Pediatric Trials Network What Is The Pediatric Trials













- Slides: 26

Pediatric Trials Network

What Is The Pediatric Trials Network PTN? n Sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) n The primary objective of the Pediatric Trials Network: Create an infrastructure for investigators to conduct trials that improve pediatric labeling and child health. n PTN is studying product formulation, drug dose, efficacy, safety, and device validation n Evidence of success will be completed trials that improve dosing, safety information, labeling, and ultimately child health 2

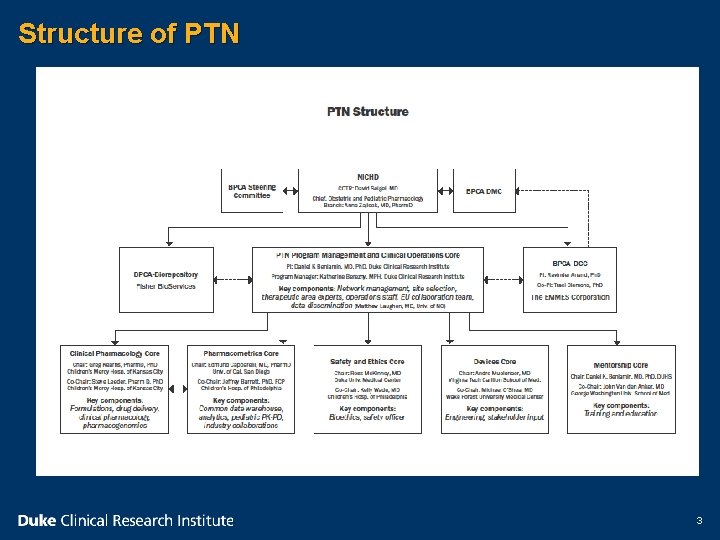
Structure of PTN 3

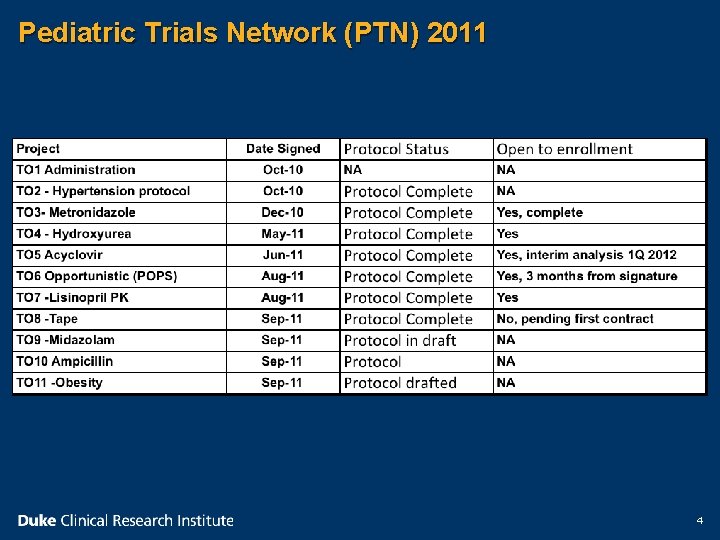
Pediatric Trials Network (PTN) 2011 4

Protocol: Metronidazole Protocol Chair: Cohen-Wolkowiez n Protocol Title: Safety and Pharmacokinetics of Multiple Dose Metronidazole in Premature Infants n Objective: Evaluate the safety, PK, and surrogate PD of intravenous metronidazole in premature infants with suspected serious infection n Study Population: 16 to 32 participants <32 weeks gestational age with suspected serious infection. Participants will be divided into 2 groups based on postnatal age. n Study Duration: original target 18 months (finished in 12); each participant will participate in the study for up to 15 days: 2 -5 days of study drug administration followed by 10 days of adverse events monitoring. n Number of Sites: 3 n October, 2011 Enrollment Complete 5

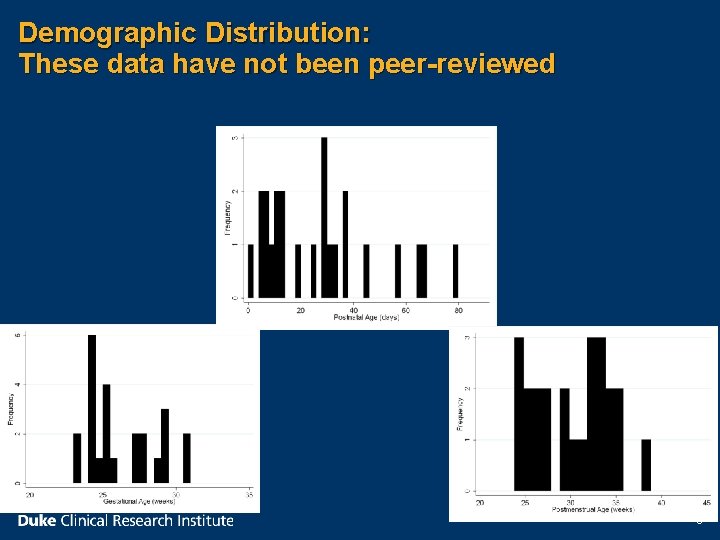
Demographic Distribution: These data have not been peer-reviewed 6

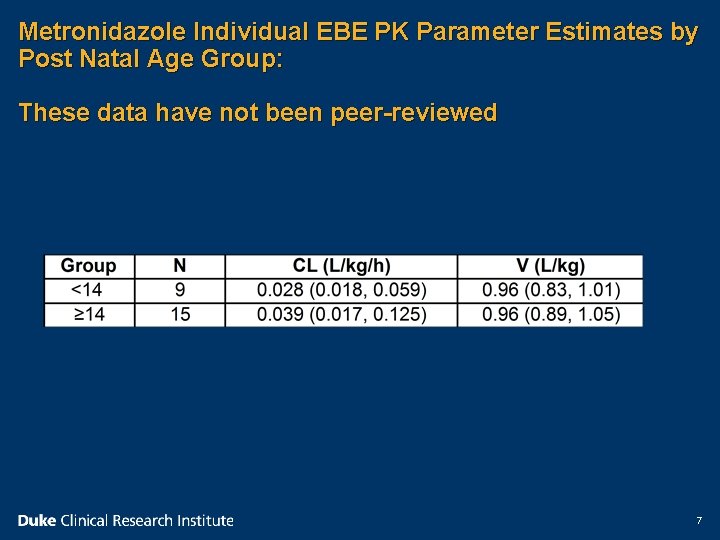
Metronidazole Individual EBE PK Parameter Estimates by Post Natal Age Group: These data have not been peer-reviewed 7

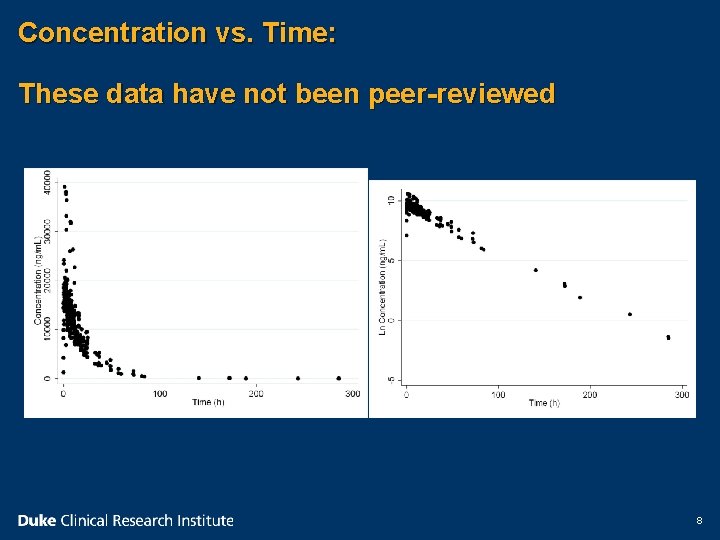
Concentration vs. Time: These data have not been peer-reviewed 8

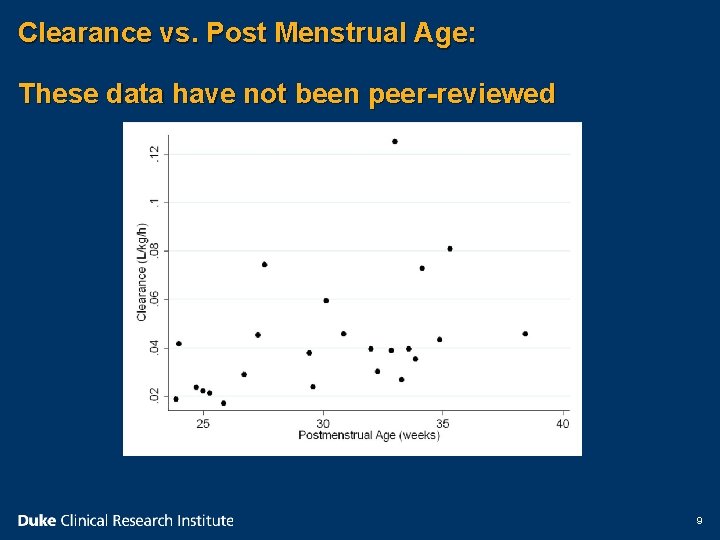
Clearance vs. Post Menstrual Age: These data have not been peer-reviewed 9

Protocol: Acyclovir Protocol Chair: Smith n Protocol Title: An Open Label Study to Describe the Pharmacokinetics of Acyclovir in Premature Infants n Objective: To evaluate the safety and PK of IV acyclovir in premature infants with suspected systemic infections. n Study Population: 20 Infants < 45 days postnatal age, suspected to have a systemic infection divided into groups by gestational and postnatal age n Study Duration: each infant will be in the study for up to 13 days; goal is to provide final component of PK data for subsequent efficacy trial n Number of Sites: 3 n First Patient Enrolled: September 19, 2011; target March 2012 10

Protocol: Hydroxyurea Protocol Chair: Neville n Protocol Title: PK & Relative Bioavailability of a Liquid Formulation of Hydroxyurea in Pediatric Patients with Sickle Cell Anemia n Objective: relative bioavailabilty study and bioequivalence study with new formulation n Study Population: 40 children ages 2 -17 with sickle cell anemia or sickle beta-zero thalassemia; two-armed study with older children (bioequivalence) enrolled first n Study duration: a subset of patients in each age cohort will receive single dose and a subset will receive multiple doses n Number of Sites: Six n First Patient: December, 2011 11

Protocol: POPS Pediatric Opportunistic PK Study n Protocol Title: Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care n Objectives: l Evaluate the PK of understudied drugs currently being administered to children. n Study Population: 500 children (birth-20 years) who are receiving understudied drugs of interest per standard of care as prescribed by their treating caregiver n Study Duration: each child will participate in the study for up to 90 days per drug; study conduct for 3 years n Number of Sites: 15 n First Patient Enrolled: November, 2011 12

Protocol: Lisinopril Protocol Chair: Trachtman n Protocol Title: Safety and Pharmacokinetics of Lisinopril in Pediatric Kidney Transplant Recipients n Objective: initial description of the PK-PD and safety of lisinopril following transplantation n Study population: 24 children ages 2 -18 with kidney transplant and stable allograft function n Study participation: Up to 51 days; enrolled children will receive multiple doses with multiple assessments for potential endpoints for subsequent efficacy trial n Number of Sites: 8 n Target to Enroll First Patient: January 2012 13

Protocol: TAPE Protocol Chair: Rahman n Protocol Title: Taking the Guesswork out of Pediatric Weight Estimation (TAPE): Validation of the Mercy TAPE n Objective: device trial to provide more accurate, rapid estimation of weight in the acute care setting—e. g. , use in emergency setting or resource poor countries for quick dosing calculations n Study population: 625 children 2 months to 16 years old enrolled into 17 strata n Protocol Final and target first enrollment January 2012 14

Other Task Orders n n n Midazolam n Analysis of previously collected data n Provide supplemental data to support of the current prescription labeling to include the treatment of seizures Ampicillin n Original written request, PK study and efficacy study in infants n PPRU (Pediatric Pharmacology Research Unit) collected samples Obesity n Analysis to provide preliminary data and held application for dosing in obese children 15

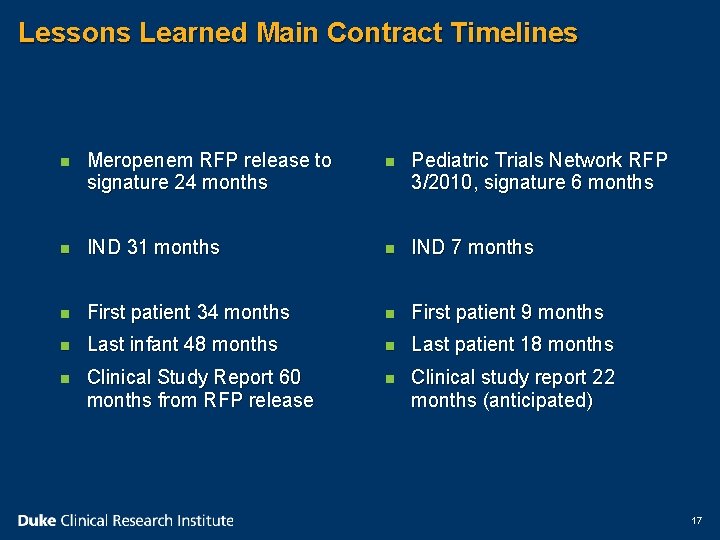
Lessons Learned Main Contract Timelines n Meropenem RFP NIH-NICHD-2005 -18 released August 2005, submitted October 2005 n Signed September 2007 (24 months) n Protocol in written as part of application; IND granted March 2008 (31 months) n Site contracts, IRB, investigators meeting n First patient June 2008 n 200 infants; last infant enrolled September 2009 16

Lessons Learned Main Contract Timelines n Meropenem RFP release to signature 24 months n Pediatric Trials Network RFP 3/2010, signature 6 months n IND 31 months n IND 7 months n First patient 34 months n First patient 9 months n Last infant 48 months n Last patient 18 months n Clinical Study Report 60 months from RFP release n Clinical study report 22 months (anticipated) 17

Differences in timelines n IRB vs. Contracts n Single entity of PTN mitigates l Risk to NIH l Risk to investigators n Contracts with sites l Opportunistic study n Contracts with vendors 18

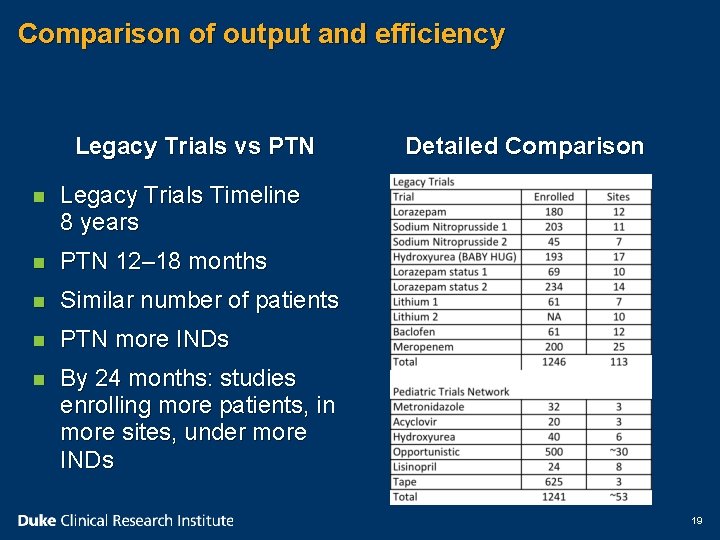
Comparison of output and efficiency Legacy Trials vs PTN n Legacy Trials Timeline 8 years n PTN 12– 18 months n Similar number of patients n PTN more INDs n By 24 months: studies enrolling more patients, in more sites, under more INDs Detailed Comparison 19

Pricing differential n Per patient pricing reduced 30– 50% n Faculty (thought leadership) l Winning a grant, conduct of the grant l Junior faculty l K 23 awardees and young investigators n Operations (staff) efficiency 20

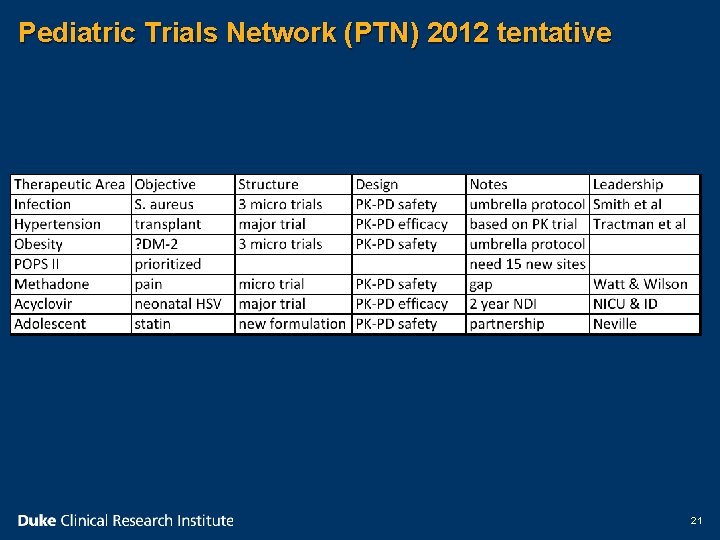
Pediatric Trials Network (PTN) 2012 tentative 21

How Do I Participate in the PTN? n The POPS study: l children interact with the health care system (e. g. , admitted to the PICU or seen in the ER) l on a prioritized off-patent therapeutic that has insufficient dosing information in their clinical stratum age-based: e. g. , premature neonates acuity based: e. g. , resuscitation meds clinical-based: e. g. , ethnicity, obesity l ask for consent to take blood at pre-specified times based on dosing interval (Q 4 vs. Q 24) 22

PTN and POPS Continued n 15 or more therapeutics bundled into one protocol n Samples stored locally and sent in batch n Flexibility to add molecules n Provide preliminary and supportive data for subsequent trials l Compare to epi-data l Metronidazole example n Provide a testing ground for sites—enrollment n Facilitate contracts and infrastructure—enrollment in between more traditional trials 23

Contacting the PTN for the POPS trial n POPS Protocol Chair: Micky Cohen-Wolkowiez michael. cohenwolkowiez@duke. edu n POPS project lead: Barrie Harper barrie. harper@duke. edu n www. pediatrictrials. org 24

Limits of the mechanism Opportunistic PK and PK-PD Safety Efficacy 25
