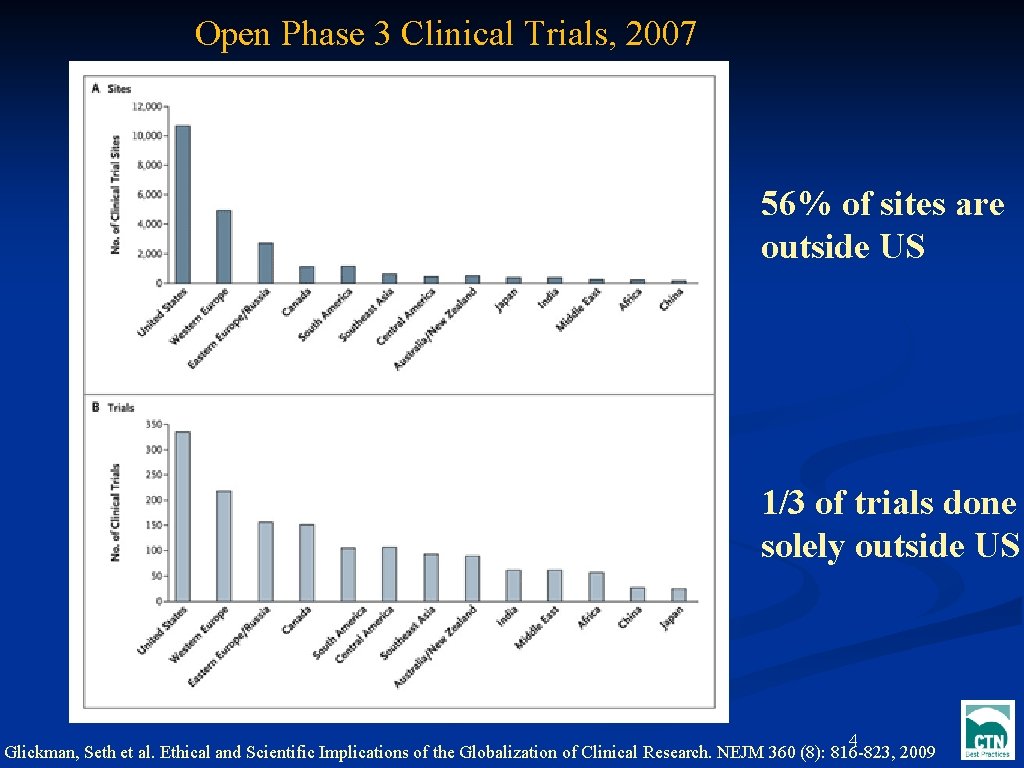
Open Phase 3 Clinical Trials, 2007 56% of sites are outside US Text 1/3 of trials done solely outside US 4 Glickman, Seth et al. Ethical and Scientific Implications of the Globalization of Clinical Research. NEJM 360 (8): 816 -823, 2009

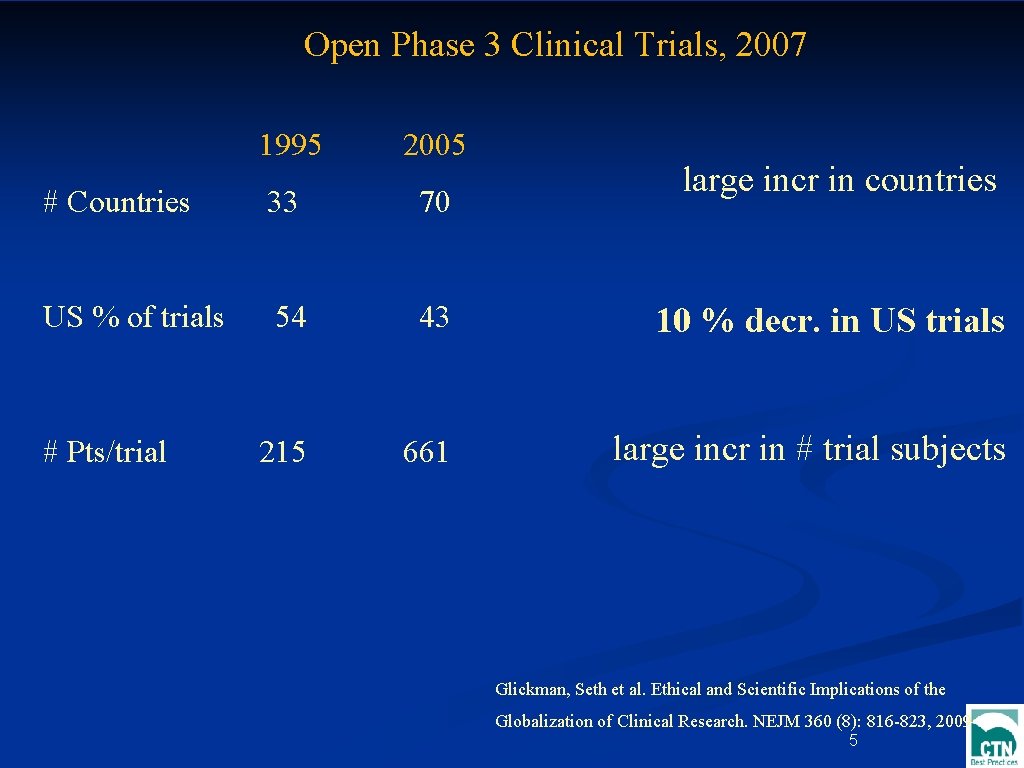
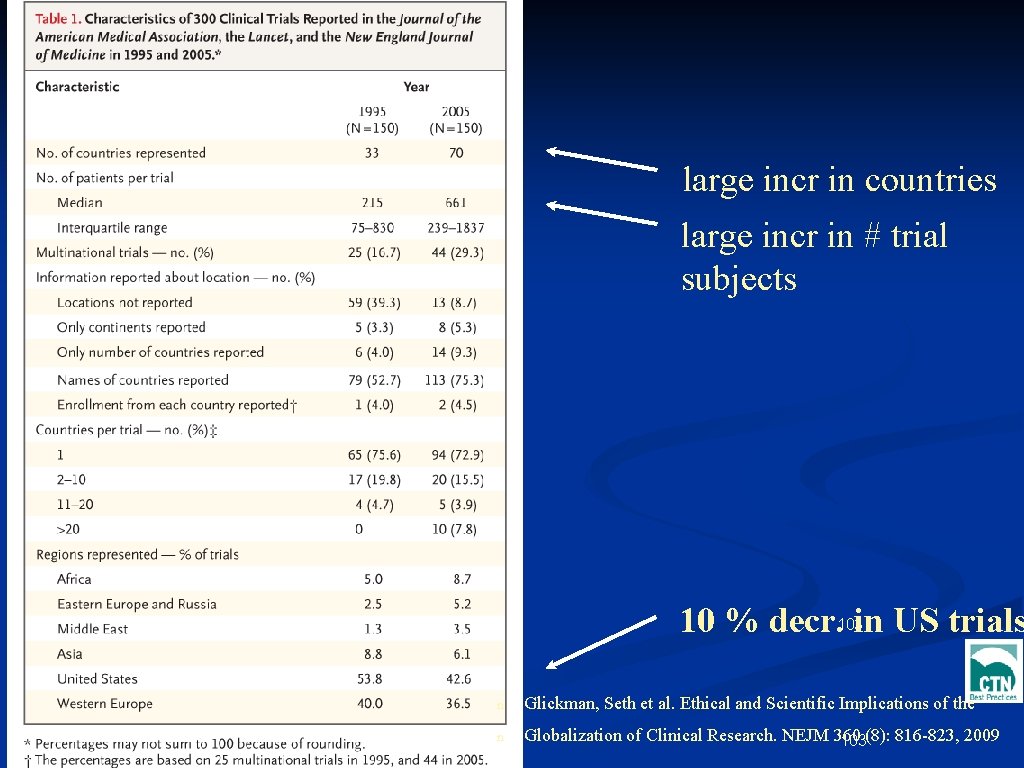
Open Phase 3 Clinical Trials, 2007 1995 2005 # Countries 33 70 US % of trials 54 43 10 % decr. in US trials # Pts/trial 215 661 large incr in # trial subjects large incr in countries Glickman, Seth et al. Ethical and Scientific Implications of the Globalization of Clinical Research. NEJM 360 (8): 816 -823, 2009 5

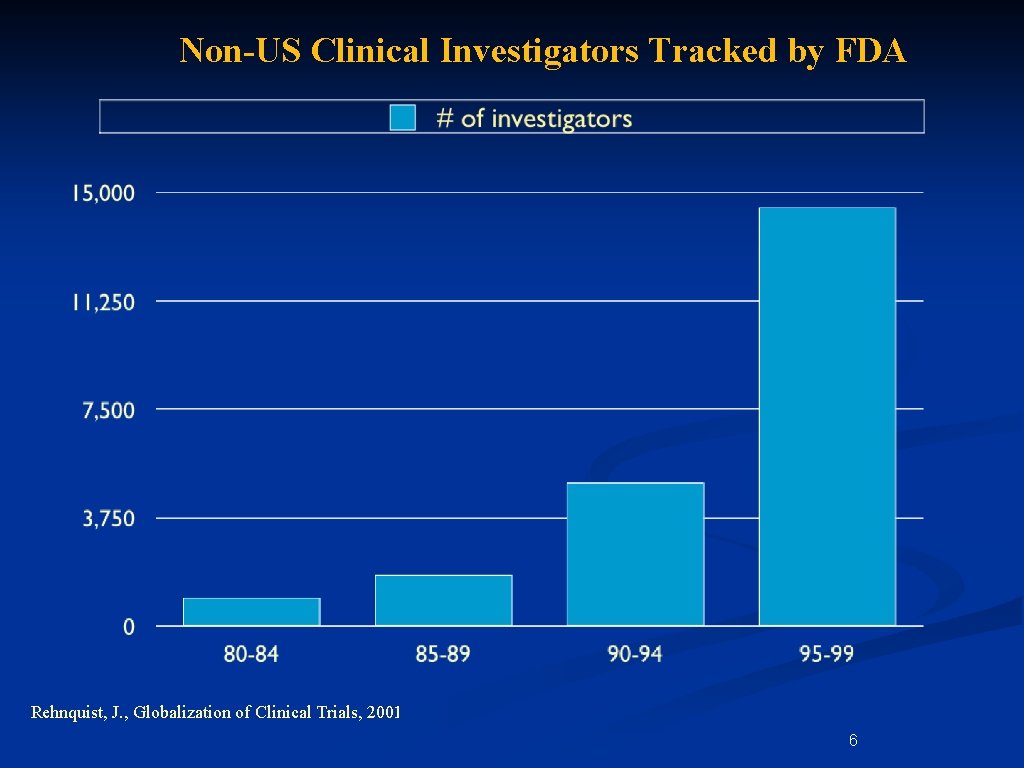
Non-US Clinical Investigators Tracked by FDA Rehnquist, J. , Globalization of Clinical Trials, 2001 6

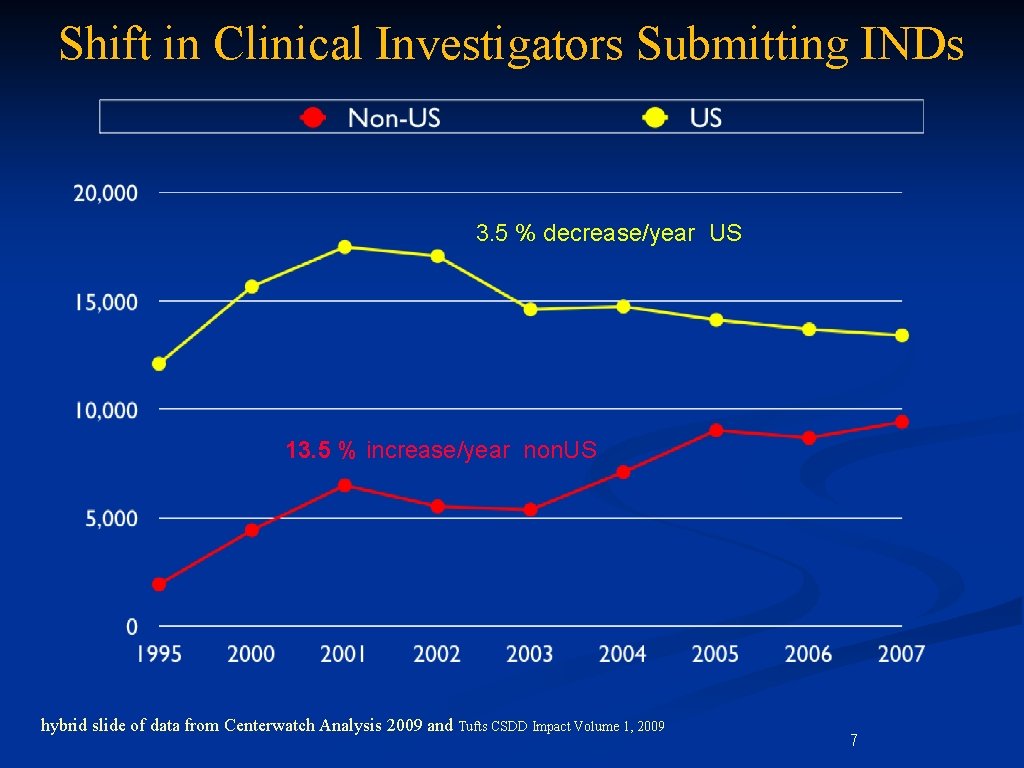
Shift in Clinical Investigators Submitting INDs 3. 5 % decrease/year US 13. 5 % increase/year non. US hybrid slide of data from Centerwatch Analysis 2009 and Tufts CSDD Impact Volume 1, 2009 7

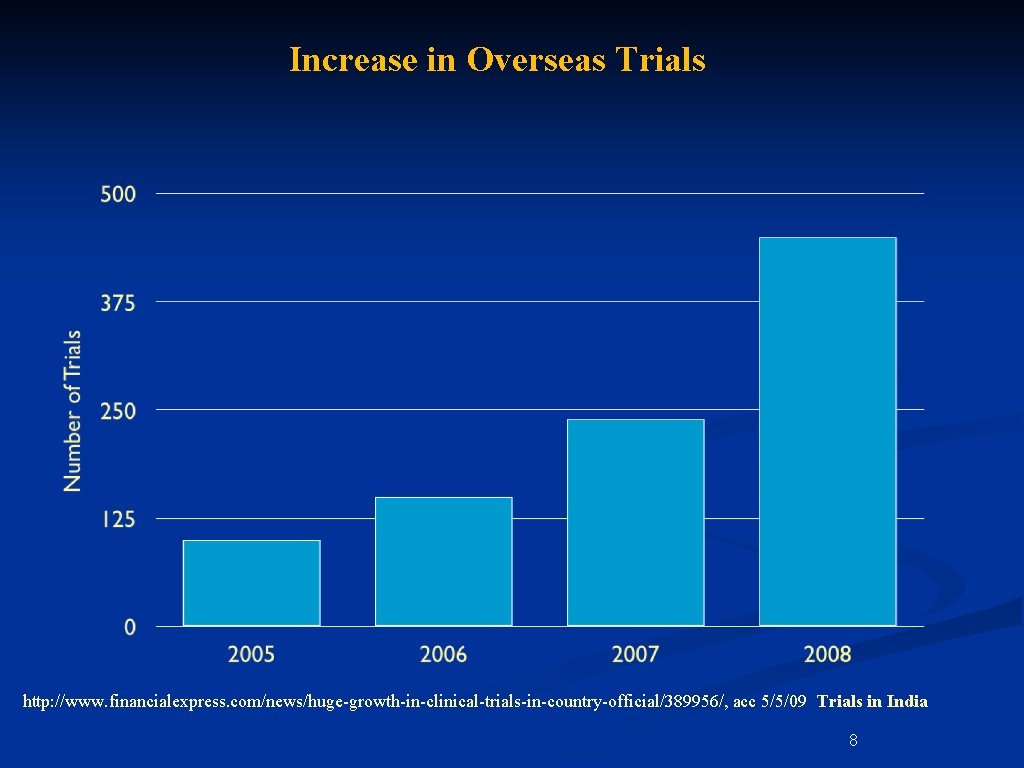
Increase in Overseas Trials http: //www. financialexpress. com/news/huge-growth-in-clinical-trials-in-country-official/389956/, acc 5/5/09 Trials in India 8

What Non-US Country is Most Desirable for doing Trials? ARS a) Japan b) China c) India d) Brazil e) Nigeria 9

What Non-US Country is Most Desirable for doing Trials? a) Japan b) China c) India d) Brazil e) Nigeria 10

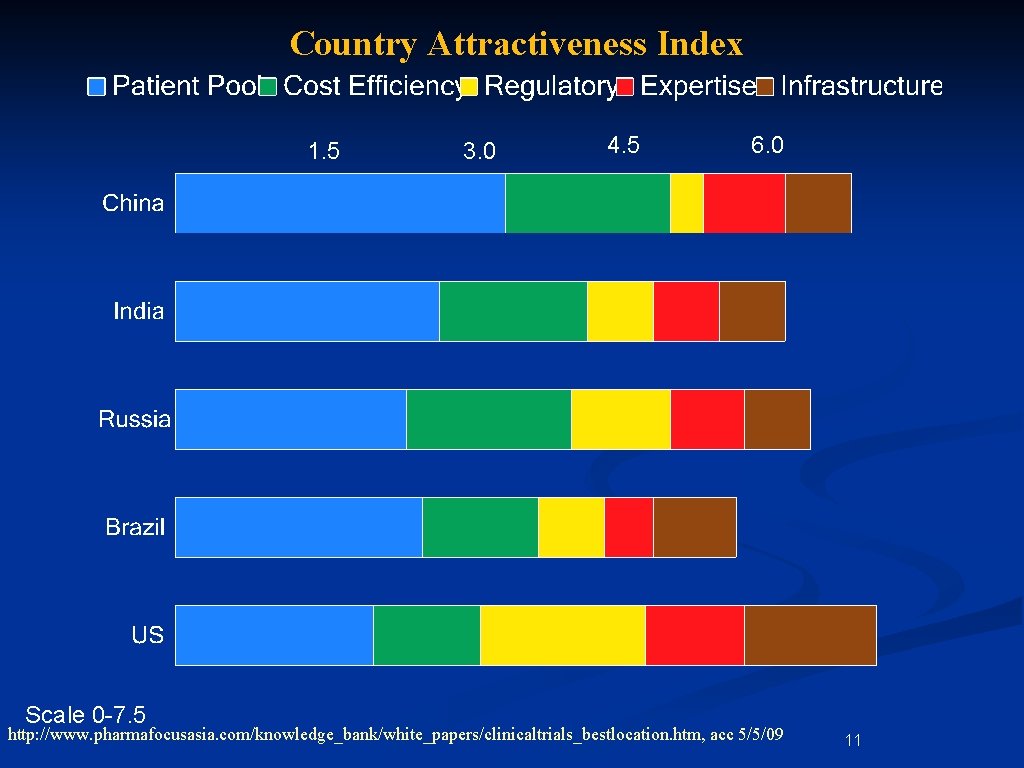
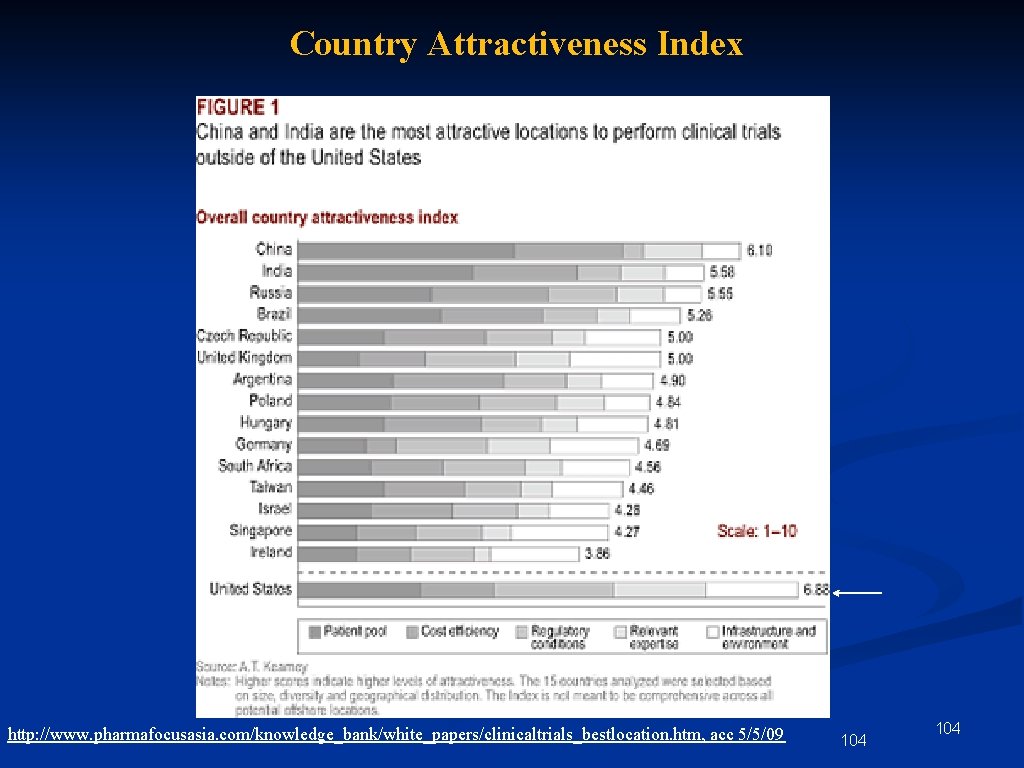
Country Attractiveness Index 1. 5 3. 0 4. 5 6. 0 Scale 0 -7. 5 http: //www. pharmafocusasia. com/knowledge_bank/white_papers/clinicaltrials_bestlocation. htm, acc 5/5/09 11

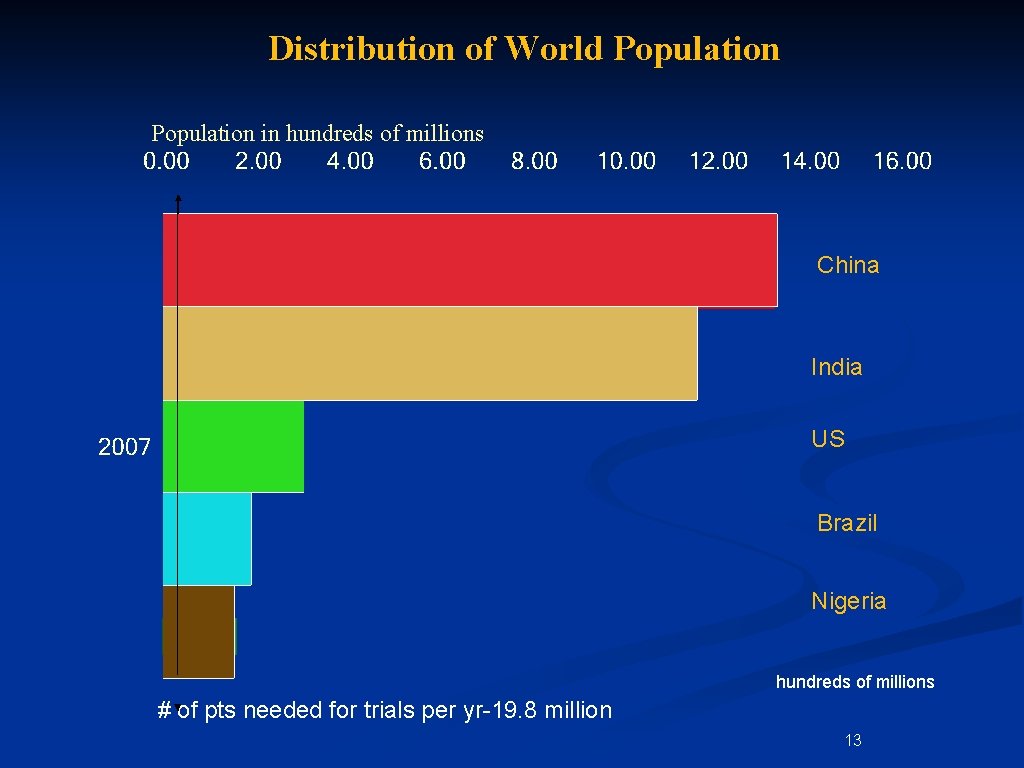
What is Driving the Shift Overseas? Access to patients • • § # of pts needed for trials rose: § 2. 8 million people in 1999 to § 19. 8 million in 2005 only 1 -2% participation rate in trials in US http: //www. boston. com/news/nation/articles/2007/12/29 12

Distribution of World Population in hundreds of millions China India US Brazil Nigeria hundreds of millions # of pts needed for trials per yr-19. 8 million 13

What is Driving the Shift Overseas? • Need for: Large numbers with a specific condition* • Specialized centers • • • Phase 2 “supercenters” with up to 9000 outpt visits/day** Treatment naive patients Different ethnic/racial groups • e. g. , Iressa effective in Asia, not US • lg Japanese pop in Brazil; less competition **http: //www. dddmag. com/clinical-trials-on-the-move. aspx 14

What is Driving the Shift Overseas? Bureaucracy • § § § delays in protocol development phase III ECOG trial protocols require about 800 days from conception to activation* contracts and grants negotiation now the longest part of starting a study o at one institution, 87 steps and 29 signatures were required to launch a trial *Sandler, Alan in http: //www. cancernetwork. com/display/article/10165/1382200, acc 5/25/09 15

What is Driving the Shift Overseas? Availability of patients • § § • • • Slow recruitment causes 85 -90% of delay in trials 80% of studies run over by 30 -42%, or avg. 6 mo* Recruitment speed may be 25 x or more higher** Cost Need to shorten development time… Trials in the Fast Lane: Accelerating Clinical Trials: Budgets, Patient Recruitment, and Productivity, Cutting Edge Information, acc 3/14/09 16 **www. ngpsummit. com/pdf/Clinstar. pdf

Costs of Delay • direct cost of delay $37, 000 per day in out-of -pocket expenses • indirect cost for a billion dollar drug is 83 million/month or 2. 8 million/day* (another estimate is $15. 6 million/day**) *Excel-China CW **www. ngpsummit. com/pdf/Clinstar. pdf 17

What is the most Expensive Place to Conduct Trials? n n a) Britain b) Japan c) Germany d) US 18

What is the most Expensive Place to Conduct Trials? n a) Britain n b) Japan n c) Germany n d) US 19

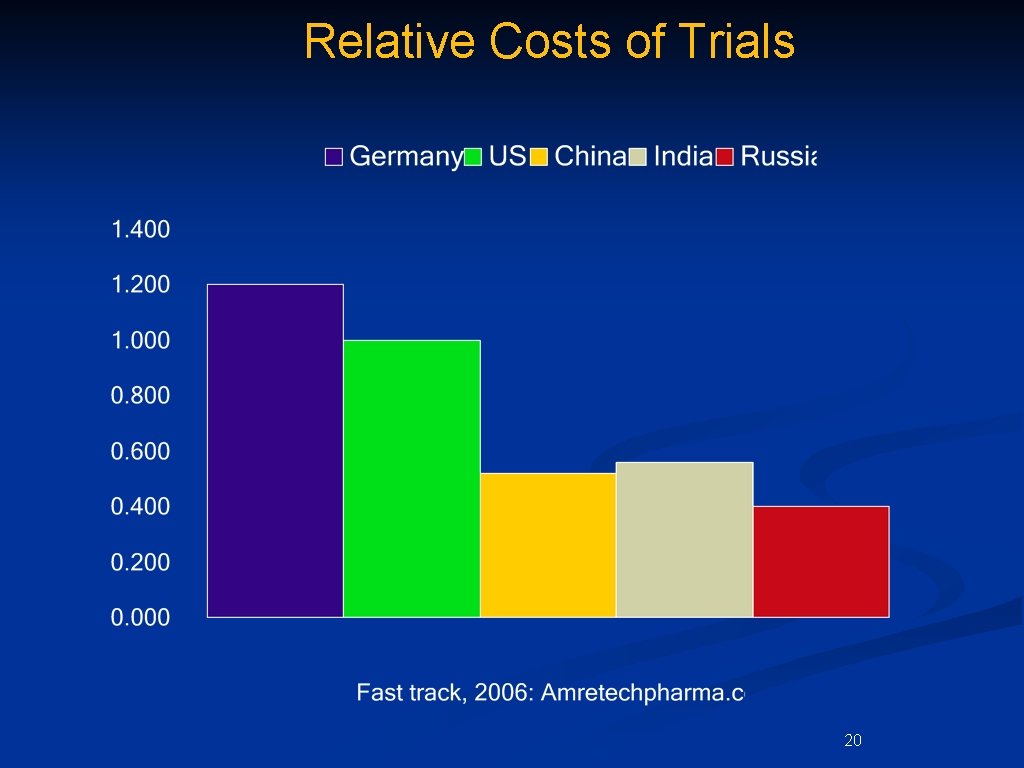
Relative Costs of Trials 20

What Else is Driving the Shift Overseas? Grooming markets in developing countries n n n Huge population of potential buyers Reduce regulatory barriers 21

Weaknesses in Ethical Review in Asia, Pacific, Africa--WHO Lack of: n n Resources n n n n DCGI (like FDA) in India staff: 3 pharmacists, no MD Procedures for protocol and informed consent review Trained IRB members Monitoring Quorum req Independence Nundy S. N Engl J Med 2005; 352: 1633 -6. Rehnquist, J. , Globalization of Clinical Trials, 2001 22

Problems in US Trials Difficulty recruiting patients in US n n n Low volunteer rate, <5% even for cancer trials Liability Mistrust of pharma Less risk-tolerant patients More competition for pts as more drugs in development n n n restrictive inclusion-exclusion req for larger trials HIPAA 23

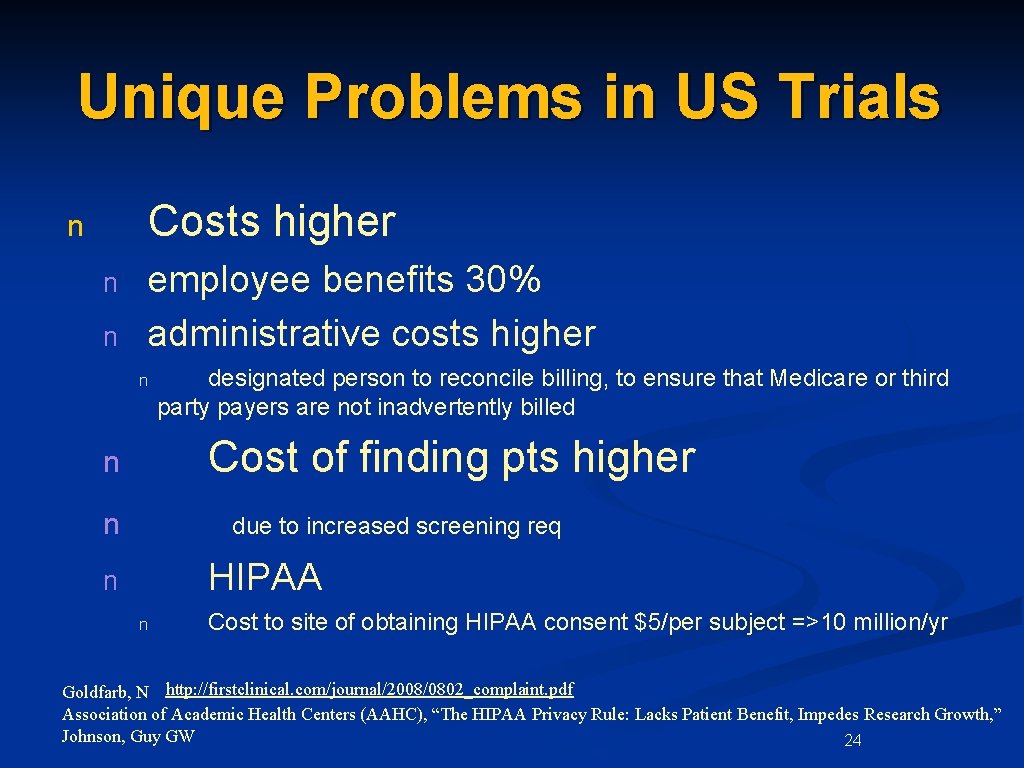
Unique Problems in US Trials Costs higher n n n employee benefits 30% administrative costs higher n designated person to reconcile billing, to ensure that Medicare or third party payers are not inadvertently billed Cost of finding pts higher n n due to increased screening req HIPAA n n Cost to site of obtaining HIPAA consent $5/per subject =>10 million/yr Goldfarb, N http: //firstclinical. com/journal/2008/0802_complaint. pdf Association of Academic Health Centers (AAHC), “The HIPAA Privacy Rule: Lacks Patient Benefit, Impedes Research Growth, ” Johnson, Guy GW 24

Unique Problems in US Trials n n Fewer feel need to participate higher standard of care n n Insurance precluding participation Goldfarb, N http: //firstclinical. com/journal/2008/0802_complaint. pdf Association of Academic Health Centers (AAHC), “The HIPAA Privacy Rule: Lacks Patient Benefit, Impedes Research Growth, ”, Johnson, Guy GW 25

What is Driving Move to Conduct Clinical Trials in Developing Countries? Summary n n n Costs are 50+ % lower outside of US More readily available “naïve” patients More compliant patients Less regulatory oversight Less litigious climate* n n *“Patients in Western countries—and in the United States especially—have an overdeveloped sense of their rights and a fear of being harmed. ” 26

Problems with shift. . . n Regulatory climate and Institutional Review Boards Protocol review may differ in emphasis on pt protections Little information on foreign IRBs and no oversight n Difficulty auditing foreign Pis n n Monitoring is more difficult More ethical issues may be raised n n n Levels of understanding and education Informed consent 27

Decline in US Investigators Why? n n Lack of exposure in training Enormous time commitment n Increasing difficulties recruiting pts n n HIPAA, insurance restrictions, incl-excl criteria Liability issues Financial incentive 28

How Profitable are Clinical Trials for a Site? a) 2 % b) 5 % c) 10% d) 20 % 29

How Profitable are Clinical Trials for a Site? a) 2 % b) 5 % c) 10% d) 20 % 30 30

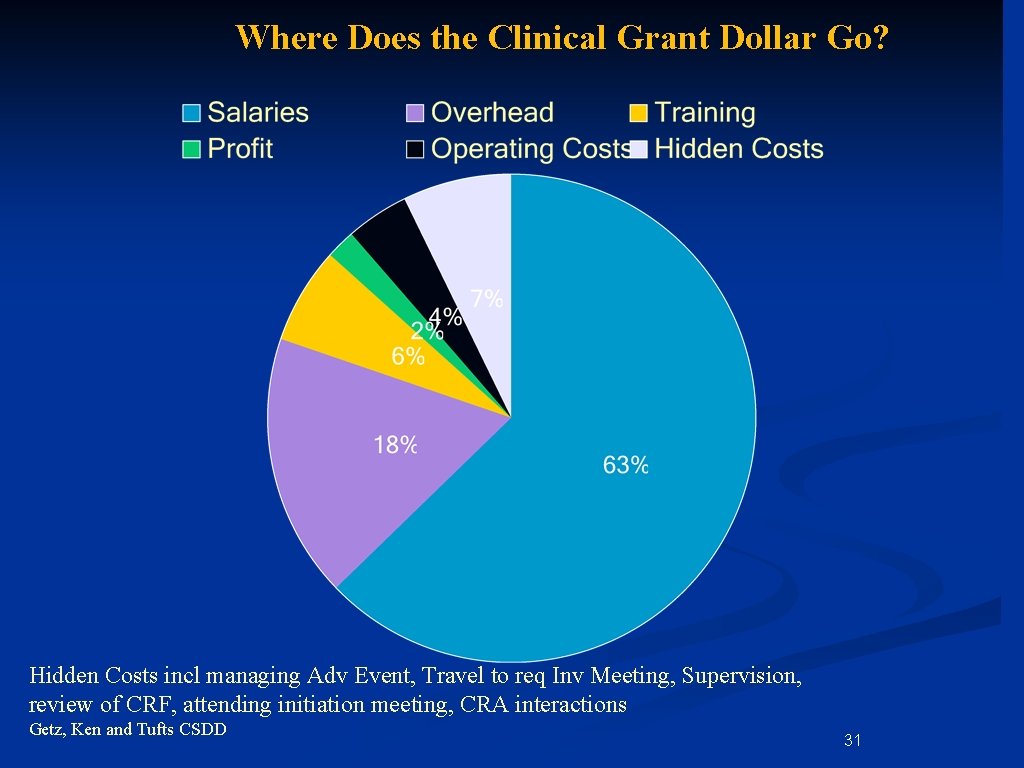
Where Does the Clinical Grant Dollar Go? Hidden Costs incl managing Adv Event, Travel to req Inv Meeting, Supervision, review of CRF, attending initiation meeting, CRA interactions Getz, Ken and Tufts CSDD 31

Shifting Research to Developing Countries Ethical Issues 32

News Flash! Genzyme’s Business Model Heuser, Stephen. One girl's hope, a nation's dilemma. Boston Globe, 6/14/09 33

Genzyme’s Business Model Pits one child vs 600, 000 hypertensive patients, 120, 000 diabetics “searching out patients…then successfully pressing their governments, even poor ones, to pay full price for the most expensive drugs in the world… Genzyme created divisions within the company to find overseas patients; it hired experts to cajole balky governments into paying for the patients' … Helped families sue the federal government” Access to healthcare in many countries’ constitution Proposal: force drug companies to scale their prices to a country's GDP Heuser, Stephen. One girl's hope, a nation's dilemma. Boston Globe, 6/14/09 34

n n Shifting Research to Developing Countries: Gender & Racial Issues There are some known metabolic differences between men & women => differences in response to drugs. Also differences in intracellular metabolism (e. g. , Cytochrome P 450 enzyme) between different races. So it is important to test drugs in men and women, and different ethnic groups… But… 35

Perception of Overseas 36

AIDS study survey 88 % of the women felt they had to participate n ~1/3 feared repercussions if they refused n Almost all unaware they could withdraw from a trial n n Inherent coercion from extreme poverty and lack of alternatives, as well as power inequities “Body Hunters” Washington Post 2000 37

Shifting Research to Developing Countries, cont. . . n Many developing countries want the financial gains and business from conducting trials 38

39

Why did Pfizer choose Nigeria for its Meningitis Trial? a) imperialism b) savings c) expedience d) subject accrual possibilities e) ease of regulatory approval 40

Why did Pfizer choose Nigeria for its Meningitis Trial? a) imperialism b) savings c) expedience d) subject accrual possibilities e) ease of regulatory approval 41

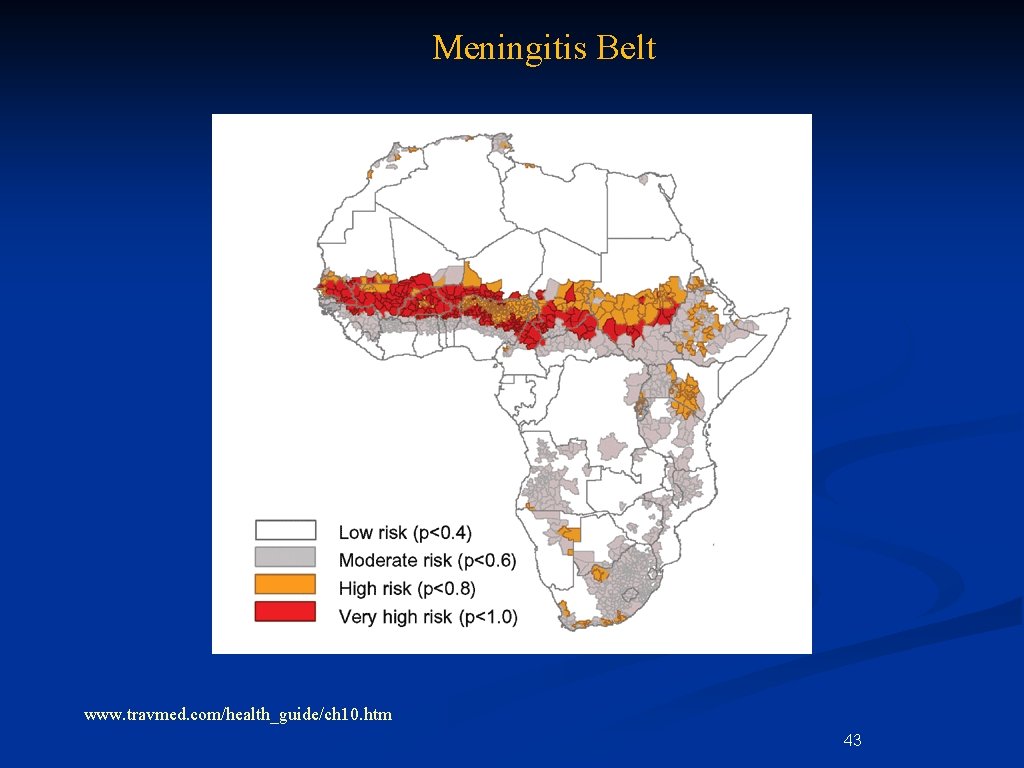
Shifting Research to Developing Countries n Some illnesses are more common in dev. countries—e. g. , meningitis belt and tropical infections 42

Meningitis Belt www. travmed. com/health_guide/ch 10. htm 43

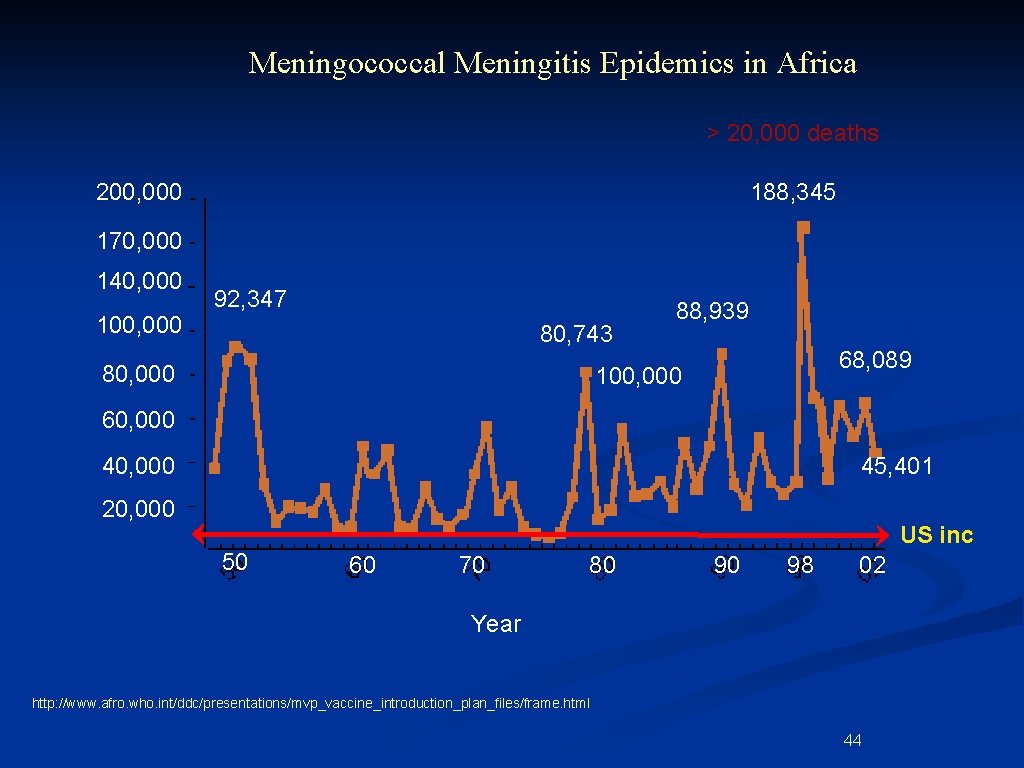
Meningococcal Meningitis Epidemics in Africa > 20, 000 deaths 200, 000 188, 345 170, 000 140, 000 100, 000 92, 347 80, 743 80, 000 88, 939 68, 089 100, 000 60, 000 45, 401 20, 000 50 US inc 60 70 80 90 98 02 Year http: //www. afro. who. int/ddc/presentations/mvp_vaccine_introduction_plan_files/frame. html 44

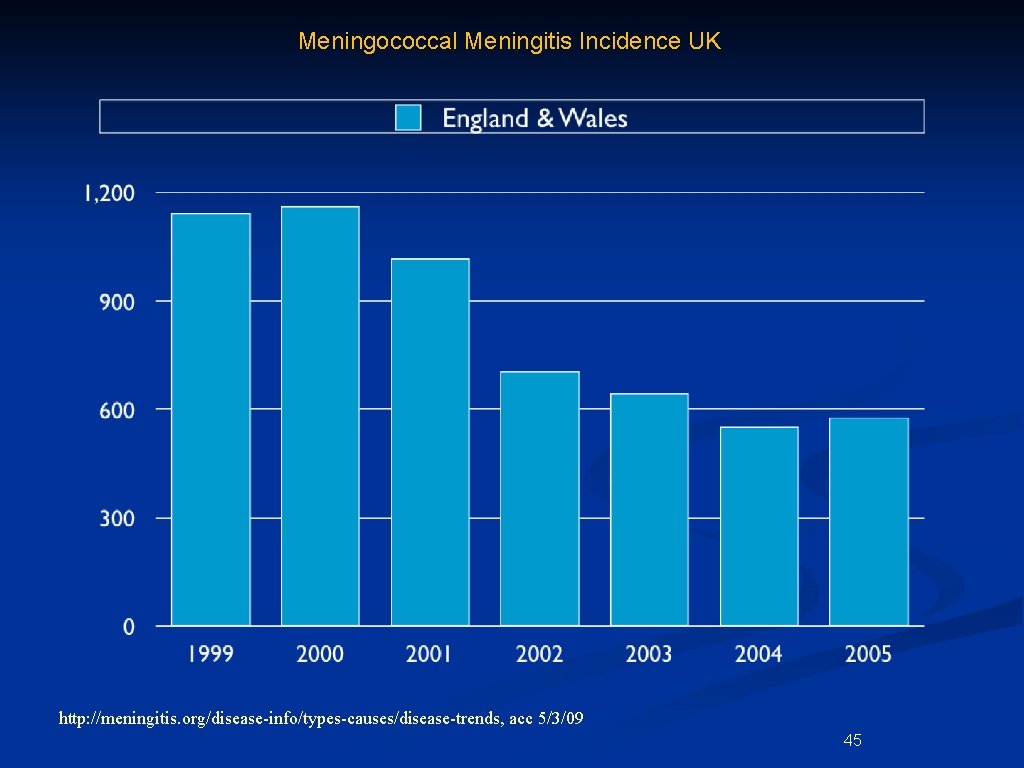
Meningococcal Meningitis Incidence UK http: //meningitis. org/disease-info/types-causes/disease-trends, acc 5/3/09 45

Nigerian Meningitis Trial 1996 meningococcal meningitis outbreak n n ~12, 000 children died over 6 months Ceftriaxone IM vs. Trovafloxacin orally n Pfizer criticized re informed consent documentation and IRB approval--not without justification n But… n 46

Nigerian Meningitis Trial n n n What is rarely mentioned: Need to do study where the illness occurs-not practical in US Value of developing oral rx Survival rate was 94. 4% Trovan vs. 93. 8 Ceftriaxone vs 89. 9% non-study hosp pts Trovan was also being studied for meningitis in the US n n 79%vs. 81% clinical success Ped ID 21(1): 14 -22, January 2002. 47

Nigerian Meningitis Trial’s Unintended Consequence n n Decline in polio immunization due to mistrust Nigerian Tuskeegee 48

Cultural Sensitivity n Culture: traditions, ethnicity, religious beliefs n Individual autonomy (Western) vs. relational, or membership in community n What defines a specific Community? n n Deference to elders, authority Community Assent/Consent-FDA 21 Part 50 Who defines what is acceptable within the community? NIH Human Participants Protections Education for Research Teams, phrp. nihtraining. com/ 49

What Concepts May Differ Internationally n Village vs. Individual n n n n Values Sense of Self Spirit/Religion Sense of Body Meaning/Sense of Illnesses Natural Courses v. Intervention Rose, Susan Ethics of International Research www. usc. edu/admin/provost/oprs/private/docs/oprs/powerpoint/Intl_Research. ppt 50

Examples of Conflicts Due to Cultural Sensitivities Tribal structure Patriarchy Fear of signing a paper Female/male issues Blood draws or surgery Touch Interviews Language difference Rose, Susan Ethics of International Research www. usc. edu/admin/provost/oprs/private/docs/oprs/powerpoint/Intl_Research. ppt 51

Vietnamese breast cancer study Oophorectomy vs Tamoxifen Unique problems as: a) Pts don’t generally decide their care b) Also unacceptable for physician to openly express uncertainty re best rx 52

Vietnamese breast cancer study What would you do? a) not do the trial in Vietnam as the woman is not consenting? b) have the patient’s husband provide consent c) rely on data from other countries d) have the doctor be the patient surrogate e) have the IRB be patient surrogate 53

Vietnamese breast cancer study Oophorectomy vs Tamoxifen Decision was made to use physician as surrogate, then tell the pt after randomization which rx they would receive 54

Another Ethical Dilemma n n n Should the absolute standards of care available in more developed countries be applied even when adequate resources are not available in the host country? Or is it perhaps more realistic and ethical to conduct trials with reference to the local standard of care? 55

Maternal-Fetal HIV Transmission n Should the intervention be compared to the local standard, which may be to do nothing, n or to provide another level of care which appears substandard to us? 56

Distributive Justice Dilemmas n n Should trials of new medicines or technologies be undertaken where the study population will not be able to benefit afterwards? e. g. , Latin American trials on surfactant “The Body Hunters” Washington Post series; Flaherty, 2/23/2001 57

Surfaxin Trial n n Surfaxin vs. old drug vs. placebo Placebo group would be unethical in US, where there is a marketed Rx. n Placebo control provides best data for all (better than non-inferiority trials) n Hosp was to get ventilators and antibiotics, improved care over their baseline. Babies would get Rx not otherwise available or affordable? Is such a trial ethical where there is no Rx? n 58

VOTE a) Surfaxin trial was ethical b) Surfaxin trial was not kosher 59

An Ethical Dilemma n Helsinki Declaration requirements revised in 2000, requiring uniform standards: n To provide each participant with the “best current” diagnostic tests and therapy. n What if the drugs or interventions may not be the “best” in the world but are better than the locally available treatments? 60

Declaration of Helsinki n Developed in response to WW 2/Nuremburg n as an ethical & moral authority n ICH-GCP Guidelines require adherence to “the principles that have their origin in the Do. H” n FDA required foreign studies be conducted in accordance with Helsinki 61

Declaration of Helsinki 2007 revisions: n requires comparison against “best current” methods and discourages use of placebos n n n requires access to benefits of study n As of 2008, FDA dropped req that studies must be in compliance with the Do. H n Studies must now just be in compliance with GCP 62

Helsinki vs ICH-GCP n GCP: n provides assurance that the data and reported results are credible and accurate, and that the rights, integrity, and confidentiality of trial subjects are protected. * * Williams, John R. Helsinki 2008 63

Helsinki vs ICH-GCP n “GCP is not an ethical code, but a procedural regulatory manual based on the regulatory frameworks of the US, Japan, and Europe. Thus, it is a description of existing procedures, not an aspirational document… It is not the procedural nuances that are at stake, but rather the moral reasoning that forms the basis of a culture of ethically responsible research. ”** ** Goodyear et al. Does the FDA have the authority to trump the Declaration of Helsinki? . BMJ 338: b 1559 64

Helsinki vs ICH n Concerns about US’ unilateral decision withdrawing from Helsinki has been depicted as n entrenching different standards for different parts of the world (ethical pluralism) n establishing the US’s right to unique policies (exceptionalism) n and one country imposing standards on others (moral imperialism). ** Goodyear et al. Does the FDA have the authority to trump the Declaration of Helsinki? . BMJ 338: b 1559 65

Distributive Justice Dilemmas n n n Should gov’t research $ be directed toward basic science research (such as mechanisms of disease) rather than applications? Should research $ be spent on future drugs or on “designer” drugs that may only benefit a small segment of the population, or on basic 66

Developing Countries By 2020, US. will have decreased to 4 percent of the world’s population n Developing countries will grow to 84 percent n Of $2 trillion annual health expenditures: n n 90% go to the top countries (US, Japan, Europe, Canada, Australia, Hong Kong, Singapore, Israel) n 10% resources for 90% of global disease burden 67

References Glickman, Seth et al. Ethical and Scientific Implications of the Globalization of Clinical Research. NEJM 360 (8): 816 -823, 2009 Rehnquist, Janet. The Globalization of Clinical Trials: A Growing Challenge in Protecting Human Subjects, 2001 OEI-01 -00 -00190 http: //oig. hhs. gov/oei Anthes, Emily; Allen, Scott. US cancer researchers go abroad for trials. Boston Globe, 12/29/07 Normile, Dennis. The Promise and Pitfalls of Clinical Trials Overseas DOI: 10. 1126/science. 322. 5899. 214, 10/08; www. sciencemag. org 68

References Nundy S, Gulhati CM. A new colonialism? Conducting clinical trials in India. N Engl J Med 2005; 352: 1633 -6 Rose, Susan Ethics of International Research www. usc. edu/admin/provost/oprs/private/docs/oprs/powerpoint/Intl_Research. ppt NIH Human Participants Protections Education for Research Teams, phrp. nihtraining. com/ Williams, John R. The 2008 Declaration of Helsinki web. wits. ac. za/NR/rdonlyres/9 A 4 CDFCC-9 E 5 A-4 DB 9 -ADC 4 -0 CD 165 D 1 DEE 5/0/Helsinkideclaration 2008. pdf Does the FDA have the authority to trump the Declaration of Helsinki? - Goodyear, Michael DE, et al. 338: b 1559 -- BMJ 69

70

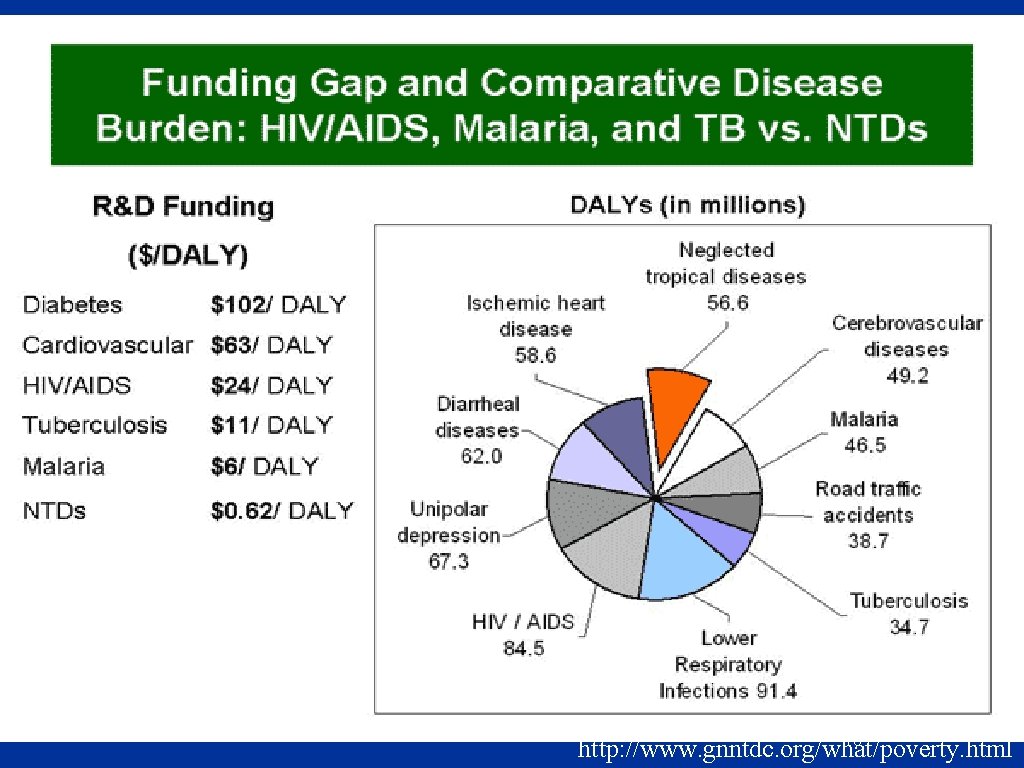
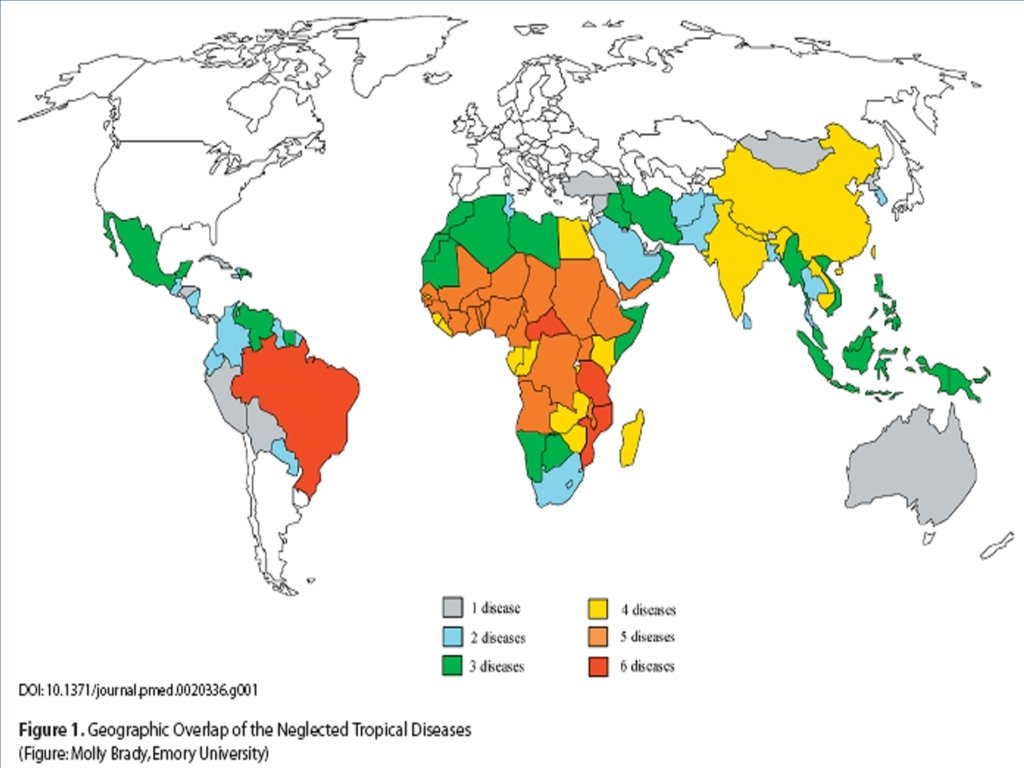
Common Features of the Neglected Tropical Diseases “Biblical diseases”-- afflictions that have burdened humanity for centuries n They affect the most vulnerable, impoverished, marginalized populations n Rural areas of low-income countries and urban slums n 2. 7 billion people on < $2 per day n n Disabling and very deforming => stigma Molyneux, Hotez, and Fenwick PLo. S Med 2(11): e 336 doi: 10. 1371/journal. pmed. 0020336 71

Common Features of the Neglected Tropical Diseases Poverty-promoting conditions n n Child development; pregnancy; worker productivity No commercial markets for products that target these diseases n Interventions, when applied, have a history of success n Molyneux, Hotez, and Fenwick PLo. S Med 2(11): e 336 doi: 10. 1371/journal. pmed. 0020336 72

Neglected Tropical Diseases Helminth Infections n n Soil Transmitted Helminth Infections n n n n Ascariasis Trichuriasis Hookworm infection Schistosomiasis (Bilharziasis) Lymphatic filariasis (Elephantiasis) Onchocerciasis (River blindness) Dracunculiasis (Guinea Worm) 73

Neglected Tropical Diseases Protozoan Infections n n African trypanosomiasis (Sleeping Sickness) Chagas disease Kala-azar (visceral leishmaniasis) Bacterial Infections n n Trachoma Leprosy Buruli ulcer 74

The Disability Adjusted Life Year (DALY) n Total amount of healthy life lost, to all causes, whether from premature mortality or from some degree of disability during a period of time 75

76 http: //www. gnntdc. org/what/poverty. html

77

Neglected Tropical Diseases n Soil-transmitted Helminths Ascariasis (round) n Trichuriasis (whip) n Hookworm n Despommier, Gwadz, Hotez, Knirsch, Parasitic Diseases 5 th Edition and www. sabin. org/ 78

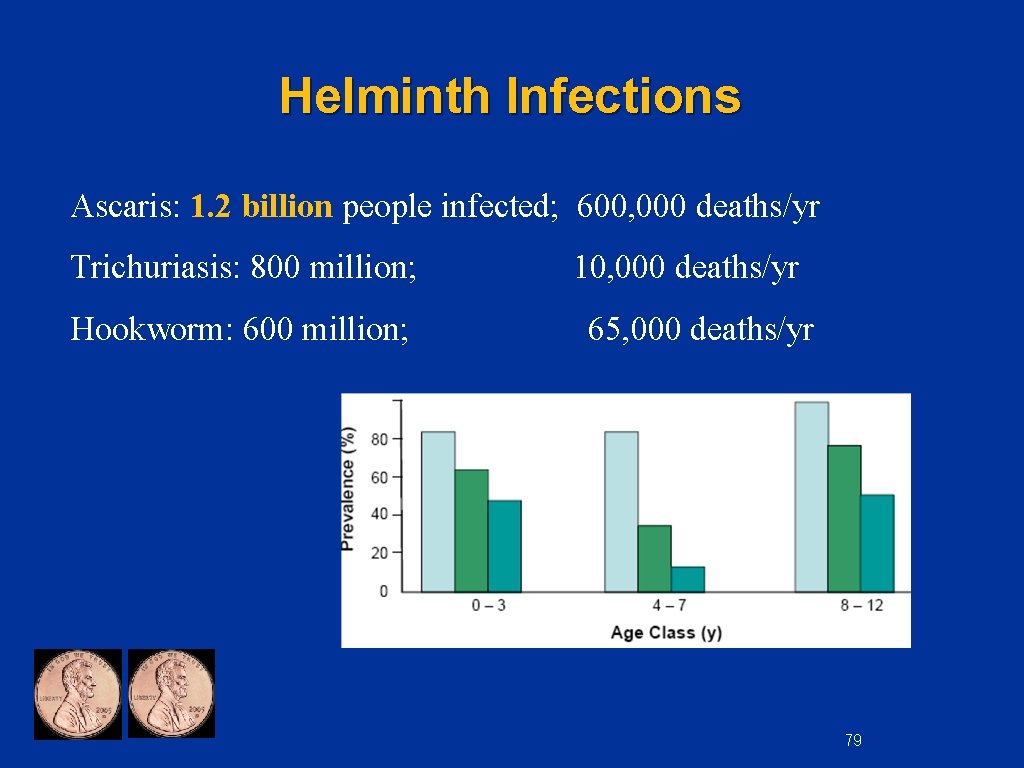
Helminth Infections Ascaris: 1. 2 billion people infected; 600, 000 deaths/yr Trichuriasis: 800 million; 10, 000 deaths/yr Hookworm: 600 million; 65, 000 deaths/yr 79

Helminth Infections Rx with Albendazole or Mebendazole only 1 -3 x/yr improves nutrition & health=> Increased ability to learn and earn => Helping break the cycle of poverty Why have we waited? 80

“Rapid Impact Package” for NTDs n n n n n Albendazole or Mebendazole drugs only 4 Diethylcarbamazine or Ivermectin Praziquantal Azithromycin Treats 7 NTDs: Helminths (Ascaris/Hookworm/Whipworm) Schistosomiasis Filariasis River Blindness Trachoma 81

n + = Rapid Impact Package Including n Drugs n Delivery n Equipment n Distribution costs n Health education materials n Training n Monitoring and Smart evaluation n It’s and just the right thing to do… 82

Treating NTDs Costs: 40 cents/per person/yr Only $200 million/yr, treats 500 million people What are we waiting for? 83

Collateral Benefits Worm Control Skin disease Reduced Blindness Prevented Control of Malaria and HIV/AIDS Anemia Prevalence reduced Lower maternal-infant mortality Nutritional Status improved Growth Enhanced Improved educational performance Improved economic performance Less time lost from school and work Better earning capacity cost effective return on investment All of this, for < 50 cents/per person/year. Why haven’t we done this? 84

Our Priorities? Few drugs for sleeping sickness: n Suramin, Pentamidine, Melarsoprol (arsenic deriv), n Eflornithine n Aventis stopped production of IV eflornithine in 1999 due to lack of profit n 2001 production resumed after agreement with WHO and n Doctors Without Borders vs: n Vaniqa ad*: “What a burden that has been lifted from my life! I feel so free now to be who I really am. *Vaniqa website 9/11/2004; accessed with wayback machine 2/21/07 I’m not at all self-conscious with people. ” 85 n

86

Priorities n n Vaniqa $1 -2/day for Vaniqa n n n Viagra $2 -3/dose Value Pak- “Buy 3, get 1 free” “Want to improve your sex life? ” AIDS Healthcare Foundation sues Pfizer 1/07 for ads promoting sex=> incr. STDs (and HIV) 87

Disparities n n 1 latte or 1 “little blue pill” vs. $ to feed 3 people for 1 day 88

Justice? n < 50 cents/ n person/ vs n per year to control 7 major NTDs Lifestyle drugs 89

Cosmetic Expenditures $35 billion/yr in US Ø Ø n n http: //www. flickr. com/photos/yoshie 231/386003324/ http: //www. cfsan. fda. gov/~comm/cp 29002. html Or > $100/person/yr vs. < 50 cents/ person/ per year to control 7 major NTDs 90

Perspective 91

Why are we not doing more? 92 http: //www. gnntdc. org/resource/conf_rsc/docs/(13 a)%20 PPPs%20 Panel_Amazigo. pdf

-". . . let us recognize that extreme poverty anywhere is a threat to human security everywhere. Let us recall that poverty is a denial of human rights. For the first time in history, in this age of unprecedented wealth and technical prowess, we have the power to save humanity from this shameful scourge. Let us summon the will to do it. " -- Kofi Annan 93

Photo: Michael Fenichel, with permission 94

Bill & Melinda Gates Foundation $1. 5 billion for vaccine preventable diseases 2006 new pledge: $ 68 million for: Leishmania vaccine Hookworm vaccine Leishmania and Trypanosome therapies PLo. S Neglected Tropical Diseases 95

96

Whatever you choose to do, Support Research Remember and Work for the Forces of Good 97

For more information, see Global Network for Neglected Tropical Diseases http: //www. GNNTDC. org 98

References-NTDs n n n Molyneux. DH, Hotez. PJ, Fenwick A. PLo. SMedicine 2005; 2: e 336 Hotez. PJ, Molyneux. DH, et al PLo. SMedicine 2006; 3: e 102 Guerrant, R. Why America Must Care about Tropical Medicine. Am. J. Trop. Med. Hyg. , 59(1), 1998, pp. 3– 16 99

References-NTDs, cont. n WHO: WHO_CDS_NTD_2006. 2_eng. pdf “Hidden successes, emerging opportunities” n http: //www. who. int/neglected_diseases/en/ n WHO: Intensified control of NTD, 2006 n 100

Global Network for Neglected Tropical Diseases n n http: //www. GNNTDC. org Schistosomiasis Control Initiative www. schisto. org n Trachoma Initiative www. trachoma. org n n Liverpool School www. filariasis. org Human Hookworm Vaccine Initiative www. sabin. org n n Earth Institute @ Columbia University Task Force for Child Survival www. taskforce. org 101

Background: FDA Oversight IND (Investigational New Drug Application) Prior to starting studies FDA can make suggestions for change NDA (New Drug Application) After trials are done, seeking FDA approval for marketing Foreign research must have been done under IND or under Declaration of Helsinki standards Helsinki req abandoned by USA in 2008* 102

large incr in countries large incr in # trial subjects 10 % decr. 103 in US trials n Glickman, Seth et al. Ethical and Scientific Implications of the n Globalization of Clinical Research. NEJM 360 (8): 816 -823, 2009 103

Country Attractiveness Index http: //www. pharmafocusasia. com/knowledge_bank/white_papers/clinicaltrials_bestlocation. htm, acc 5/5/09 104

What is Driving the Shift Overseas? n Access to patients n 1 2 3 5 8 9 19. 8 million needed in 2005** % of country’s pop China India US Brazil Nigeria Russia 1, 337, 722, 000 1, 162, 850, 000 306, 360, 000 191, 182, 188 154, 729, 000 141, 833, 393 1. 5 1. 7 6. 4 10. 4 12. 8 13. 9 • % of world population • **http: //www. boston. com/news/nation/articles/2007/12/29 105