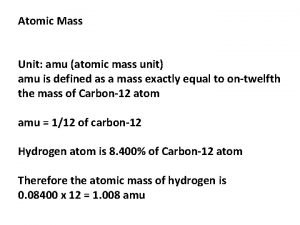Unit 4 Atomic Theory History Greeks n Democritus







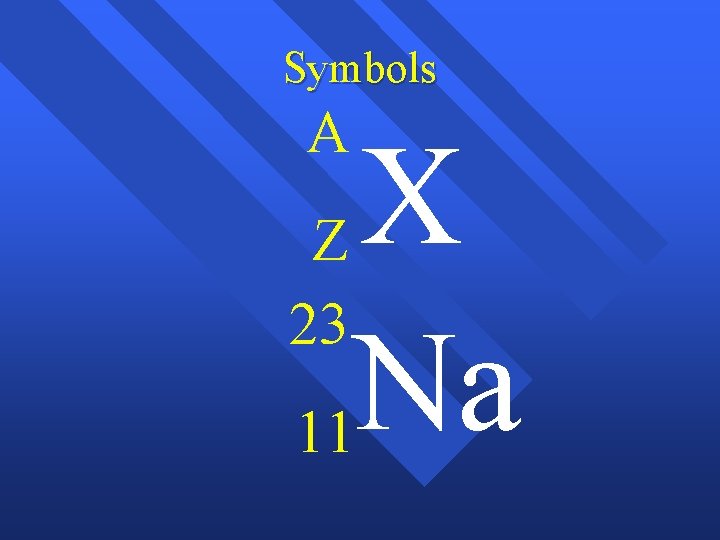





























- Slides: 64

Unit # 4 Atomic Theory

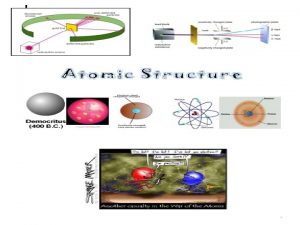
History Greeks n Democritus (460 -370 BC) – Matter is made of atoms. - Different kinds of shapes and sizes n Aristotle ( 364 -322 BC) - didn’t believe of atoms. - - Matter is made of earth, fire, air, and water.

Dalton’s Atomic Theory 1) Elements are made up of atoms 2) Atoms of each element are identical. Atoms of different elements are different. 3) Compounds are formed when atoms combine. Each compound has a specific number and kinds of atom. 4) Chemical reactions are rearrangement of atoms. Atoms are not created or destroyed.

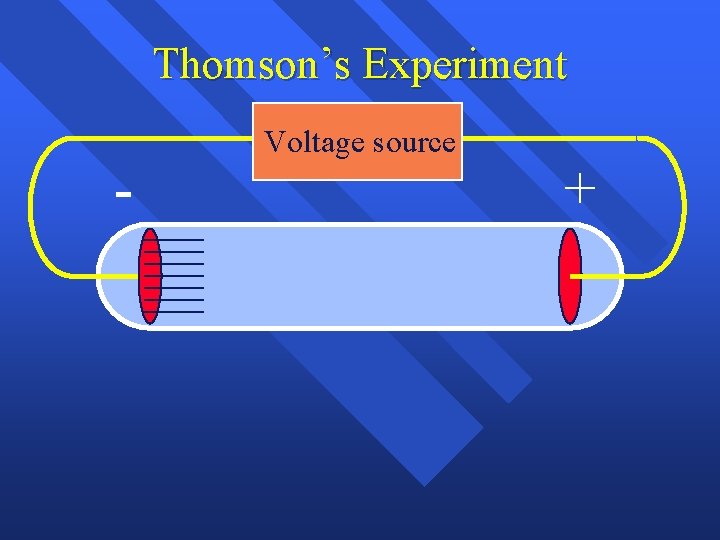
Experiments to determine what an atom was n J. J. Thomson- used Cathode ray tubes

Thomson’s Experiment - Voltage source +

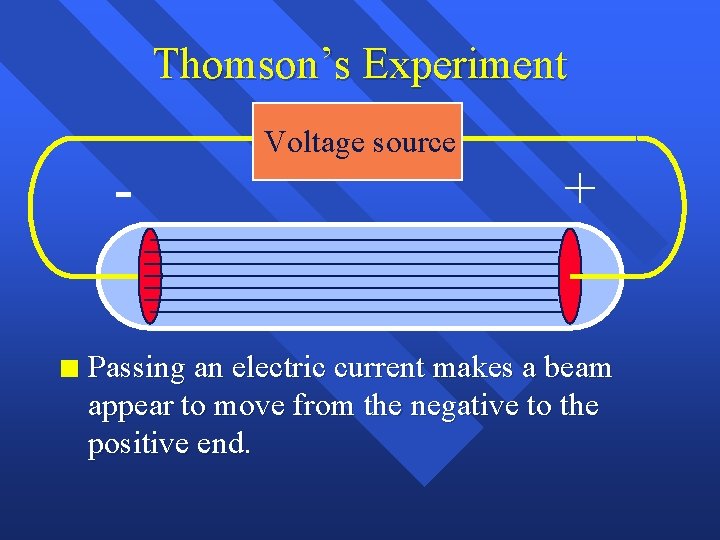
Thomson’s Experiment n Voltage source + Passing an electric current makes a beam appear to move from the negative to the positive end.

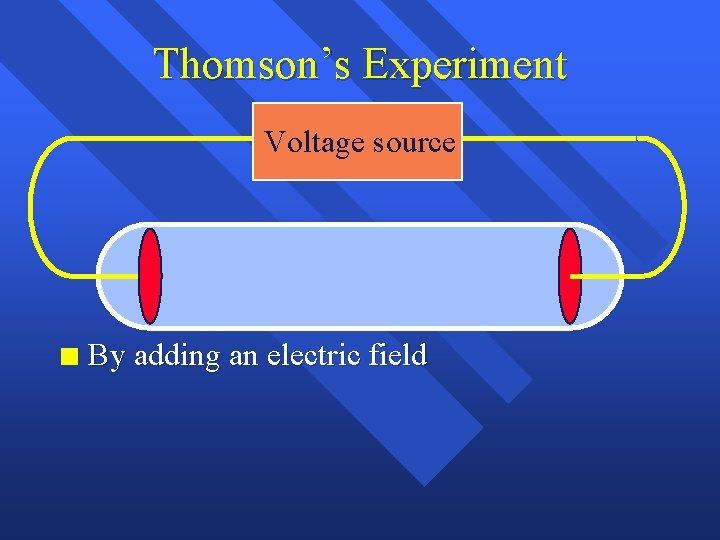
Thomson’s Experiment Voltage source n By adding an electric field

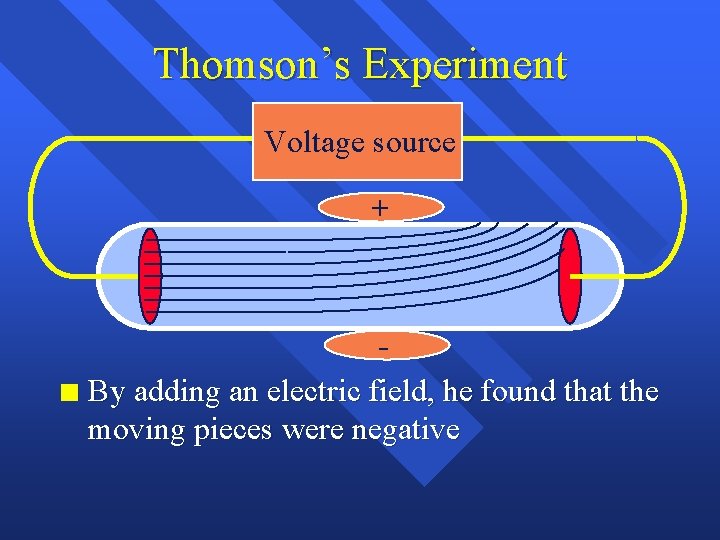
Thomson’s Experiment Voltage source + n By adding an electric field, he found that the moving pieces were negative

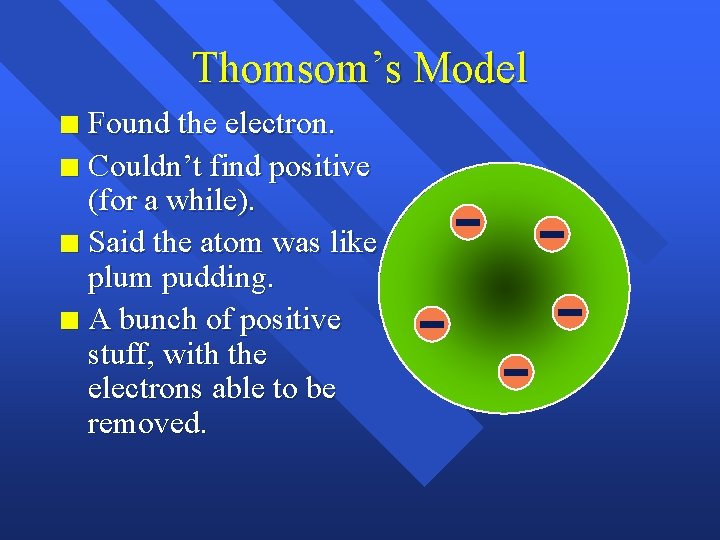
Thomsom’s Model Found the electron. n Couldn’t find positive (for a while). n Said the atom was like plum pudding. n A bunch of positive stuff, with the electrons able to be removed. n

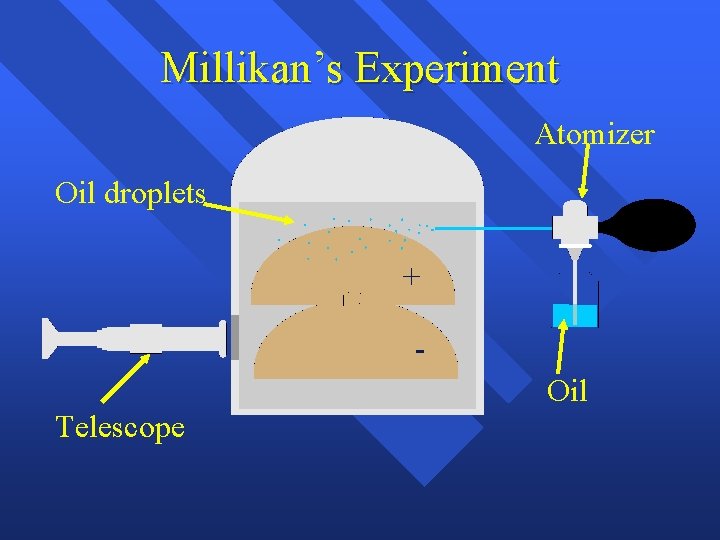
Millikan’s Experiment Atomizer Oil droplets + Oil Telescope

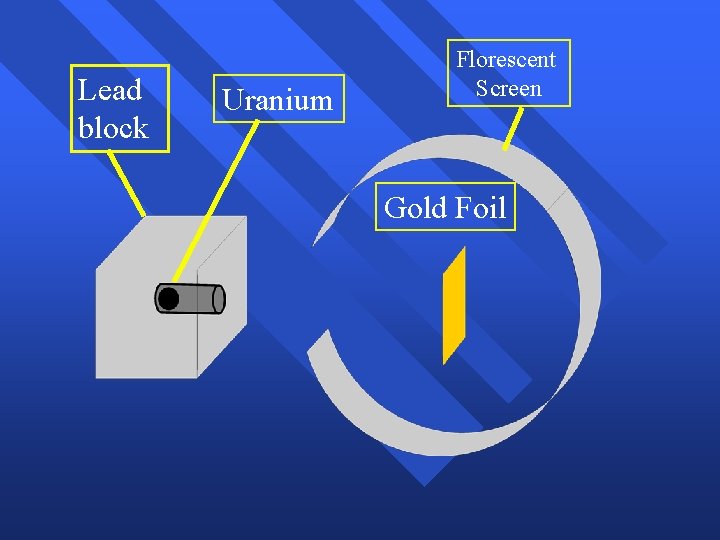
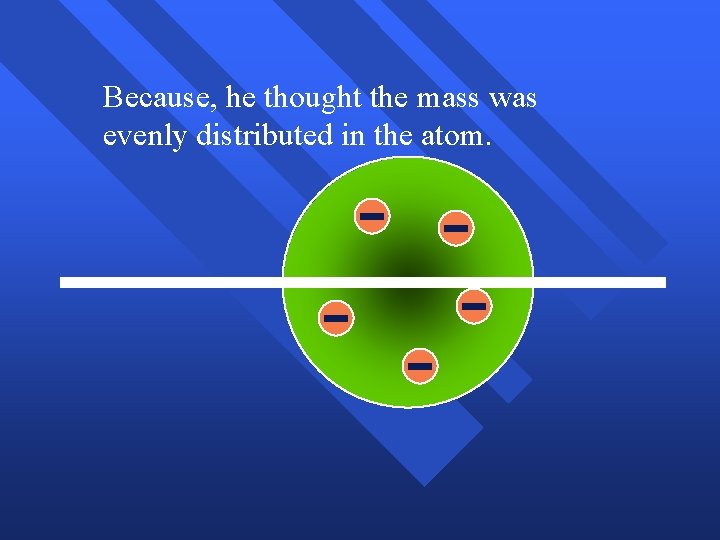
Rutherford’s Experiment Used uranium to produce alpha particles. n Aimed alpha particles at gold foil by drilling hole in lead block. n Since the mass is evenly distributed in gold atoms alpha particles should go straight through. n Used gold foil because it could be made atoms thin. n

Lead block Uranium Florescent Screen Gold Foil

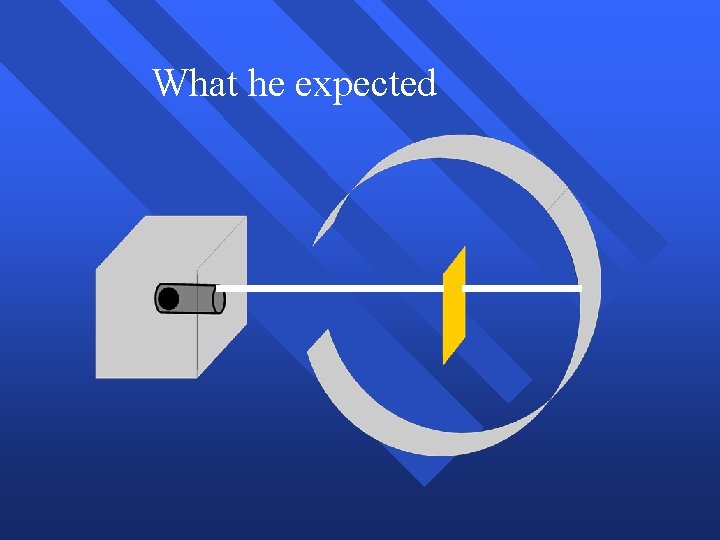
What he expected

Because

Because, he thought the mass was evenly distributed in the atom.

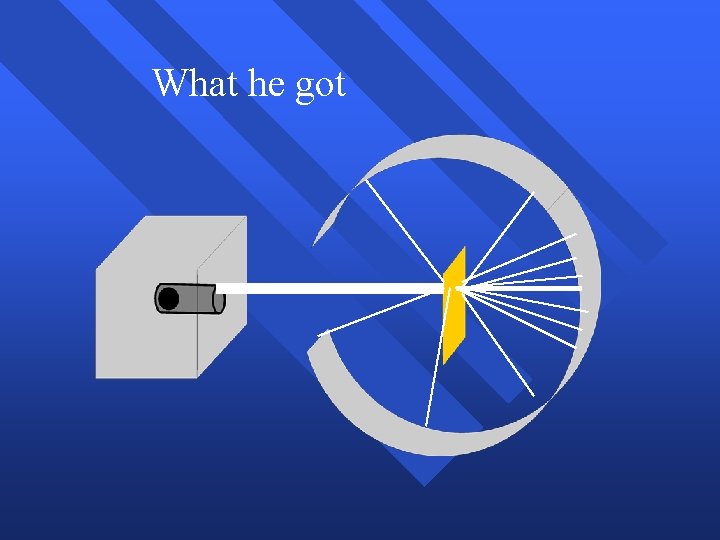
What he got

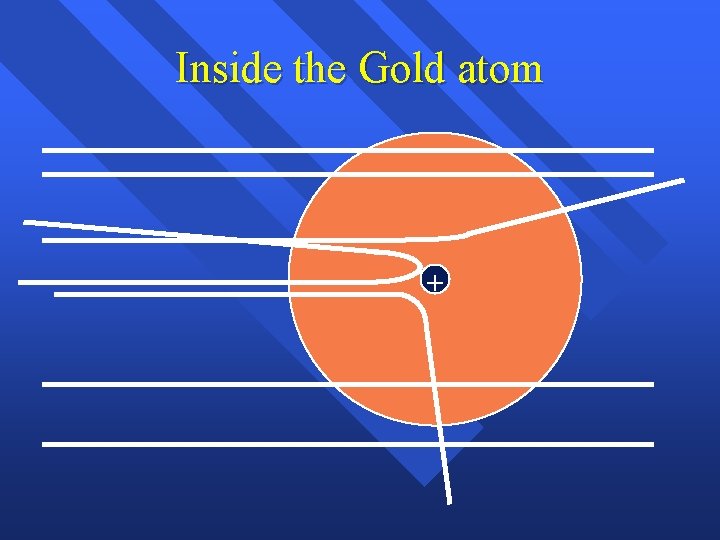
How he explained it Atom is mostly empty n Small dense, positive piece at center. n Alpha particles are deflected by it if they get close enough. n +

Inside the Gold atom +

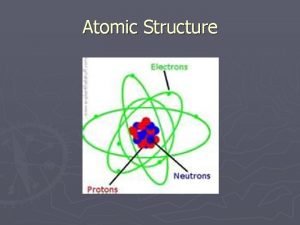
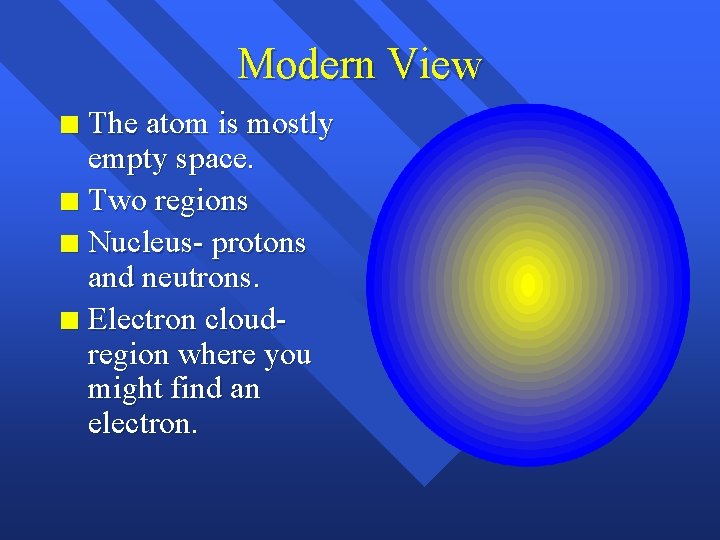
Modern View The atom is mostly empty space. n Two regions n Nucleus- protons and neutrons. n Electron cloudregion where you might find an electron. n

Chadwick 1932 James Chadwick proves the existence of neutrons n He fired protons at Lithium nuclei, splitting them into helium nuclei and releasing energy. n

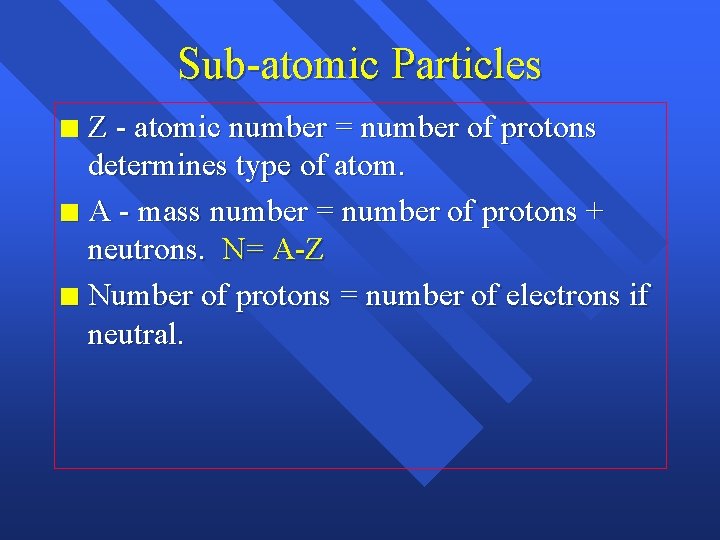
Sub-atomic Particles Z - atomic number = number of protons determines type of atom. n A - mass number = number of protons + neutrons. N= A-Z n Number of protons = number of electrons if neutral. n
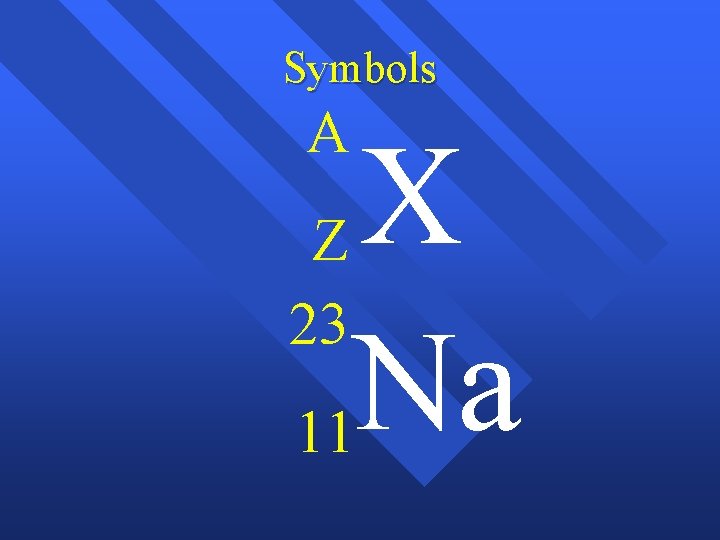
Symbols A X Z 23 Na 11

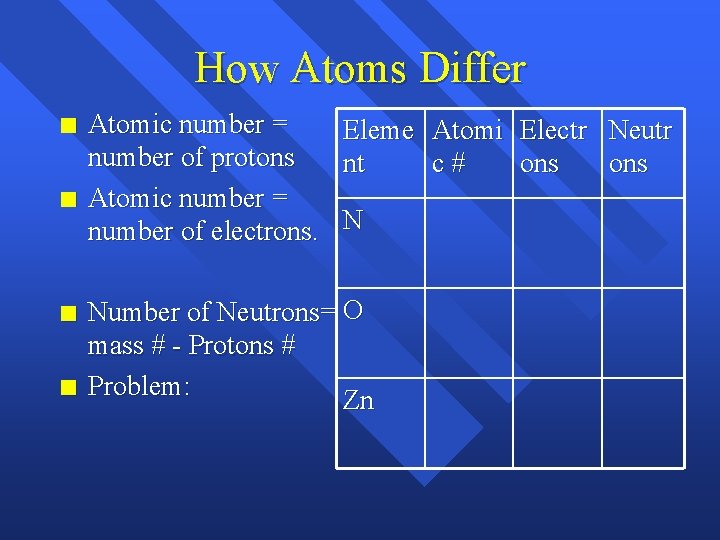
How Atoms Differ n n Atomic number = Eleme Atomi Electr Neutr number of protons nt c# ons Atomic number = number of electrons. N Number of Neutrons= O mass # - Protons # Problem: Zn

Homework 1. 2. 3. How many electrons and protons are in each atom? Radon ; Magnesium An atom contains 66 electrons. Which element is it? An atom of an element contains 14 protons. Which element is it?

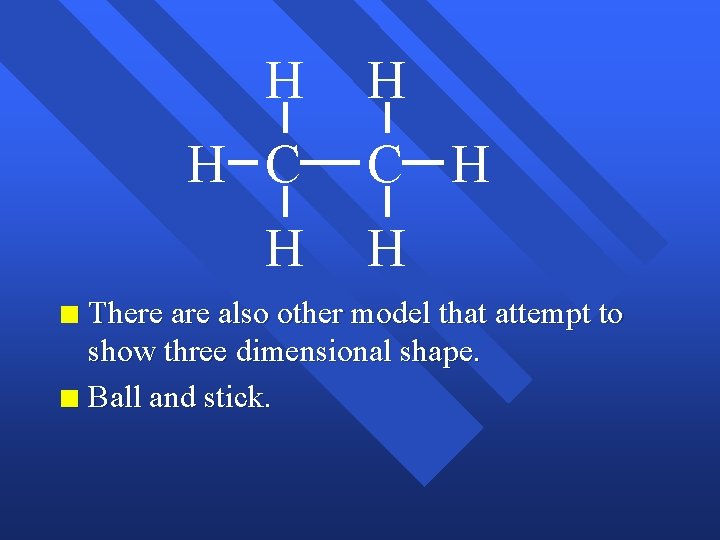
Chemical Bonds The forces that hold atoms together. n Covalent bonding - sharing electrons. n Makes molecules. n Chemical formula- the number and type of atoms in a molecule. n C 2 H 6 - 2 carbon atoms, 6 hydrogen atoms, n n Structural formula shows the connections, but not necessarily the shape.

H H C H H There also other model that attempt to show three dimensional shape. n Ball and stick. n

Ions Atoms or groups of atoms with a charge. n Cations- positive ions - get by losing electrons(s). n Anions- negative ions - get by gaining electron(s). n Ionic bonding- held together by the opposite charges. n Ionic solids are called salts. n

Polyatomic Ions Groups of atoms that have a charge. n Yes, you have to memorize them. n List on page 65 n

Periodic Table

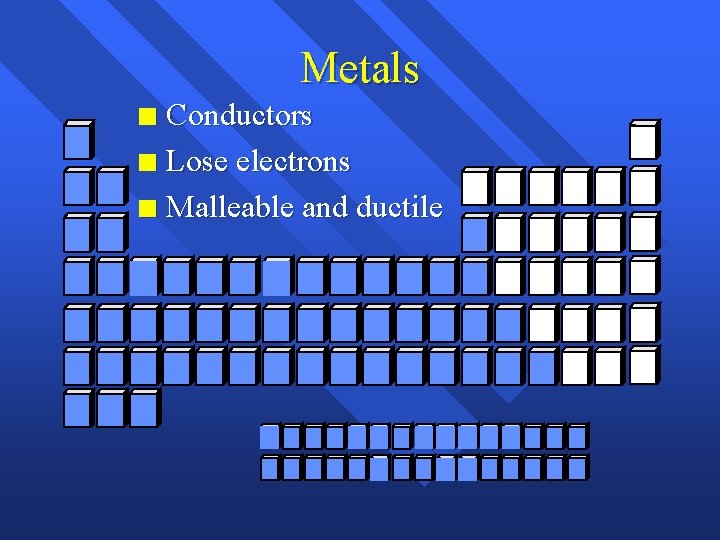
Metals Conductors n Lose electrons n Malleable and ductile n

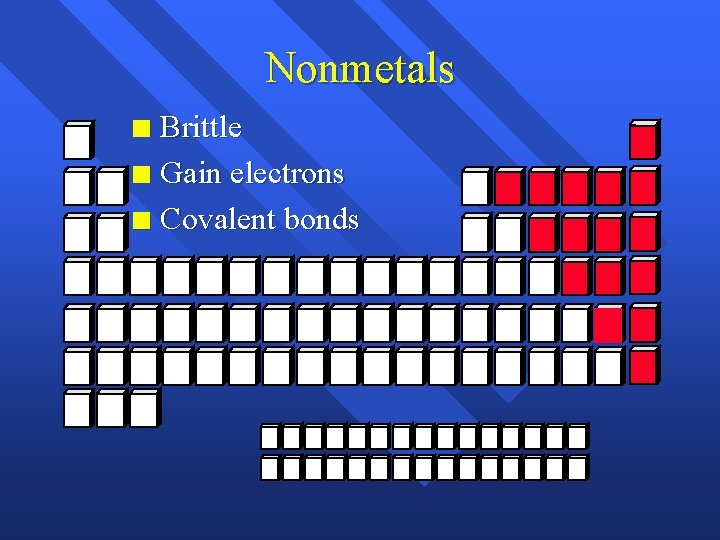
Nonmetals Brittle n Gain electrons n Covalent bonds n

Semi-metals or Metalloids

Alkali Metals

Alkaline Earth Metals

Halogens

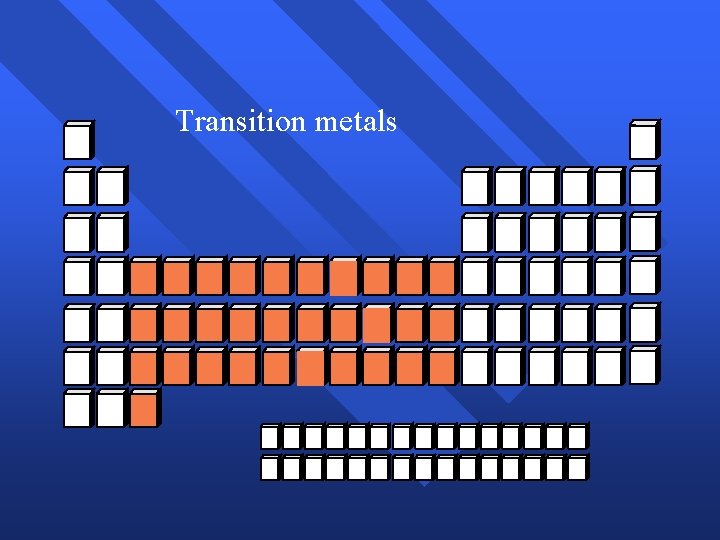
Transition metals

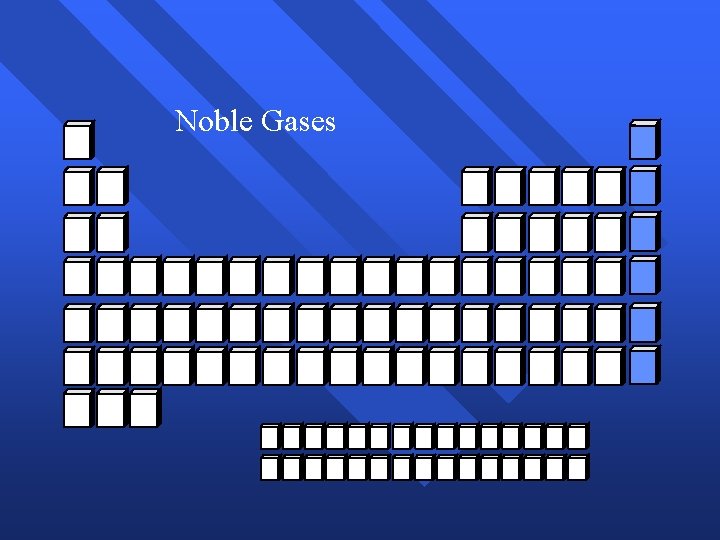
Noble Gases

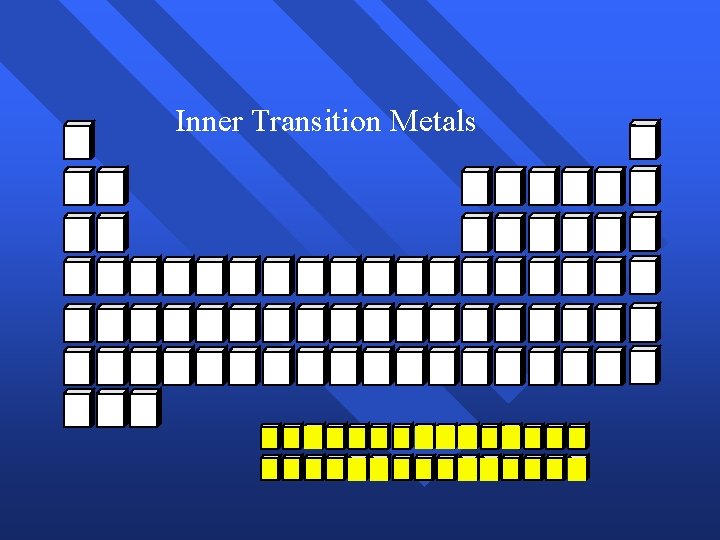
Inner Transition Metals

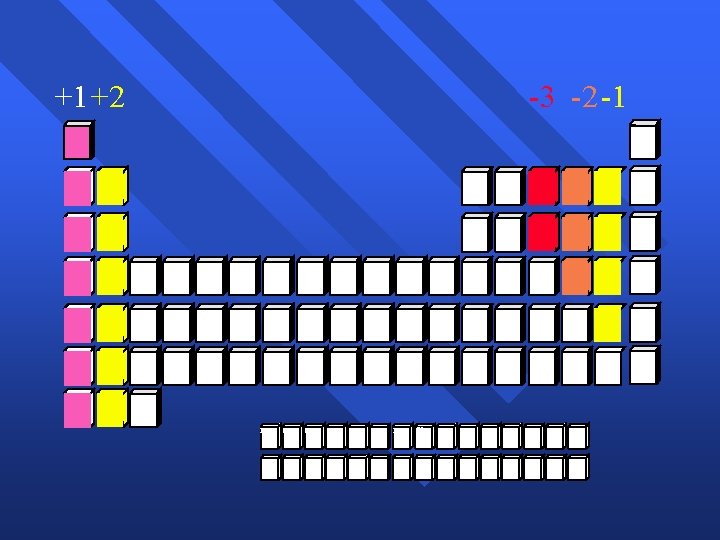
+1+2 -3 -2 -1

Naming compounds Two types n Ionic - metal and non metal or polyatomics. n Covalent- we will just learn the rules for 2 non-metals. n

Ionic compounds If the cation is monoatomic- Name the metal (cation) just write the name. n If the cation is polyatomic- name it. n If the anion is monoatomic- name it but change the ending to –ide. n If the anion is poly atomic- just name it n Practice. n

Covalent compounds Two words, with prefixes. n Prefixes tell you how many. n mono, di, tri, tetra, penta, hexa, septa, nona, deca n First element whole name with the appropriate prefix, except mono. n Second element, -ide ending with appropriate prefix. n Practice n

More Naming

Ionic compounds If the cation is monoatomic- Name the metal (cation) just write the name. n If the cation is polyatomic- name it n If the anion is monoatomic- name it but change the ending to -ide n If the anion is poly atomic- just name it n practice n

Ionic Compounds Have to know what ions they form n off table, polyatomic, or figure it out n Ca. S n K 2 S n n Al. PO 4 K 2 SO 4 n Fe. S n Co. I 3 n

Ionic Compounds n Fe 2(C 2 O 4) n Mg. O n Mn. O n KMn. O 4 n NH 4 NO 3 n Hg 2 Cl 2 n Cr 2 O 3

Ionic Compounds KCl. O 4 n Na. Cl. O 3 n YBr. O 2 n Cr(Cl. O)6 n

Naming Covalent Compounds Two words, with prefixes n Prefixes tell you how many. n mono, di, tri, tetra, penta, hexa, septa, nona, deca n First element whole name with the appropriate prefix, except mono n Second element, -ide ending with appropriate prefix n Practice n

Naming Covalent Compounds CO 2 n CO n CCl 4 n N 2 O 4 n Xe. F 6 n N 4 O 4 n P 2 O 10 n

Writing Formulas Two sets of rules, ionic and covalent n To decide which to use, decide what the first word is. n If is a metal or polyatomic use ionic. n If it is a non-metal use covalent. n

Ionic Formulas Charges must add up to zero. n Get charges from table, name of metal ion, or memorized from the list. n Use parenthesis to indicate multiple polyatomics. n

Ionic Formulas Sodium nitride n sodium- Na is always +1 n nitride - ide tells you it comes from the table n nitride is N-3 n

Ionic Formulas Sodium nitride n sodium- Na is always +1 n Nitride - ide tells you it comes from the table n nitride is N-3 n Doesn’t add up to zero. n +1 Na -3 N

Ionic Formulas Sodium nitride n sodium- Na is always +1 n nitride - ide tells you it comes from the table n nitride is N-3 n Doesn’t add up to zero n Need 3 Na n +1 Na -3 N Na 3 N

Ionic Compounds Sodium sulfite n calcium iodide n Lead (II) oxide n Lead (IV) oxide n Mercury (I) sulfide n Barium chromate n Aluminum hydrogen sulfate n Cerium (IV) nitrite n

Covalent compounds The name tells you how to write the formula n duh n Sulfur dioxide n diflourine monoxide n nitrogen trichloride n diphosphorus pentoxide n

More Names and formulas

Acids Substances that produce H+ ions when dissolved in water. n All acids begin with H. n Two types of acids: n Oxyacids n Non-oxyacids n

Naming acids If the formula has oxygen in it n write the name of the anion, but change – ate to -ic acid – ite to -ous acid n Watch out for sulfuric and sulfurous n H 2 Cr. O 4 n HMn. O 4 n HNO 2 n

Naming acids If the acid doesn’t have oxygen n add the prefix hydron change the suffix -ide to -ic acid n HCl n H 2 S n HCN n

Formulas for acids Backwards from names. n If it has hydro- in the name it has no oxygen n Anion ends in -ide n No hydro, anion ends in -ate or -ite n Write anion and add enough H to balance the charges. n

Formulas for acids hydrofluoric acid n dichromic acid n carbonic acid n hydrophosphoric acid n hypofluorous acid n perchloric acid n phosphorous acid n

Hydrates Some salts trap water crystals when they form crystals. n These are hydrates. n Both the name and the formula needs to indicate how many water molecules are trapped. n In the name we add the word hydrate with a prefix that tells us how many water molecules. n

Hydrates In the formula you put a dot and then write the number of molecules. n Calcium chloride dihydrate = Ca. Cl 2· 2 H 2 O n Chromium (III) nitrate hexahydrate = Cr(NO 3)3· 6 H 2 O n
 Democritus theory of the universe
Democritus theory of the universe Democritus
Democritus Democritus (400 bc) atomic model
Democritus (400 bc) atomic model Niels bohr atomic theory timeline
Niels bohr atomic theory timeline Atomic model timeline project
Atomic model timeline project Fifth edition chemistry a molecular approach
Fifth edition chemistry a molecular approach Democritus atomic model
Democritus atomic model Teori atom democritus
Teori atom democritus History of the atom
History of the atom In what forms of writing did the greeks excel
In what forms of writing did the greeks excel The greeks based their ideal of beauty on
The greeks based their ideal of beauty on Why did the tyrants fall out of favor
Why did the tyrants fall out of favor French baroque floral design
French baroque floral design Romans and greeks
Romans and greeks Middle ages floral arrangements
Middle ages floral arrangements Ancient greece values
Ancient greece values Above all else i must be saved
Above all else i must be saved The word theatre comes from
The word theatre comes from Geocentric theory
Geocentric theory Beware of greeks bearing gifts
Beware of greeks bearing gifts Draw segment sr the bisector of the vertex angle prq
Draw segment sr the bisector of the vertex angle prq Option greeks wikipedia
Option greeks wikipedia Is atomic mass and relative atomic mass the same
Is atomic mass and relative atomic mass the same Periodic trends in properties of elements
Periodic trends in properties of elements Trend of ionic radius
Trend of ionic radius Abundance calculation chemistry
Abundance calculation chemistry Atomic
Atomic Atomic number vs atomic radius
Atomic number vs atomic radius History of atomic models
History of atomic models Atomic absorption spectroscopy history
Atomic absorption spectroscopy history Atomic structure webquest
Atomic structure webquest Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test Democritus atom model
Democritus atom model Democritus contribution
Democritus contribution Aristotle atomic structure
Aristotle atomic structure Kelemahan teori dalton
Kelemahan teori dalton 460 bc atomic structure
460 bc atomic structure Democritus milky way
Democritus milky way Democritus and dalton
Democritus and dalton Democritus
Democritus Democritus atom modeli
Democritus atom modeli Democritus periodic table
Democritus periodic table Teori atom democritus
Teori atom democritus Democritus atom modeli
Democritus atom modeli Gold foil experiment
Gold foil experimentHenry moseley atomic theory timeline
 Democritus 400 bc
Democritus 400 bc Dalton thomson rutherford bohr schrodinger
Dalton thomson rutherford bohr schrodinger What did democritus propose
What did democritus propose Teori atom democritus
Teori atom democritus Democritus
Democritus Greek philosopher democritus
Greek philosopher democritus Democritus 460 bc
Democritus 460 bc How did democritus characterize atoms?
How did democritus characterize atoms? Teori atom democritus
Teori atom democritus Democritus greek model
Democritus greek model Democritus atomos
Democritus atomos Democritus atom modeli
Democritus atom modeli Democritus believed
Democritus believed Atomic mass unit
Atomic mass unit Moles of atoms
Moles of atoms Atomic mass unit
Atomic mass unit Schrodinger
Schrodinger Atomic unit of work in hibernate
Atomic unit of work in hibernate What is amu
What is amu