Democritus atomos DALTON JJ THOMSON RUTHERFORD Atom theory











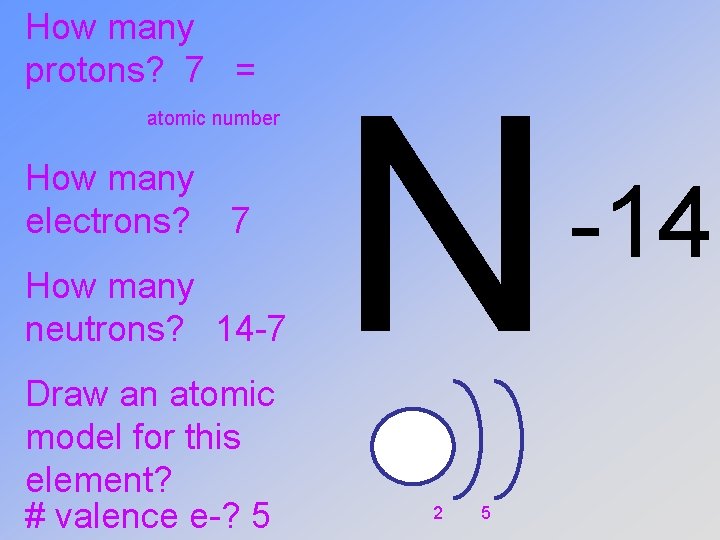





















- Slides: 59

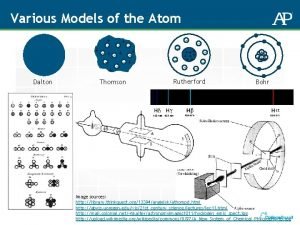
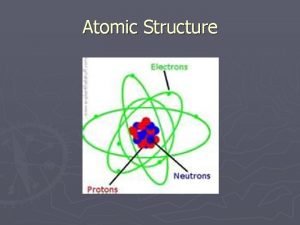
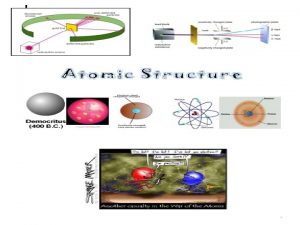
Democritus “atomos” DALTON JJ THOMSON RUTHERFORD Atom theory with hard spheres Subatomic particles Atoms mostly empty space Electron (mass and – charge) Dense nucleus in center Atomic Theory Development Experimental evidence Law of definite proportions BOHR de BROGLIE e- energy levels e- move in waves within energy levels Proton + charge CHADWICK neutron

Who first coined the term atom? 1. 2. 3. 4. 5. Dalton Democritus Rutherford Bohr Thomson

Who stated that elements are made of atoms? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

Who used experimental results and the Law of Definite Proportions to develop their atomic theory? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

Who discovered the atom was divisible since it had subatomic particles? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

Who discovered the electron? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

Who discovered that atoms are mostly empty space? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

Who discovered that atoms have a dense centrally located nucleus in atoms ? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

Who discovered the proton in the nucleus? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

Who discovered the neutron? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

Who stated that electrons are located in fixed energy levels? 1. 2. 3. 4. 5. Dalton Chadwick Rutherford Bohr Thomson

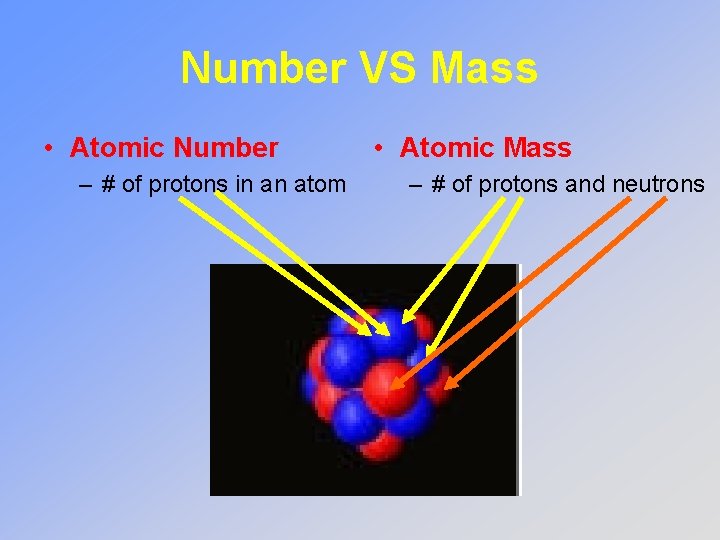
Number VS Mass • Atomic Number – # of protons in an atom • Atomic Mass – # of protons and neutrons

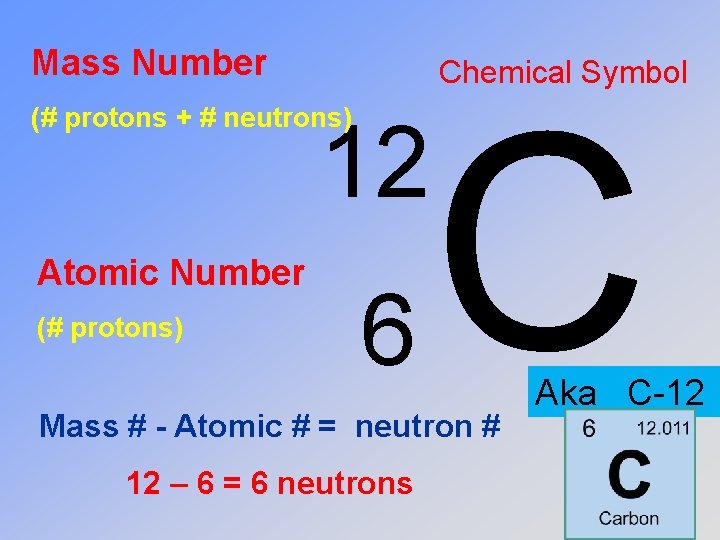
Mass Number Chemical Symbol (# protons + # neutrons) 12 Atomic Number (# protons) 6 C Mass # - Atomic # = neutron # 12 – 6 = 6 neutrons Aka C-12

Stop on yellow slides allowing students to work out all parts before going to answer slides

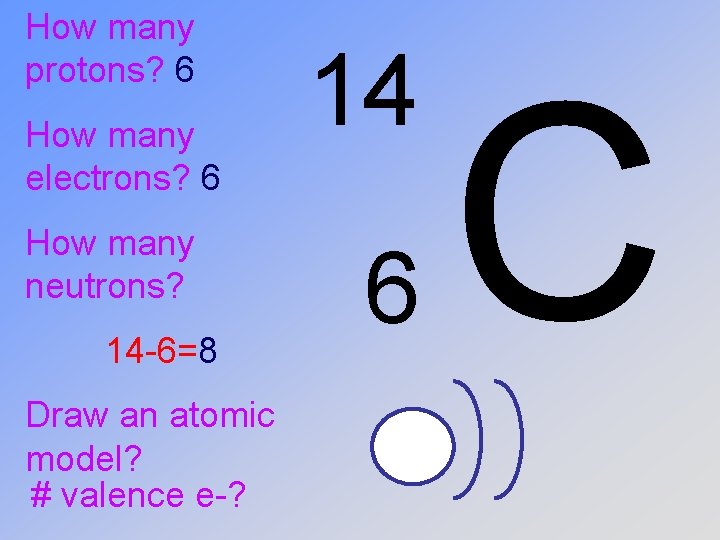
How many protons? How many electrons? How many neutrons? Draw an atomic model for this element? 14 6 C

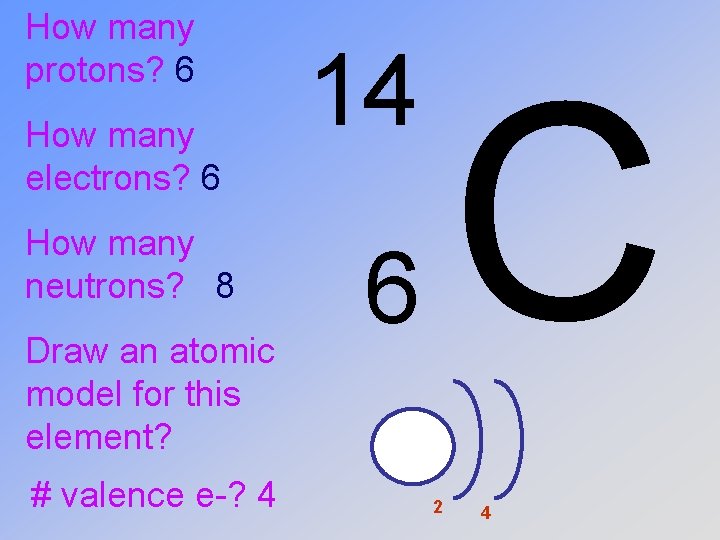
How many protons? 6 How many electrons? How many neutrons? Draw an atomic model for this element? 14 6 C

How many protons? 6 How many electrons? 6 How many neutrons? Draw an atomic model for this element? 14 6 C

How many protons? 6 How many electrons? 6 How many neutrons? 14 -6=8 Draw an atomic model? # valence e-? 14 6 C

How many protons? 6 How many electrons? 6 How many neutrons? 8 Draw an atomic model for this element? # valence e-? 4 14 C 6 2 4

How many protons? How many electrons? How many neutrons? Draw an atomic model for this element? # valence e-? 7 3 Li

How many protons? 3 How many electrons? 3 How many neutrons? 4 Draw an atomic model for this element? # valence e-? 1 7 3 Li 2 1

How many protons? How many electrons? How many neutrons? Draw an atomic model for this element? # valence e-? N -14
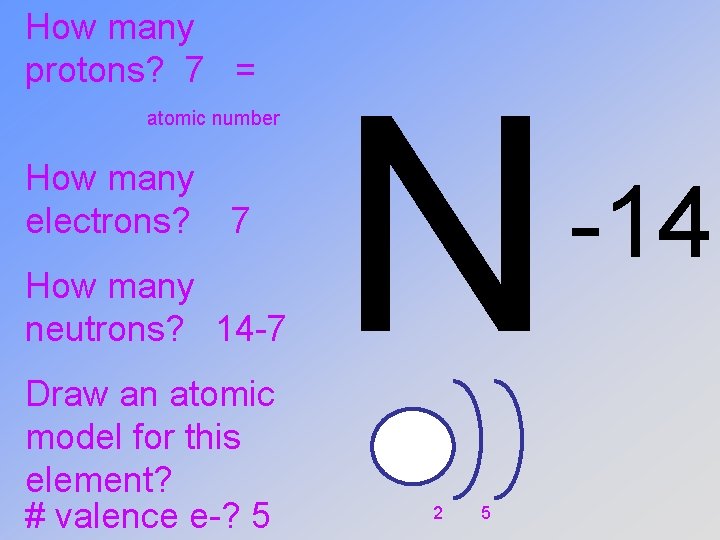
How many protons? 7 = atomic number How many electrons? 7 How many neutrons? 14 -7 Draw an atomic model for this element? # valence e-? 5 N 2 5 -14

How many protons? How many electrons? How many neutrons? Draw an atomic model for this element? # valence e-? use your periodic table Be

How many protons? 4 How many electrons? 4 How many neutrons? use your periodic table Be 9 -4 = 5 Draw an atomic model? # valence e-? 2 2 2

How many protons? How many electrons? How many neutrons? Draw an atomic model for this element? # valence e-? use your periodic table For atomic number 20

How many protons? 20 How many electrons? 20 How many neutrons? 20 use your periodic table For atomic number 20 Draw an atomic model for this element? # valence e-? 2 2 8 8 2

How many protons? How many neutrons? 238 U

How many protons? 92 How many neutrons? 146 238 U

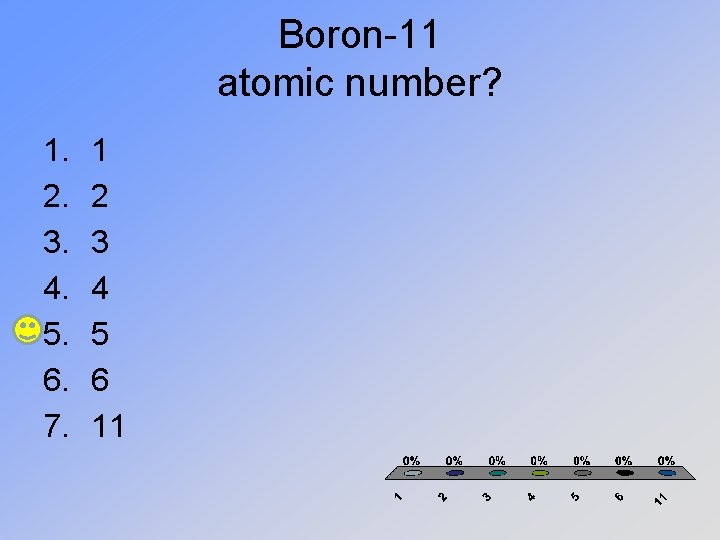
Boron-11 atomic number? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Boron-11 number of protons? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Boron-11 number of electrons? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Boron-11 number of neutrons? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Boron-11 number of energy levels? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

1. 2. 3. 4. 5. 6. 7. Boron-11 number of electrons in the first energy level? 1 2 3 4 5 6 11

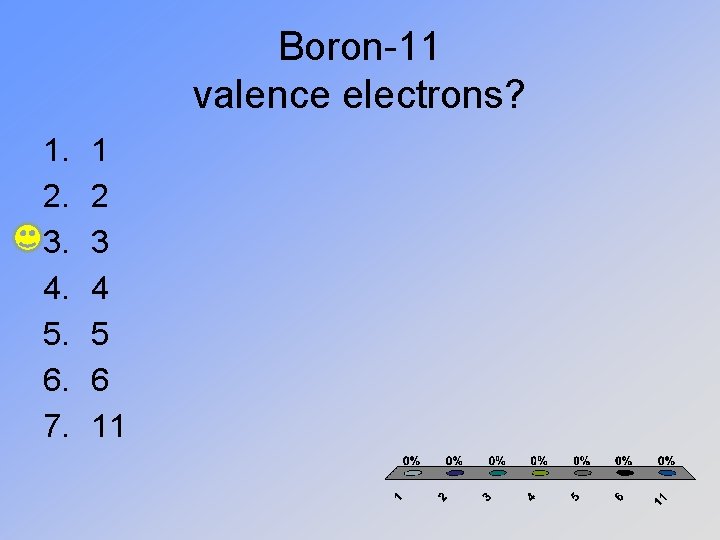
Boron-11 valence electrons? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Boron-11 most similar to ? 1. 2. 3. 4. C Be Si Al

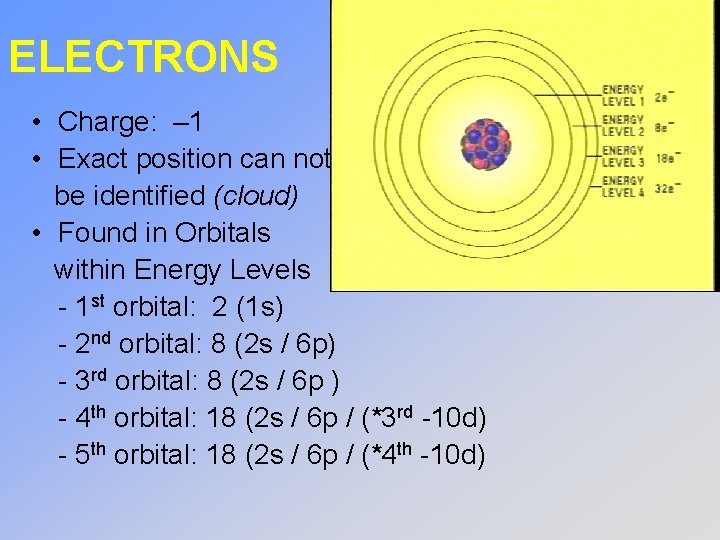
ELECTRONS • Charge: – 1 • Exact position can not be identified (cloud) • Found in Orbitals within Energy Levels - 1 st orbital: 2 (1 s) - 2 nd orbital: 8 (2 s / 6 p) - 3 rd orbital: 8 (2 s / 6 p ) - 4 th orbital: 18 (2 s / 6 p / (*3 rd -10 d) - 5 th orbital: 18 (2 s / 6 p / (*4 th -10 d)

Complete the energy levels … • # of Protons? 7 • # electrons? 7 2 • # e’ in orbital? • # e; in 2 nd orbital? 5 • # of valence e’ ? 5 1 st NITROGEN

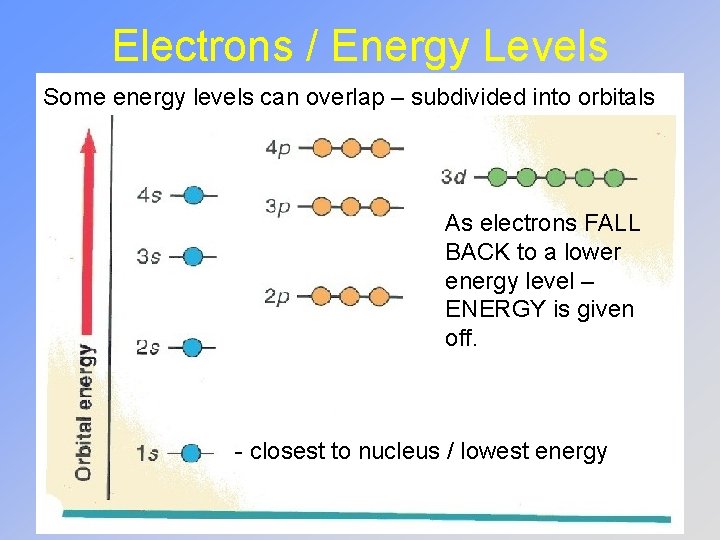
Electrons / Energy Levels Some energy levels can overlap – subdivided into orbitals As electrons FALL BACK to a lower energy level – ENERGY is given off. - closest to nucleus / lowest energy

• Imagine floor is magnetic and shoes repel. Paper could pass under shoes…. • Sub microscopic world operates this way only it is ELECTRIC, not magnetic! • Special Cases: nucleus of two atoms touch= thermal nuclear reaction. FISSION / FUSION

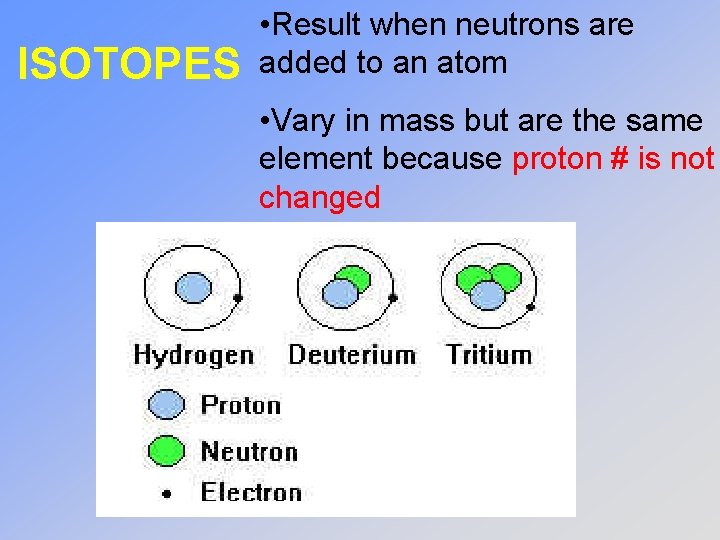
ISOTOPES • Result when neutrons are added to an atom • Vary in mass but are the same element because proton # is not changed

Atomic Mass Unit • AMU – Is equal to 1/12 th of the mass of a carbon atom – 1 AMU = isotope of carbon ( 6 protons / 6 neutrons) • Average atomic mass – weighted avg.

Center of a atom, contains most of the atom’s mass 1. 2. 3. 4. 5. 6. 7. Proton Neutron Electron Nucleus Energy levels Mass number Electromagnetic force

Positively charged particle that exists in the nucleus of an atom. 1. 2. 3. 4. 5. 6. 7. Proton Neutron Electron Nucleus Energy levels Mass number Eelctromagnetic force

The least massive of the three subatomic particles which also carries an negative net charge. 1. 2. 3. 4. 5. 6. 7. Proton Neutron Electron Nucleus Energy levels Mass number Electromagnetic force

Particle with no charge that exists in the nucleus of an atom 1. 2. 3. 4. 5. 6. 7. Proton Neutron Electron Nucleus Energy levels Mass number Electromagnetic force

Describes how electrons are arranged around an atom 1. 2. 3. 4. 5. 6. 7. Proton Neutron Electron Nucleus Energy levels Mass number Electromagnetic force

The force that holds the atom together is called 1. 2. 3. 4. 5. 6. 7. Proton Neutron Electron Nucleus Energy levels Mass number Electromagnetic force

The total number of protons and neutrons in the nucleus of an atom 1. 2. 3. 4. 5. 6. 7. Proton Neutron Electron Nucleus Energy levels Mass number Electromagnetic force

Na atomic number? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Na-23 number of protons? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Na number of electrons? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Na number of neutrons? 1. 2. 3. 4. 5. 6. 7. 10 12 3 2 5 6 11

Na number of energy levels? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

1. 2. 3. 4. 5. 6. 7. Na number of electrons in the first energy level? 1 2 3 4 6 8 11

Na number of electrons in the second energy level? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 6 8 11

Na valence electrons? 1. 2. 3. 4. 5. 6. 7. 1 2 3 4 5 6 11

Na most similar to ? 1. Ne 2. K 3. Mg
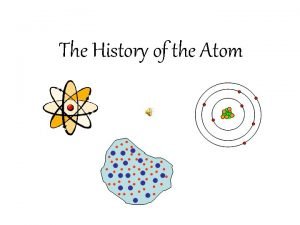
 Dalton thomson rutherford bohr schrodinger
Dalton thomson rutherford bohr schrodinger Dalton thomson rutherford
Dalton thomson rutherford Dalton thomson rutherford bohr
Dalton thomson rutherford bohr Teori atom thomson
Teori atom thomson Dalton atom modeli
Dalton atom modeli History of the atom graphic organizer
History of the atom graphic organizer Democritus atomos
Democritus atomos Empedocles atomic theory
Empedocles atomic theory Teori atom democritus
Teori atom democritus Kelemahan atom rutherford
Kelemahan atom rutherford Thomson e rutherford
Thomson e rutherford Thomson rutherford
Thomson rutherford Teori atom democritus
Teori atom democritus Democritus atomic theory
Democritus atomic theory Teori atom democritus
Teori atom democritus Niels bohr atomic theory timeline
Niels bohr atomic theory timeline What scientist discovered the electron
What scientist discovered the electron Democritus atom modeli
Democritus atom modeli ü
ü Democritus atomic theory
Democritus atomic theory Democritus atom modeli
Democritus atom modeli Teori atom democritus
Teori atom democritus Teori atom democritus
Teori atom democritus Gzle
Gzle Dalton atom modeli maket
Dalton atom modeli maket Bunyi atom bohr
Bunyi atom bohr Rhuterford
Rhuterford Atom jj thomson
Atom jj thomson Atom jj thomson
Atom jj thomson Atom jj thomson
Atom jj thomson Model atom thomson
Model atom thomson Atom thomson gambar
Atom thomson gambar Model atom thomson
Model atom thomson Thomson atom modeli
Thomson atom modeli Kelebihan atom rutherford
Kelebihan atom rutherford Thomson atom modeli
Thomson atom modeli Thomson atom modeli
Thomson atom modeli 1808 dalton
1808 dalton Thomson atom modeli eksiklikleri
Thomson atom modeli eksiklikleri Dalton atom modeli
Dalton atom modeli Dalton atom modeli maketi
Dalton atom modeli maketi Kelebihan atom john dalton
Kelebihan atom john dalton History of the atom john dalton
History of the atom john dalton Teori atom dalton
Teori atom dalton Dalton atom modeli üzümlü kek
Dalton atom modeli üzümlü kek John dalton atom modeli
John dalton atom modeli Dalton atom modelleri
Dalton atom modelleri Dalton atom modelleri
Dalton atom modelleri Dot
Dot Drawbacks of rutherford model of atom
Drawbacks of rutherford model of atom Draw/describe a model for rutherford's atom.
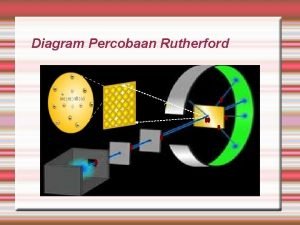
Draw/describe a model for rutherford's atom. Rutherford pictured the atom as a dense
Rutherford pictured the atom as a dense Perkembangan model atom
Perkembangan model atom E rutherford atom modeli
E rutherford atom modeli Democritus 400 bc
Democritus 400 bc Atomic theory timeline
Atomic theory timeline Jj thomson atomic theory
Jj thomson atomic theory Jj thomson theory
Jj thomson theory The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom John daltons atom
John daltons atom