Terapia ipoglicemizzante in pazienti con SCA Alessandro Scorsone

































- Slides: 69

Terapia ipoglicemizzante in pazienti con SCA Alessandro Scorsone ASP 6 Palermo PO Civico Partinico, Centro di Riferimento Regionale per il Diabete e l’impianto dei microinfusori Partinico. Direttore Vincenzo Provenzano

LA GESTIONE DEL PAZIENTE DIABETICO CON CARDIOPATIA ISCHEMICA RECENTE Quali farmaci per trattare l’iperglicemia…. • dopo l’evento acuto



Nei pazienti con SCA, l’uso della terapia insulinica può essere protratto con efficacia e sicurezza fino a tre mesi dopo la dimissione e comunque per tutto il tempo necessario a raggiungere e stabilizzare un buon compenso glicemico anche nei diabetici di tipo 2 (20).

• Quale monitoraggio seguire durante il ricovero ospedaliero? • Chi dovrebbe gestire il paziente cardiologo/diabetologo/infermiere?

Proposta AMD per la Continuità delle Cure

Cause dell’aumento di mortalità e aumento di ospedalizzazione nel paziente diabetico post SCA • Scarsa aderenza alla terapia farmacologica • Mancato cambiamento dello stile di vita • Insufficiente presa in carico dei pazienti post SCA

Organizzazione del FOLLOW-UP Al paziente dimesso dal centro ospedaliero verrà consegnata la lettera di dimissione contenente: informazioni cliniche; risultati dei principali esami eseguiti durante il ricovero; dati sulla stratificazione del rischio cardio-metabolico Quanto è importante l’utilizzo di una cartella clinica condivisa ? Quanto è importante l’utilizzo di una cartella informatizzata?

LETTERA DI DIMISSIONI: CONTENUTI MINIMI Dati anagrafici Generalità del medico curante Diagnosi principale e secondarie, con evidenza delle comorbilità e della presenza di device Obiettività cardiopolmonare all’ingresso e dimissione PA e Peso corporeo Copia dell’ecg alla dimissione Variazioni significative dei parametri ematochimici (funzionalità renale, tiroidea ed epatica, emoglobina, BNP, troponina) Copia o referti degli esami strumentali eseguiti Decorso clinico con terapia somministrata Terapia farmacologica da assumere con dosaggio, modalità e durata Indicazione dei target da raggiungere in termini di pressione arteriosa, frequenza cardiaca, LDL, emoglobina glicata Modifica dello stile di vita con particolare riferimento alle attività consentite e/o vietate Controlli successivi consigliati o programmati

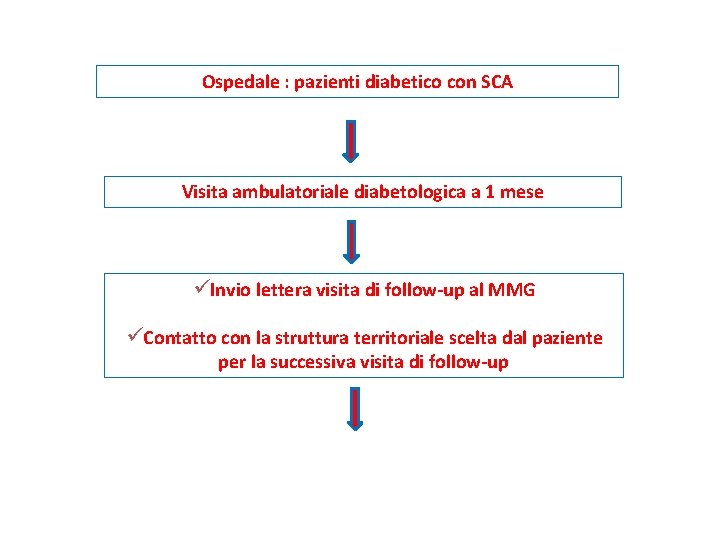
Ospedale : pazienti diabetico con SCA Visita ambulatoriale diabetologica a 1 mese üInvio lettera visita di follow-up al MMG üContatto con la struttura territoriale scelta dal paziente per la successiva visita di follow-up

RUOLO DELL’INFERMIERE PROFESSIONALE: nuovi scenari L’Infermiere Professionale svolge un ruolo di notevole importanza nel creare il collegamento tra il Paziente e le varie figure coinvolte nel progetto, divenendo attore fondamentale per assicurare la continuità assistenziale. L’Infermiere Territoriale sarà contattato dopo la dimmissione dagli infermieri dei centri ospedalieri per fissare gli appuntamenti per le visite specialistiche successive. Gli infermieri ospedalieri e territoriali saranno coinvolti nell’attività di counseling, a rinforzo di quanto già effettuato in ospedale e presso il Medico di Medicina Generale. Costituiranno un nodo di collegamento permanente tra il Paziente e la sua famiglia da un lato e il Cardiologo/Diabetologo Ospedaliero, il Diabetologo Territoriale e il Medico di Medicina Generale dall’altro. Contatteranno i pazienti che non si presentano agli appuntamenti stabiliti, chiedendone le motivazioni e favorendo il ripristino dei controlli. In caso di eventi avversi comunicheranno allo specialista l’accaduto.

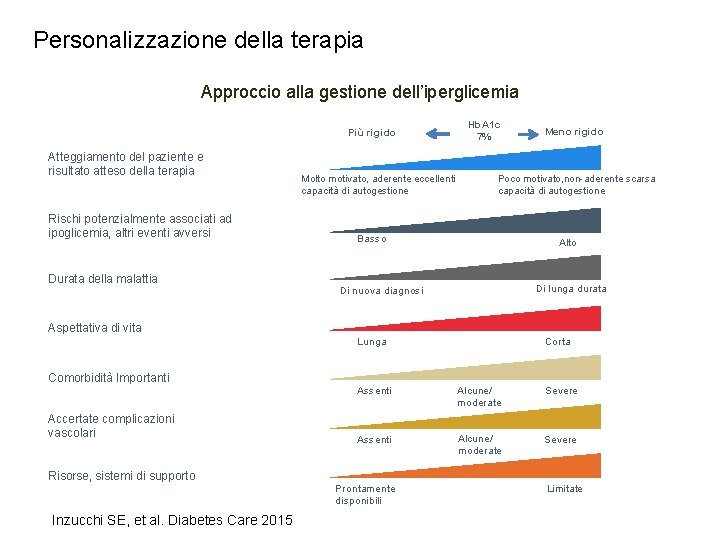
Personalizzazione della terapia Approccio alla gestione dell’iperglicemia Più rigido Atteggiamento del paziente e risultato atteso della terapia Rischi potenzialmente associati ad ipoglicemia, altri eventi avversi Durata della malattia Molto motivato, aderente eccellenti capacità di autogestione Hb. A 1 c 7% Meno rigido Poco motivato, non-aderente scarsa capacità di autogestione Basso Alto Di lunga durata Di nuova diagnosi Aspettativa di vita Lunga Comorbidità Importanti Accertate complicazioni vascolari Risorse, sistemi di supporto Inzucchi SE, et al. Diabetes Care 2015 Corta Assenti Alcune/ moderate Severe Prontamente disponibili Limitate


Sulfonylureas

SU and Heart • Tutte le SU (glimepiride compresa) riducono il flusso coronarico a riposo (Legtenberg R 1999 e 2001) • Possibile riduzione del sopraslivellamento del tratto ST nei pazienti in trattamento con SU (Huizar 2003) • Dati contrastanti su un possibile effetto proaritmico della glibenclamide (Najeed S 2002, Dhein S 2000) • Effetti negativi della glibenclamide sulla contrattilità ventricolare nel coniglio in confronto alla metformina (Ren J 1999) e nell’uomo con DM 2 in confronto all’insulina (Scognamiglio R 2002)

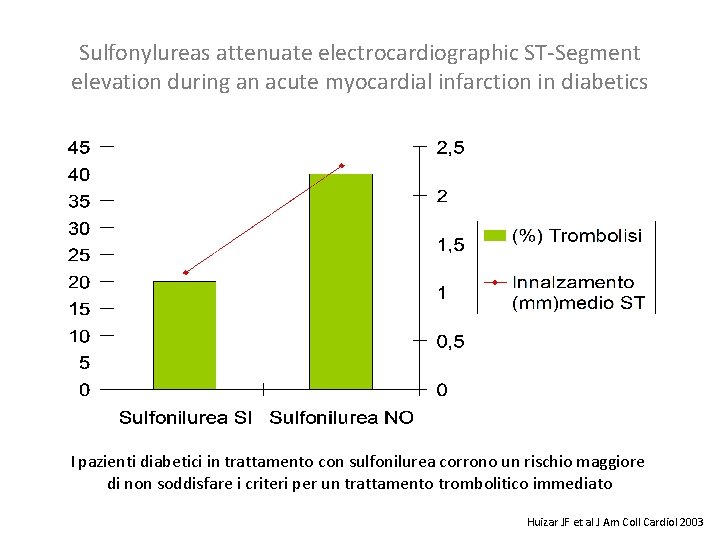
Sulfonylureas attenuate electrocardiographic ST-Segment elevation during an acute myocardial infarction in diabetics I pazienti diabetici in trattamento con sulfonilurea corrono un rischio maggiore di non soddisfare i criteri per un trattamento trombolitico immediato Huizar JF et al J Am Coll Cardiol 2003

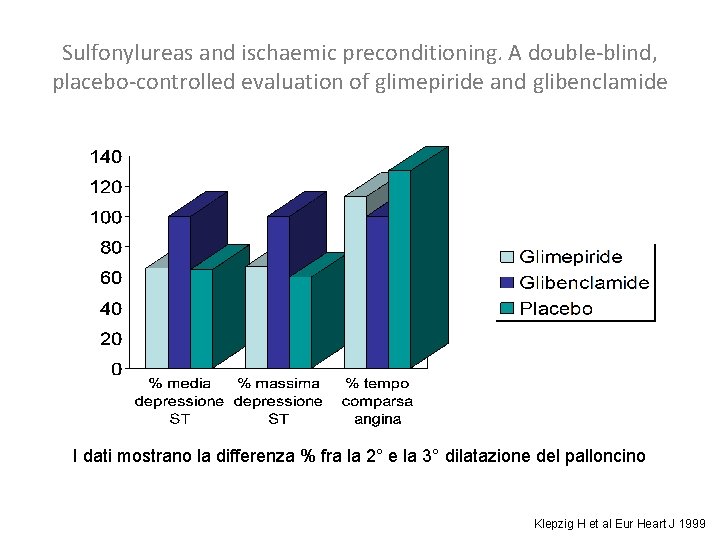
SU e precondizionamento ischemico • Le SU inibiscono i canali del potassio ATP sensibili con depolarizzazione cellulare ed innalzamento del calcio intracellulare (Terzic A 1995, Ashcroft S 1992) • In questo modo bloccano il precondizionamento (Engler R 1996, Cleveland J 1997, Tomai F 1994) • Cuori isolati di animali: le SU, tranne la glimepiride, annullano il precondizionamento, particolarmente la glibenclamide (Horimoto H 2002, Gribble F 1998, Legtenberg R 2001, Mocanu M 2001, Nieszner E 2002) • Occlusione sperimentale acuta di una coronaria nell’uomo, confronto doppio cieco fra placebo, glibenclamide e glimepiride: la glibenclamide sopprime il precondizionamento ischemico (Klepzig H 1999)

Sulfonylureas and ischaemic preconditioning. A double-blind, placebo-controlled evaluation of glimepiride and glibenclamide I dati mostrano la differenza % fra la 2° e la 3° dilatazione del palloncino Klepzig H et al Eur Heart J 1999

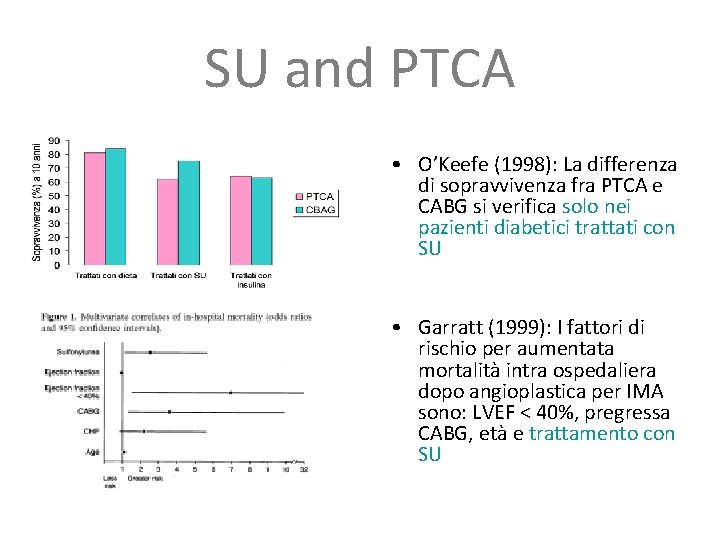
SU and PTCA • O’Keefe (1998): La differenza di sopravvivenza fra PTCA e CABG si verifica solo nei pazienti diabetici trattati con SU • Garratt (1999): I fattori di rischio per aumentata mortalità intra ospedaliera dopo angioplastica per IMA sono: LVEF < 40%, pregressa CABG, età e trattamento con SU

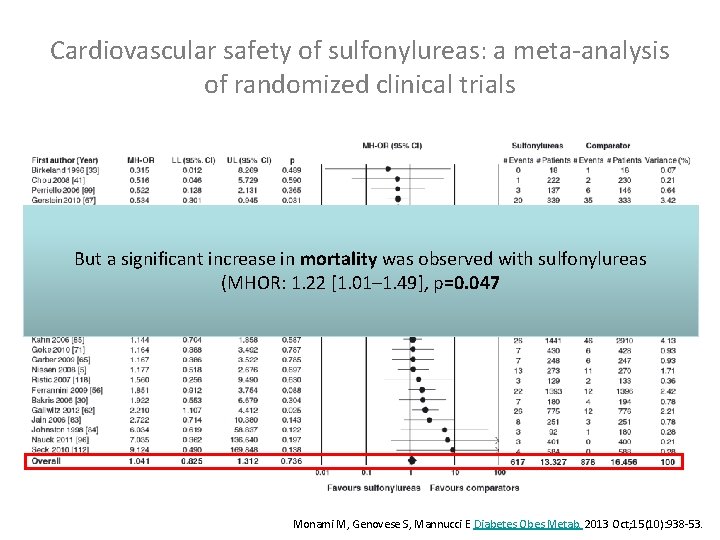
Cardiovascular safety of sulfonylureas: a meta-analysis of randomized clinical trials But a significant increase in mortality was observed with sulfonylureas (MHOR: 1. 22 [1. 01– 1. 49], p=0. 047 Monami M, Genovese S, Mannucci E Diabetes Obes Metab. 2013 Oct; 15(10): 938 -53.

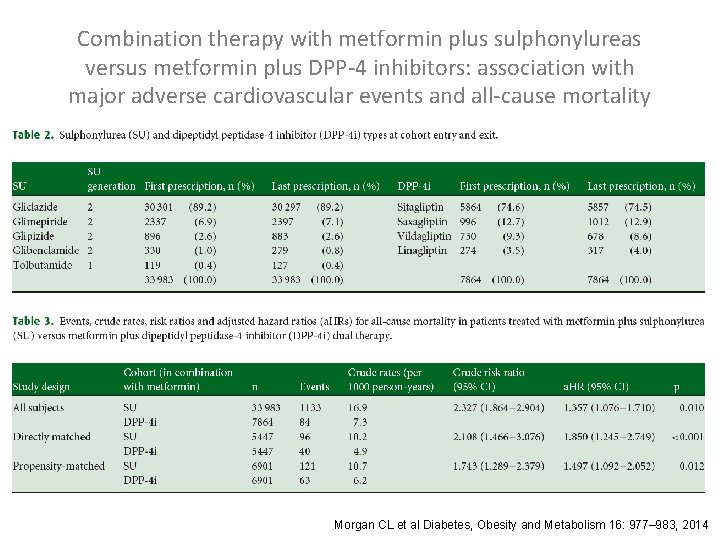
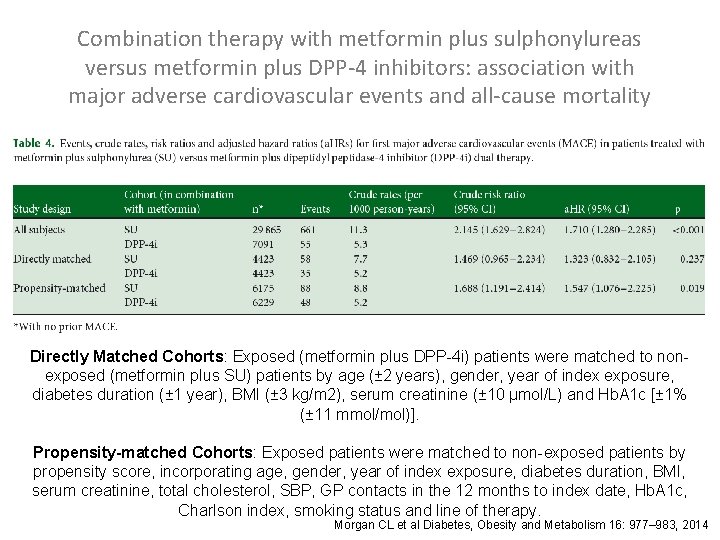
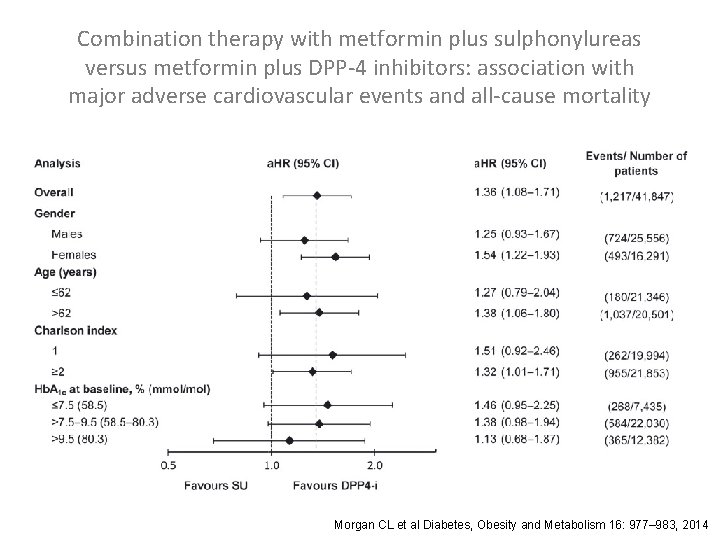
Combination therapy with metformin plus sulphonylureas versus metformin plus DPP-4 inhibitors: association with major adverse cardiovascular events and all-cause mortality Morgan CL et al Diabetes, Obesity and Metabolism 16: 977– 983, 2014

Combination therapy with metformin plus sulphonylureas versus metformin plus DPP-4 inhibitors: association with major adverse cardiovascular events and all-cause mortality Directly Matched Cohorts: Exposed (metformin plus DPP-4 i) patients were matched to nonexposed (metformin plus SU) patients by age (± 2 years), gender, year of index exposure, diabetes duration (± 1 year), BMI (± 3 kg/m 2), serum creatinine (± 10 μmol/L) and Hb. A 1 c [± 1% (± 11 mmol/mol)]. Propensity-matched Cohorts: Exposed patients were matched to non-exposed patients by propensity score, incorporating age, gender, year of index exposure, diabetes duration, BMI, serum creatinine, total cholesterol, SBP, GP contacts in the 12 months to index date, Hb. A 1 c, Charlson index, smoking status and line of therapy. Morgan CL et al Diabetes, Obesity and Metabolism 16: 977– 983, 2014

Combination therapy with metformin plus sulphonylureas versus metformin plus DPP-4 inhibitors: association with major adverse cardiovascular events and all-cause mortality Morgan CL et al Diabetes, Obesity and Metabolism 16: 977– 983, 2014

Pioglitazone

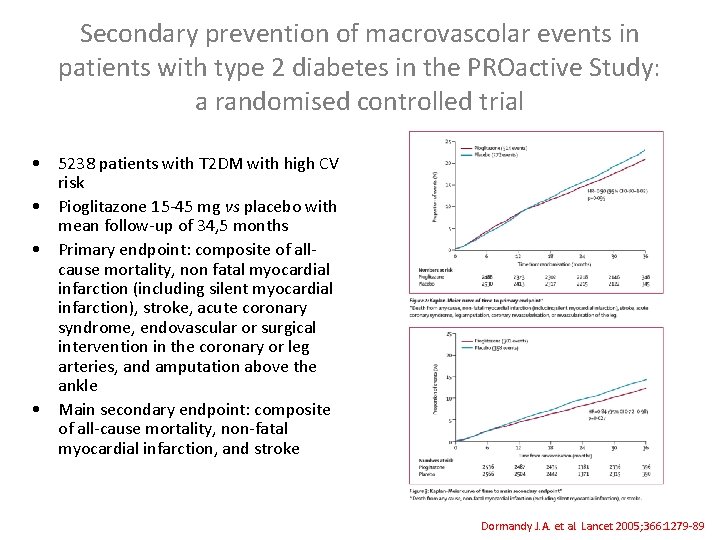
Secondary prevention of macrovascolar events in patients with type 2 diabetes in the PROactive Study: a randomised controlled trial • 5238 patients with T 2 DM with high CV risk • Pioglitazone 15 -45 mg vs placebo with mean follow-up of 34, 5 months • Primary endpoint: composite of allcause mortality, non fatal myocardial infarction (including silent myocardial infarction), stroke, acute coronary syndrome, endovascular or surgical intervention in the coronary or leg arteries, and amputation above the ankle • Main secondary endpoint: composite of all-cause mortality, non-fatal myocardial infarction, and stroke Dormandy J. A. et al. Lancet 2005; 366: 1279 -89

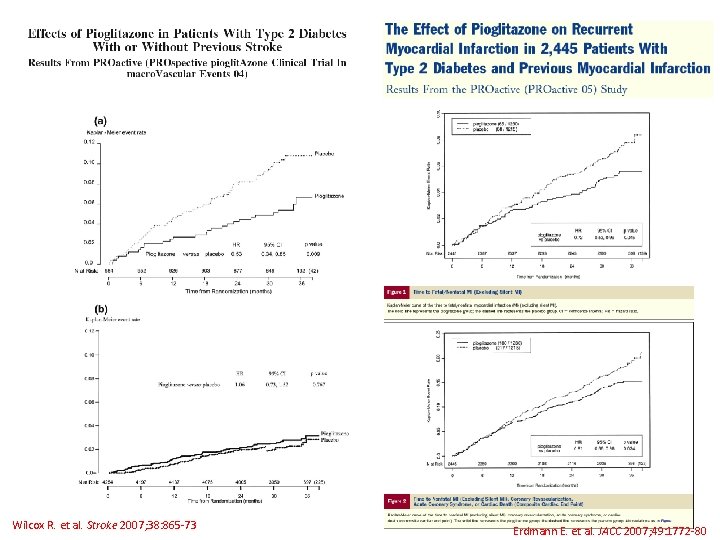
Wilcox R. et al. Stroke 2007; 38: 865 -73 Erdmann E. et al. JACC 2007; 49: 1772 -80

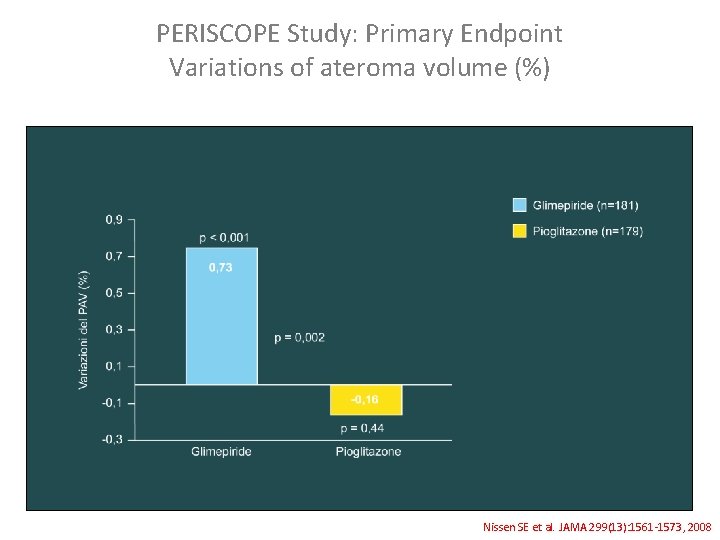
PERISCOPE Study: Primary Endpoint Variations of ateroma volume (%) Nissen SE et al. JAMA 299(13): 1561 -1573, 2008

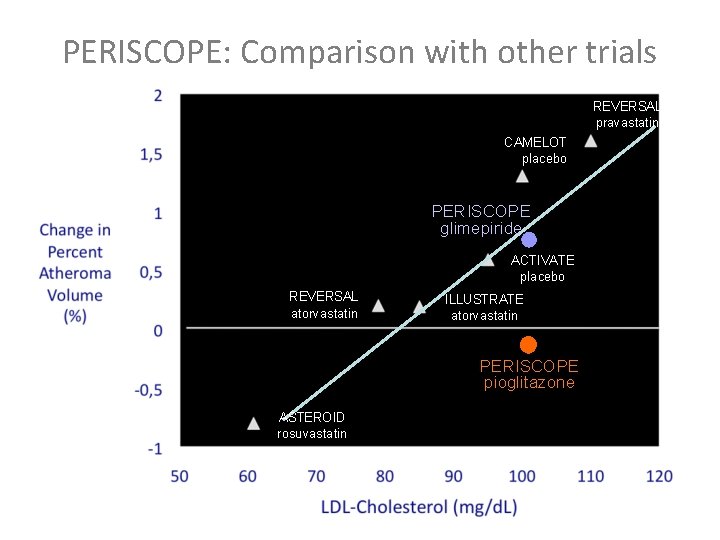
PERISCOPE: Comparison with other trials REVERSAL pravastatin CAMELOT placebo PERISCOPE glimepiride ACTIVATE placebo REVERSAL atorvastatin ILLUSTRATE atorvastatin PERISCOPE pioglitazone ASTEROID rosuvastatin

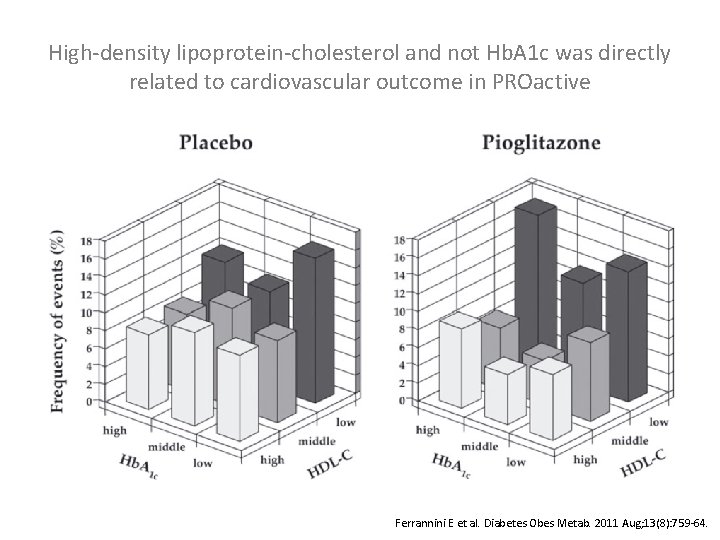
High-density lipoprotein-cholesterol and not Hb. A 1 c was directly related to cardiovascular outcome in PROactive Ferrannini E et al. Diabetes Obes Metab. 2011 Aug; 13(8): 759 -64.

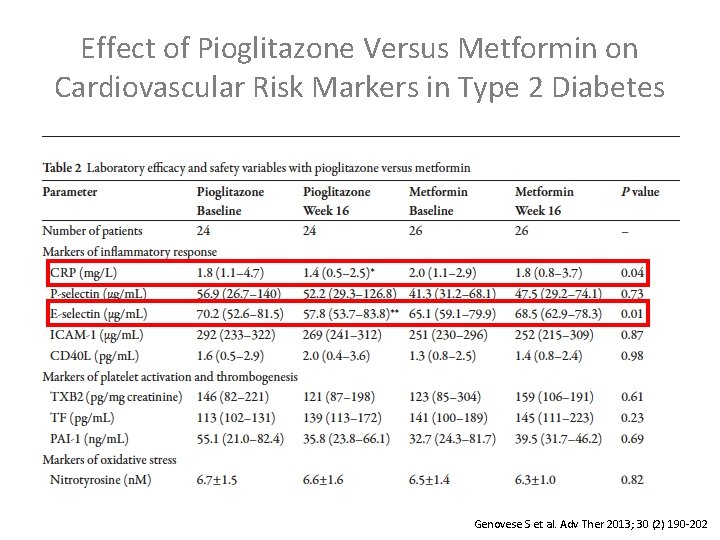
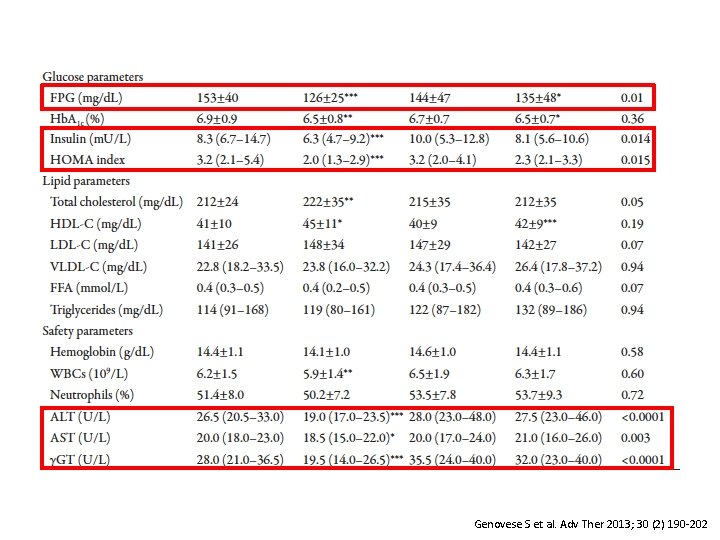
Effect of Pioglitazone Versus Metformin on Cardiovascular Risk Markers in Type 2 Diabetes Genovese S et al. Adv Ther 2013; 30 (2) 190 -202

Genovese S et al. Adv Ther 2013; 30 (2) 190 -202

GLP 1 -RA

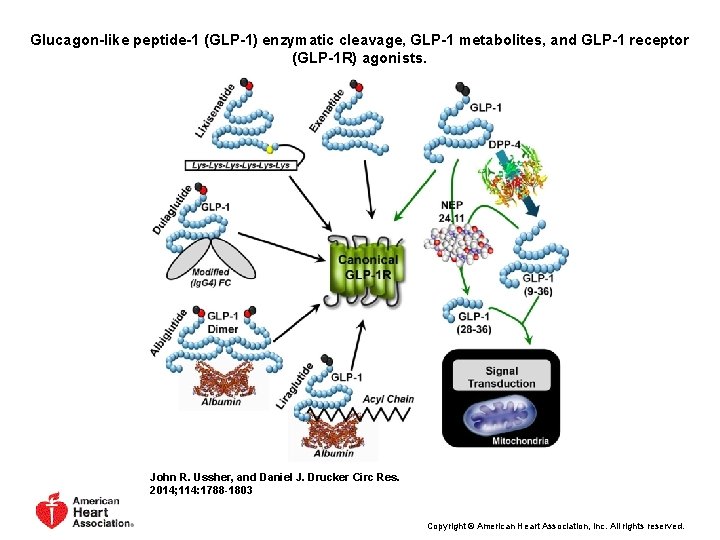
Glucagon-like peptide-1 (GLP-1) enzymatic cleavage, GLP-1 metabolites, and GLP-1 receptor (GLP-1 R) agonists. John R. Ussher, and Daniel J. Drucker Circ Res. 2014; 114: 1788 -1803 Copyright © American Heart Association, Inc. All rights reserved.

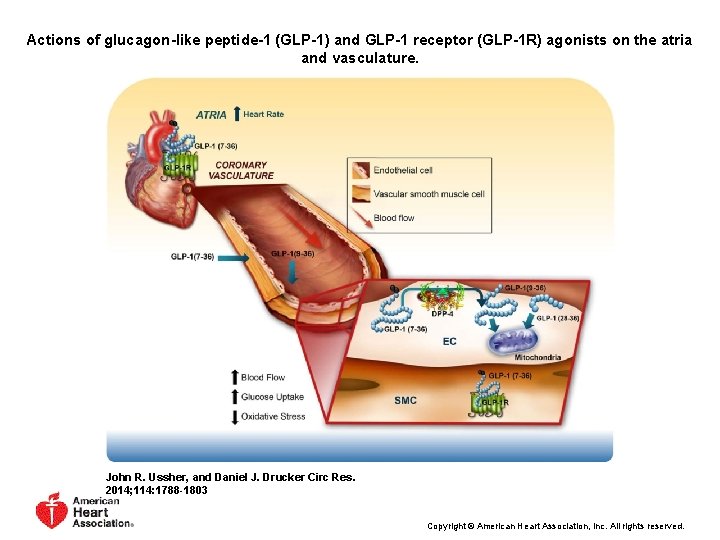
Actions of glucagon-like peptide-1 (GLP-1) and GLP-1 receptor (GLP-1 R) agonists on the atria and vasculature. John R. Ussher, and Daniel J. Drucker Circ Res. 2014; 114: 1788 -1803 Copyright © American Heart Association, Inc. All rights reserved.

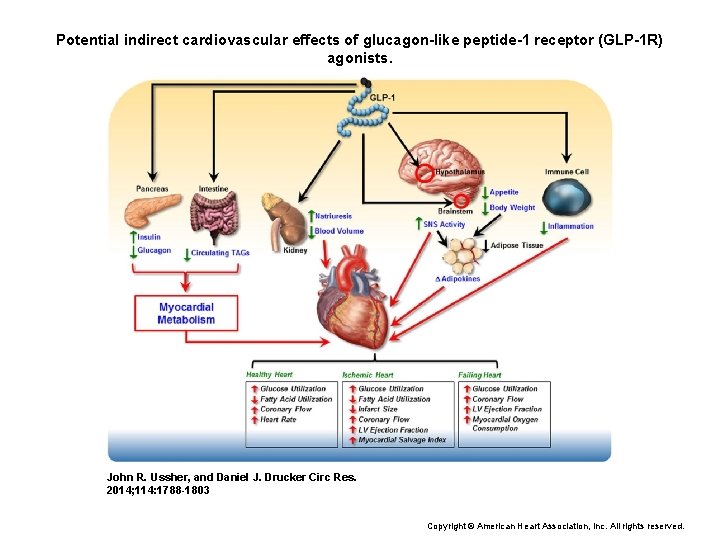
Potential indirect cardiovascular effects of glucagon-like peptide-1 receptor (GLP-1 R) agonists. John R. Ussher, and Daniel J. Drucker Circ Res. 2014; 114: 1788 -1803 Copyright © American Heart Association, Inc. All rights reserved.

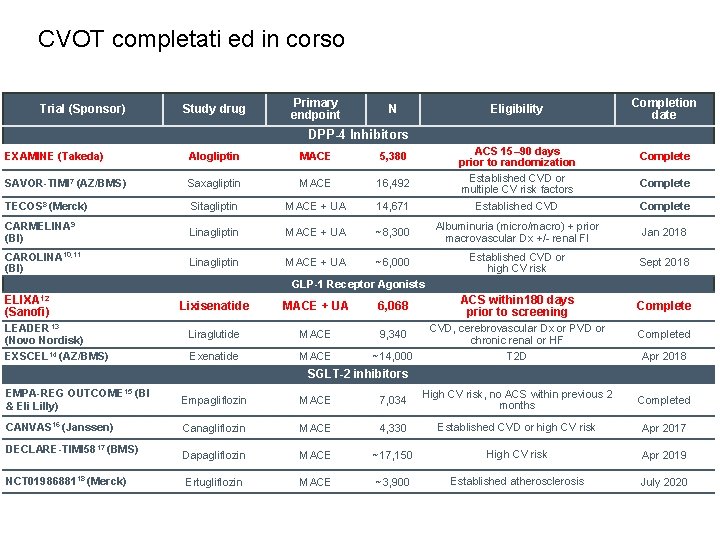
CVOT completati ed in corso Trial (Sponsor) Study drug Primary endpoint N Eligibility Completion date DPP-4 Inhibitors ACS 15– 90 days prior to randomization Established CVD or multiple CV risk factors EXAMINE (Takeda) Alogliptin MACE 5, 380 Complete SAVOR-TIMI 7 (AZ/BMS) Saxagliptin MACE 16, 492 TECOS 8 (Merck) Sitagliptin MACE + UA 14, 671 Established CVD Complete CARMELINA 9 (BI) Linagliptin MACE + UA ~8, 300 Albuminuria (micro/macro) + prior macrovascular Dx +/- renal FI Jan 2018 CAROLINA 10, 11 (BI) Linagliptin MACE + UA ~6, 000 Established CVD or high CV risk Sept 2018 Complete GLP-1 Receptor Agonists ELIXA 12 (Sanofi) Lixisenatide MACE + UA 6, 068 ACS within 180 days prior to screening Complete LEADER 13 (Novo Nordisk) Liraglutide MACE 9, 340 CVD, cerebrovascular Dx or PVD or chronic renal or HF Completed EXSCEL 14 (AZ/BMS) Exenatide MACE ~14, 000 T 2 D Apr 2018 SGLT-2 inhibitors EMPA-REG OUTCOME 15 (BI & Eli Lilly) Empagliflozin MACE 7, 034 High CV risk, no ACS within previous 2 months Completed CANVAS 16 (Janssen) Canagliflozin MACE 4, 330 Established CVD or high CV risk Apr 2017 Dapagliflozin MACE ~17, 150 High CV risk Apr 2019 Ertugliflozin MACE ~3, 900 Established atherosclerosis July 2020 DECLARE-TIMI 5817 (BMS) NCT 0198688118 (Merck)









Original Article Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus Benjamin M. Scirica, M. D. , M. P. H. , Deepak L. Bhatt, M. D. , M. P. H. , Eugene Braunwald, M. D. , P. Gabriel Steg, M. D. , Jaime Davidson, M. D. , Boaz Hirshberg, M. D. , Peter Ohman, M. D. , Robert Frederich, M. D. , Ph. D. , Stephen D. Wiviott, M. D. , Elaine B. Hoffman, Ph. D. , Matthew A. Cavender, M. D. , M. P. H. , Jacob A. Udell, M. D. , M. P. H. , Nihar R. Desai, M. D. , M. P. H. , Ofri Mosenzon, M. D. , Darren K. Mc. Guire, M. D. , Kausik K. Ray, M. D. , Lawrence A. Leiter, M. D. , Itamar Raz, M. D. , for the SAVOR-TIMI 53 Steering Committee and Investigators N Engl J Med Volume 369(14): 1317 -1326 October 3, 2013

Study Overview • Saxagliptin, a new oral antihyperglycemic drug in the DPP-4 inhibitor class, had no effect on the risk of cardiovascular events in patients with type 2 diabetes. • Although the drug does not increase cardiovascular risk, it also does not provide cardiovascular benefit.

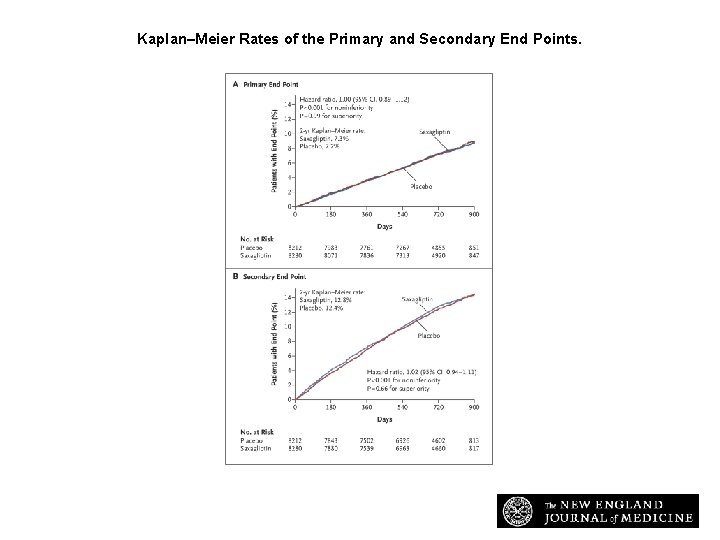
Kaplan–Meier Rates of the Primary and Secondary End Points. Scirica BM et al. N Engl J Med 2013; 369: 1317 -1326

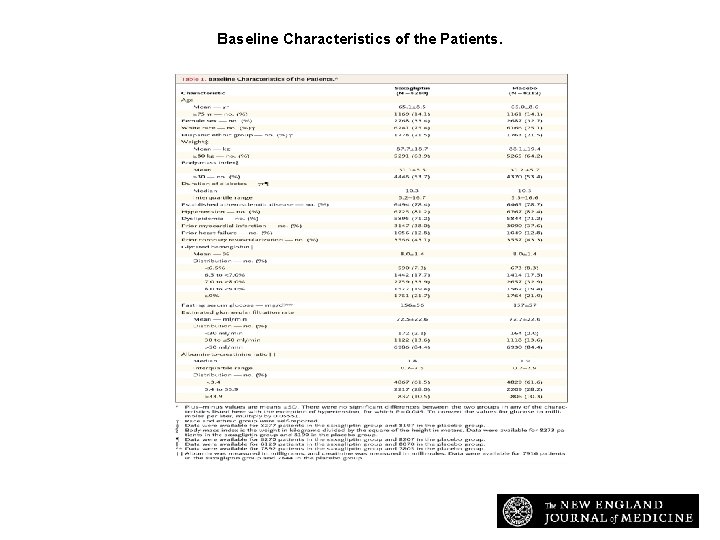
Baseline Characteristics of the Patients. Scirica BM et al. N Engl J Med 2013; 369: 1317 -1326

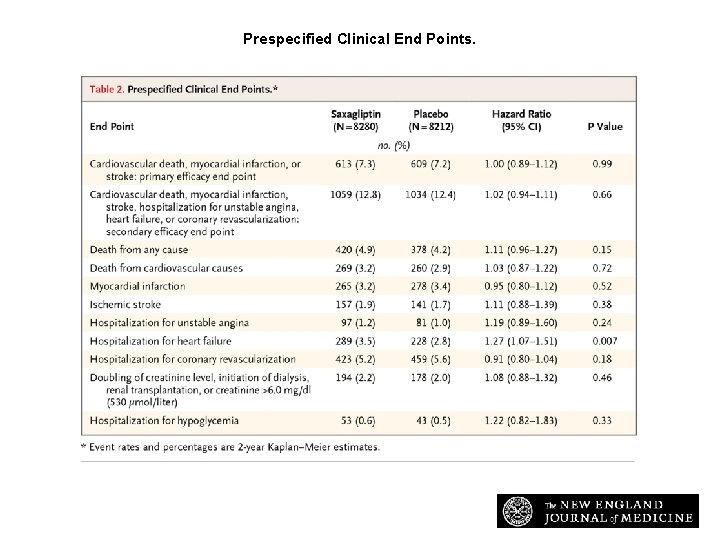
Prespecified Clinical End Points. Scirica BM et al. N Engl J Med 2013; 369: 1317 -1326

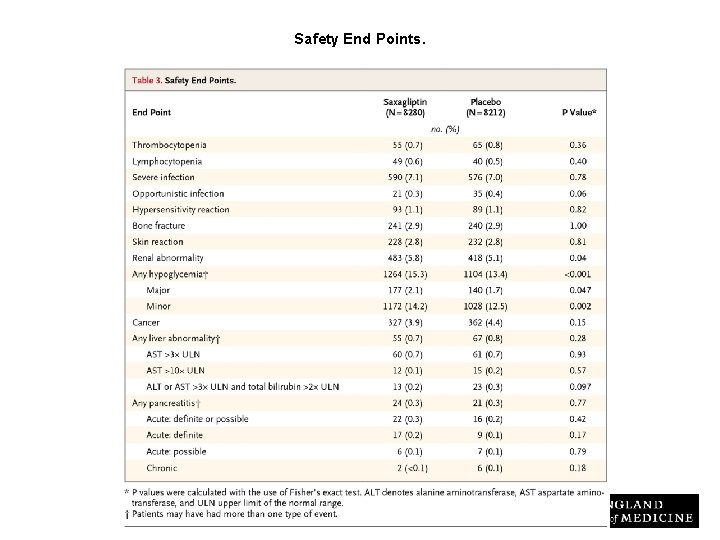
Safety End Points. Scirica BM et al. N Engl J Med 2013; 369: 1317 -1326

Conclusions • DPP-4 inhibition with saxagliptin did not increase or decrease the rate of ischemic events, though the rate of hospitalization for heart failure was increased. • Although saxagliptin improves glycemic control, other approaches are necessary to reduce cardiovascular risk in patients with diabetes.

Original Article Lixisenatide in Patients with Type 2 Diabetes and Acute Coronary Syndrome Marc A. Pfeffer, M. D. , Ph. D. , Brian Claggett, Ph. D. , Rafael Diaz, M. D. , Kenneth Dickstein, M. D. , Ph. D. , Hertzel C. Gerstein, M. D. , Lars V. Køber, M. D. , Francesca C. Lawson, M. D. , Lin Ping, M. D. , Xiaodan Wei, Ph. D. , Eldrin F. Lewis, M. D. , M. P. H. , Aldo P. Maggioni, M. D. , John J. V. Mc. Murray, M. D. , Ph. D. , Jeffrey L. Probstfield, M. D. , Matthew C. Riddle, M. D. , Scott D. Solomon, M. D. , Jean-Claude Tardif, M. D. , for the ELIXA Investigators N Engl J Med Volume 373(23): 2247 -2257 December 3, 2015

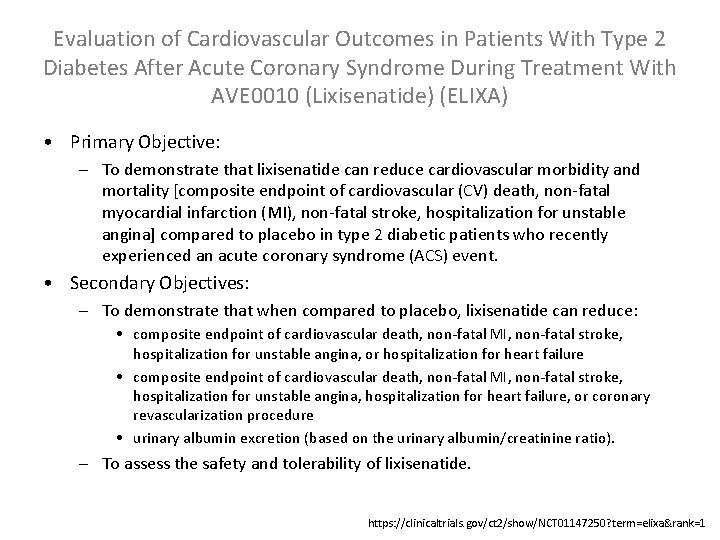
Evaluation of Cardiovascular Outcomes in Patients With Type 2 Diabetes After Acute Coronary Syndrome During Treatment With AVE 0010 (Lixisenatide) (ELIXA) • Primary Objective: – To demonstrate that lixisenatide can reduce cardiovascular morbidity and mortality [composite endpoint of cardiovascular (CV) death, non-fatal myocardial infarction (MI), non-fatal stroke, hospitalization for unstable angina] compared to placebo in type 2 diabetic patients who recently experienced an acute coronary syndrome (ACS) event. • Secondary Objectives: – To demonstrate that when compared to placebo, lixisenatide can reduce: • composite endpoint of cardiovascular death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina, or hospitalization for heart failure • composite endpoint of cardiovascular death, non-fatal MI, non-fatal stroke, hospitalization for unstable angina, hospitalization for heart failure, or coronary revascularization procedure • urinary albumin excretion (based on the urinary albumin/creatinine ratio). – To assess the safety and tolerability of lixisenatide. https: //clinicaltrials. gov/ct 2/show/NCT 01147250? term=elixa&rank=1

Study Overview • In this randomized study, the addition of lixisenatide, a glucagon-like peptide 1–receptor agonist, to usual care in patients with type 2 diabetes and a recent cardiovascular event did not alter the rate of subsequent major cardiovascular or other serious adverse events.

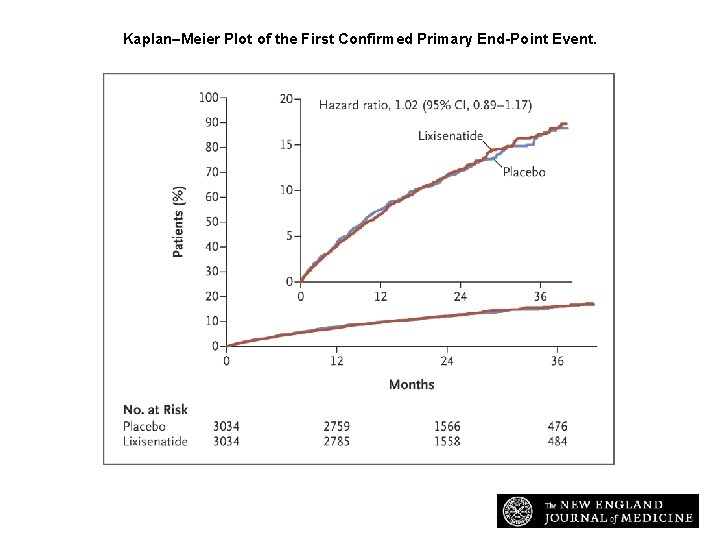
Kaplan–Meier Plot of the First Confirmed Primary End-Point Event. Pfeffer MA et al. N Engl J Med 2015; 373: 2247 -2257

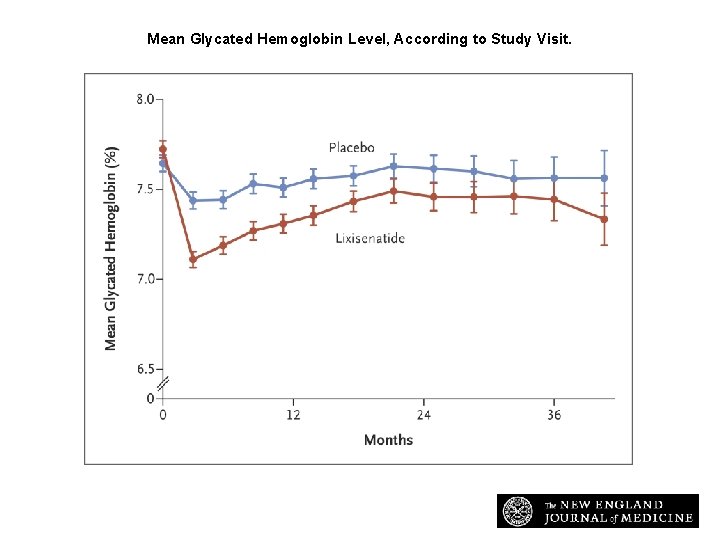
Mean Glycated Hemoglobin Level, According to Study Visit. Pfeffer MA et al. N Engl J Med 2015; 373: 2247 -2257

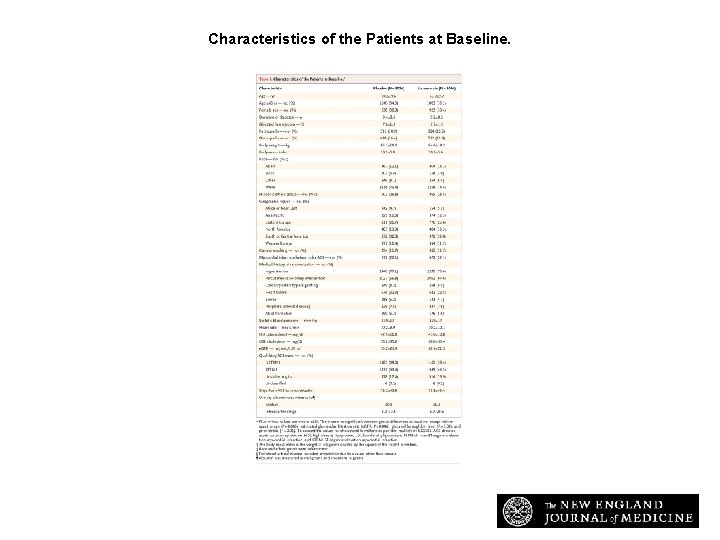
Characteristics of the Patients at Baseline. Pfeffer MA et al. N Engl J Med 2015; 373: 2247 -2257

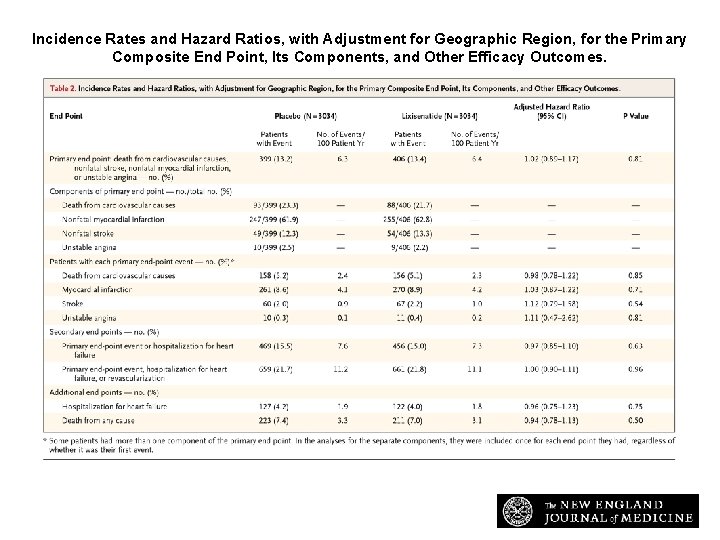
Incidence Rates and Hazard Ratios, with Adjustment for Geographic Region, for the Primary Composite End Point, Its Components, and Other Efficacy Outcomes. Pfeffer MA et al. N Engl J Med 2015; 373: 2247 -2257

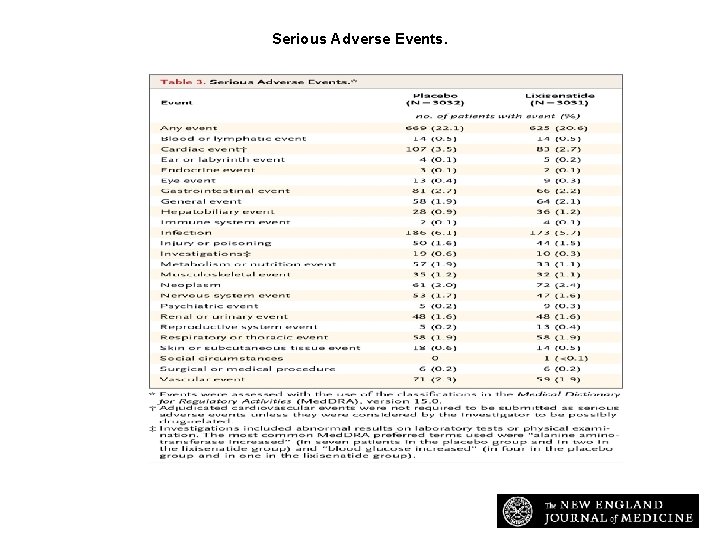
Serious Adverse Events. Pfeffer MA et al. N Engl J Med 2015; 373: 2247 -2257

Conclusions • In patients with type 2 diabetes and a recent acute coronary syndrome, the addition of lixisenatide to usual care did not significantly alter the rate of major cardiovascular events or other serious adverse events.

DPPIV-Inhibitors

EXAMINE Cardiovascular Outcomes With Alogliptin in Patients With Type 2 Diabetes Mellitus and Recent Acute Coronary Syndrome William B. White, MD for the EXAMINE Investigators Cardiology Center, University of Connecticut School of Medicine, Farmington, Connecticut USA September 2013 EUCAN/ALO/2013 -10101

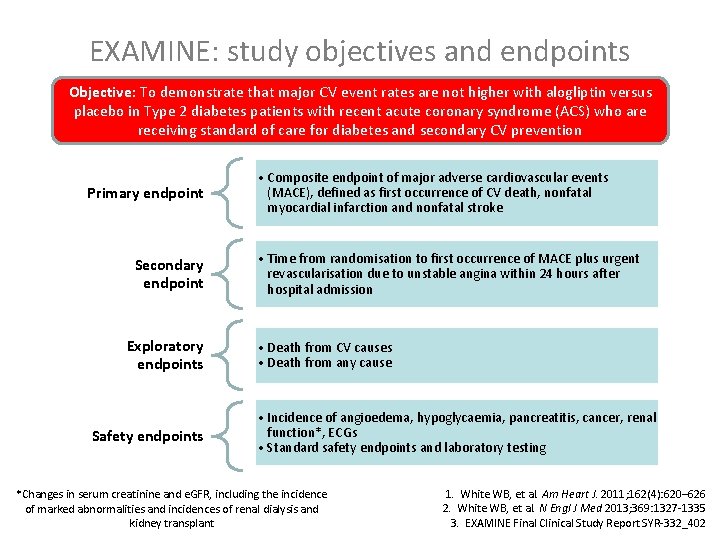
EXAMINE: study objectives and endpoints Objective: To demonstrate that major CV event rates are not higher with alogliptin versus placebo in Type 2 diabetes patients with recent acute coronary syndrome (ACS) who are receiving standard of care for diabetes and secondary CV prevention Primary endpoint Secondary endpoint Exploratory endpoints Safety endpoints • Composite endpoint of major adverse cardiovascular events (MACE), defined as first occurrence of CV death, nonfatal myocardial infarction and nonfatal stroke • Time from randomisation to first occurrence of MACE plus urgent revascularisation due to unstable angina within 24 hours after hospital admission • Death from CV causes • Death from any cause • Incidence of angioedema, hypoglycaemia, pancreatitis, cancer, renal function*, ECGs • Standard safety endpoints and laboratory testing *Changes in serum creatinine and e. GFR, including the incidence of marked abnormalities and incidences of renal dialysis and kidney transplant 1. White WB, et al. Am Heart J. 2011; 162(4): 620– 626 2. White WB, et al. N Engl J Med 2013; 369: 1327 -1335 3. EXAMINE Final Clinical Study Report SYR-332_402

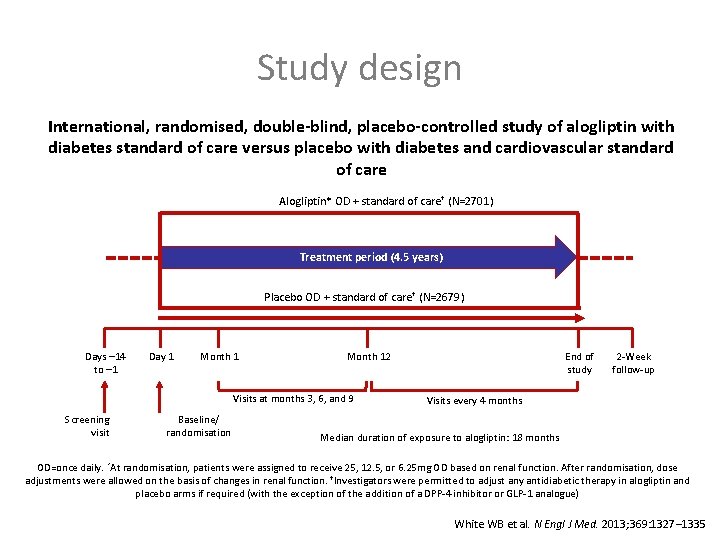
Study design International, randomised, double-blind, placebo-controlled study of alogliptin with diabetes standard of care versus placebo with diabetes and cardiovascular standard of care Alogliptin* OD + standard of care† (N=2701) Treatment period (4. 5 years) Placebo OD + standard of care† (N=2679) Days – 14 to – 1 Day 1 Month 12 Visits at months 3, 6, and 9 Screening visit Baseline/ randomisation End of study 2 -Week follow-up Visits every 4 months Median duration of exposure to alogliptin: 18 months OD=once daily. *At randomisation, patients were assigned to receive 25, 12. 5, or 6. 25 mg OD based on renal function. After randomisation, dose adjustments were allowed on the basis of changes in renal function. †Investigators were permitted to adjust any antidiabetic therapy in alogliptin and placebo arms if required (with the exception of the addition of a DPP-4 inhibitor or GLP-1 analogue) White WB et al. N Engl J Med. 2013; 369: 1327– 1335

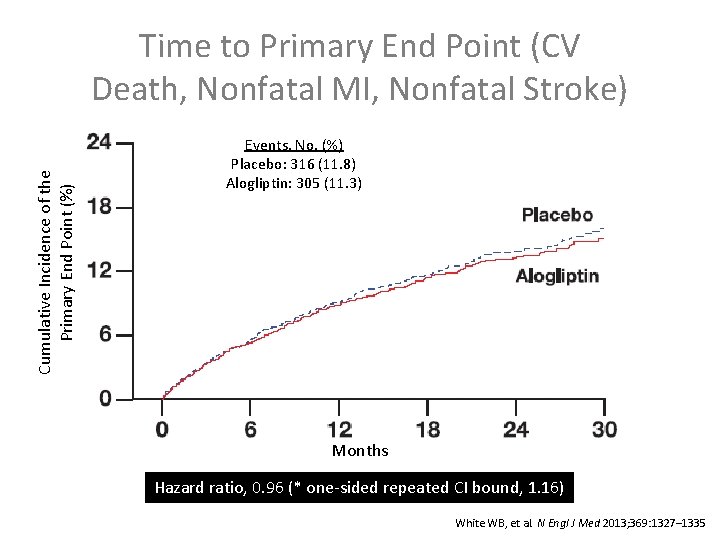
Time to Primary End Point (CV Death, Nonfatal MI, Nonfatal Stroke) Cumulative Incidence of the Primary End Point (%) Events, No. (%) Placebo: 316 (11. 8) Alogliptin: 305 (11. 3) Placebo (n): 2679 2299 1891 Months 1375 805 286 Alogliptin (n): 2701 2316 1899 1394 821 296 Hazard ratio, 0. 96 (* one-sided repeated CI bound, 1. 16) * Using alpha=0. 01. White WB, et al. N Engl J Med 2013; 369: 1327– 1335

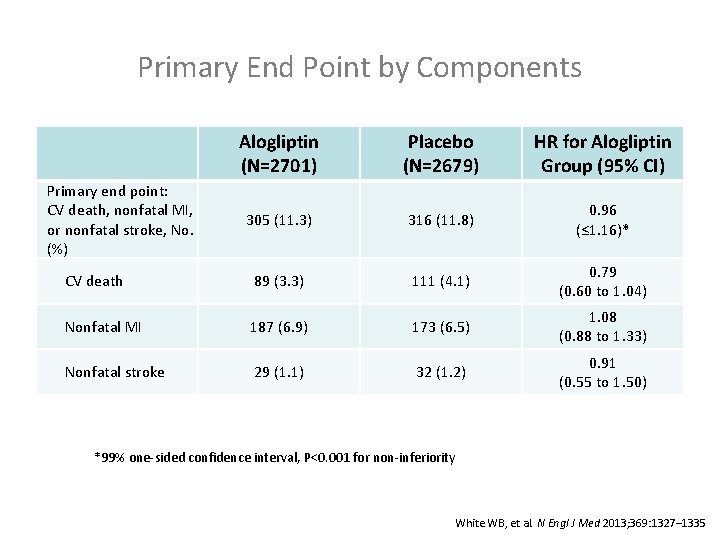
Primary End Point by Components Alogliptin (N=2701) Placebo (N=2679) HR for Alogliptin Group (95% CI) 305 (11. 3) 316 (11. 8) 0. 96 (≤ 1. 16)* CV death 89 (3. 3) 111 (4. 1) 0. 79 (0. 60 to 1. 04) Nonfatal MI 187 (6. 9) 173 (6. 5) 1. 08 (0. 88 to 1. 33) Nonfatal stroke 29 (1. 1) 32 (1. 2) 0. 91 (0. 55 to 1. 50) Primary end point: CV death, nonfatal MI, or nonfatal stroke, No. (%) *99% one-sided confidence interval, P<0. 001 for non-inferiority White WB, et al. N Engl J Med 2013; 369: 1327– 1335

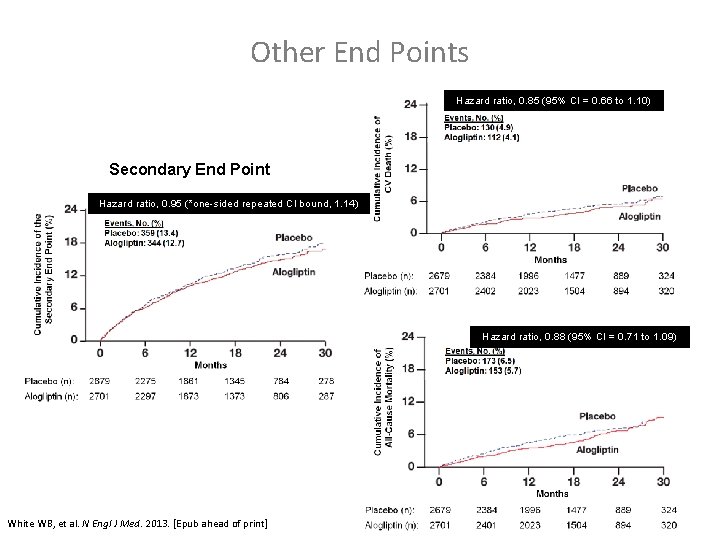
Other End Points Any CV Death Hazard ratio, 0. 85 (95% CI = 0. 66 to 1. 10) Secondary End Point Hazard ratio, 0. 95 (*one-sided repeated CI bound, 1. 14) All-Cause Mortality Hazard ratio, 0. 88 (95% CI = 0. 71 to 1. 09) * Using alpha=0. 01. White WB, et al. N Engl J Med. 2013. [Epub ahead of print]

In conclusione… • In aggiunta alla efficacia sul compenso metabolico, alcune categorie di farmaci ipoglicemizzanti sembrano influenzare positivamente la prognosi dei pazienti con cardiopatia ischemica recente • Questa capacità è documentata per i farmaci primariamente attivi sulla insulino-resistenza (metformina, pioglitazone) ed è verosimile per quelli che agiscono sul sistema del GLP-1 • In a very high-CV-risk population with type 2 diabetes and recent history of acute coronary syndrome, EXAMINE demonstrated cardiovascular safety of alogliptin and no other safety signals emerged
 ü
ü Tvb
Tvb Suoni duri gl
Suoni duri gl Sca edvance360
Sca edvance360 Sca jakt och fiske
Sca jakt och fiske Jaktrapport holmen
Jaktrapport holmen Bjrk
Bjrk Sca compliance principles
Sca compliance principles Sca scb scc
Sca scb scc Sca 2021 calendar
Sca 2021 calendar Sca business strategy
Sca business strategy Aica pica sca
Aica pica sca Sca compliance principles
Sca compliance principles Hp fortify mobile application security
Hp fortify mobile application security Scä väntetid
Scä väntetid Sca 101
Sca 101 Command injection fortify
Command injection fortify Sca
Sca Service oriented architechture
Service oriented architechture Fortify awb
Fortify awb Sca compliance principles
Sca compliance principles Sca for dummies
Sca for dummies Sca galec
Sca galec Sca jakt och fiske
Sca jakt och fiske Sca packaging
Sca packaging Sca compliance principles
Sca compliance principles International currency technologies corp
International currency technologies corp Fortify webinspect tutorial
Fortify webinspect tutorial Sca nokia
Sca nokia Sca jakt
Sca jakt ôi vì yêu mà chúa không tiếc đời
ôi vì yêu mà chúa không tiếc đời Monica terapia clark
Monica terapia clark Modelos sistemicos terapia familiar
Modelos sistemicos terapia familiar Paraplegia spastica ereditaria
Paraplegia spastica ereditaria Ibft terapeuta
Ibft terapeuta Salvador minuchin terapia familiar
Salvador minuchin terapia familiar Terapia educazionale del diabetico
Terapia educazionale del diabetico Que es un autorregistro
Que es un autorregistro Terapia neuroacustica
Terapia neuroacustica Terapia w piaskownicy
Terapia w piaskownicy Terapia centrada en el cliente
Terapia centrada en el cliente Przeciwprzeniesienie w terapii pacjentów borderline
Przeciwprzeniesienie w terapii pacjentów borderline Activitati de meloterapie
Activitati de meloterapie Saba laba sama lama
Saba laba sama lama Terapia triple helicobacter
Terapia triple helicobacter Rakowski sierosław
Rakowski sierosław Funciones de la terapia dialéctico conductual
Funciones de la terapia dialéctico conductual Terapia intensiva cardiochirurgica monaldi
Terapia intensiva cardiochirurgica monaldi Kognitywna terapia behawioralna
Kognitywna terapia behawioralna Nutriia
Nutriia Frases de auto perdão
Frases de auto perdão Terapia aversiva
Terapia aversiva Soggetti con panipopituitarismo
Soggetti con panipopituitarismo Arto fantasma terapia
Arto fantasma terapia Alcalosi ruminale
Alcalosi ruminale Terapia ocupationala la varstnici
Terapia ocupationala la varstnici Escala de glasgow
Escala de glasgow Iperpotassiemia
Iperpotassiemia Egzema mkurnaloba
Egzema mkurnaloba Anamnese idoso terapia ocupacional
Anamnese idoso terapia ocupacional Modelo de terapia de strong como influencia interpersonal
Modelo de terapia de strong como influencia interpersonal Urea breath test
Urea breath test Terapia de pareja
Terapia de pareja Terapia electroconvulsiva
Terapia electroconvulsiva Terapia electroconvulsiva
Terapia electroconvulsiva Terapia awersyjna
Terapia awersyjna Laso terapia
Laso terapia Terapia racional emotiva de beck
Terapia racional emotiva de beck Terapia inotropica
Terapia inotropica Terapia mrt
Terapia mrt