Sympathetic Nerve Denervation Sympathetic Nerve for Treatment of









- Slides: 39

Sympathetic Nerve Denervation Sympathetic Nerve for Treatment of Hypertension Denervation for Treatment of Hypertension Ron Waksman, MD, FACC Professor of Medicine (Cardiology) Georgetown University, Associate Director Division of Cardiology Washington Hospital Center Washington DC

Disclosures • Consultant / Advisory Board: Abbott Vascular, Biotronik, Medtronic, Boston Scientific. • Speaker Biotronik, Boston Scientific, Medtronic, Abbott Vascular • Research Grants: Biotronik, Medtronic, Boston Scientific, Elli Lilly, Atrium, GSK, Abbott Vascular

Need for Novel Therapy v Approximately 50% of pts with hypertension remain hypertensive despite: Combination of pharmaceutical agents v Improved compliance v Lifestyle changes v Vs

Rational for Renal Sympathectomy v Contribution of the renal sympathetic activity in the development of hypertension has been demonstrated in both animal and human experiments v The human transplant experiment has demonstrated that the denervated kidney reliably supports electrolyte and volume homeostasis

Rational for Renal Sympathectomy l Pts with essential hypertension exhibit increased renal NA spillover into plasma l Increased renal and cardiac NA spillover is consistent with the hemodynamic profile of essential hypertension n l Increased heart rate Increased cardiac output Renovascular resistence Essential hypertension has been recognized as a NEUROGENIC phenomenon

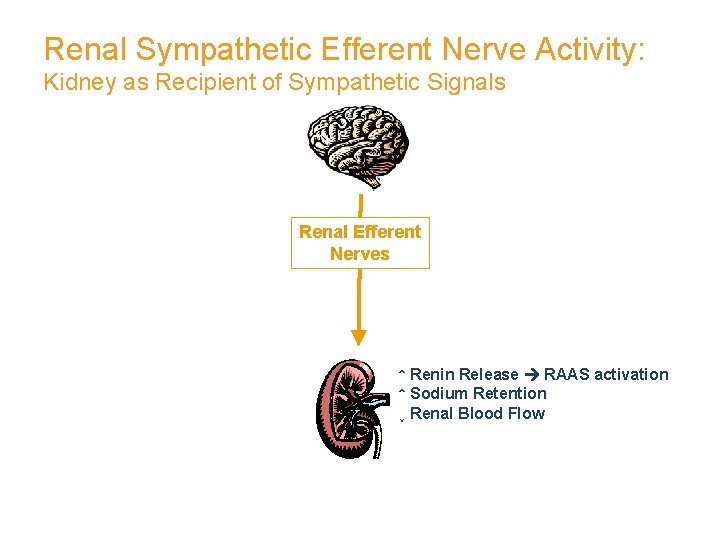
Renal Sympathetic Efferent Nerve Activity: Kidney as Recipient of Sympathetic Signals Renal Efferent Nerves ↑ Renin Release RAAS activation ↑ Sodium Retention ↓ Renal Blood Flow

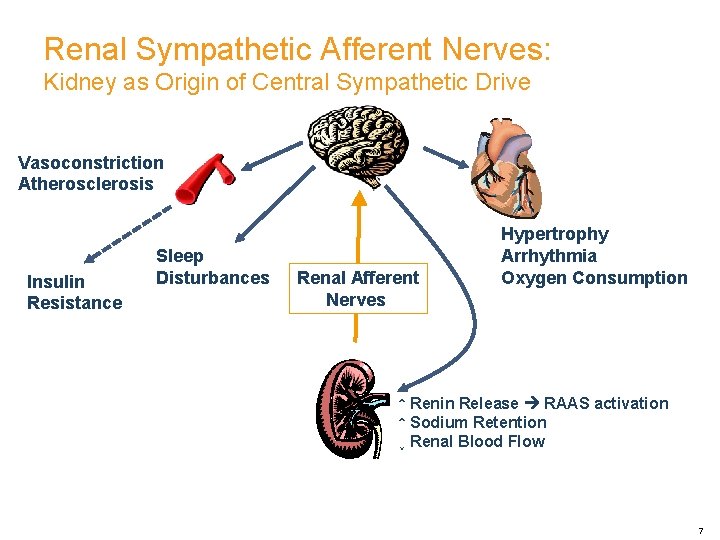
Renal Sympathetic Afferent Nerves: Kidney as Origin of Central Sympathetic Drive Vasoconstriction Atherosclerosis Insulin Resistance Sleep Disturbances Renal Afferent Nerves Hypertrophy Arrhythmia Oxygen Consumption ↑ Renin Release RAAS activation ↑ Sodium Retention ↓ Renal Blood Flow 7

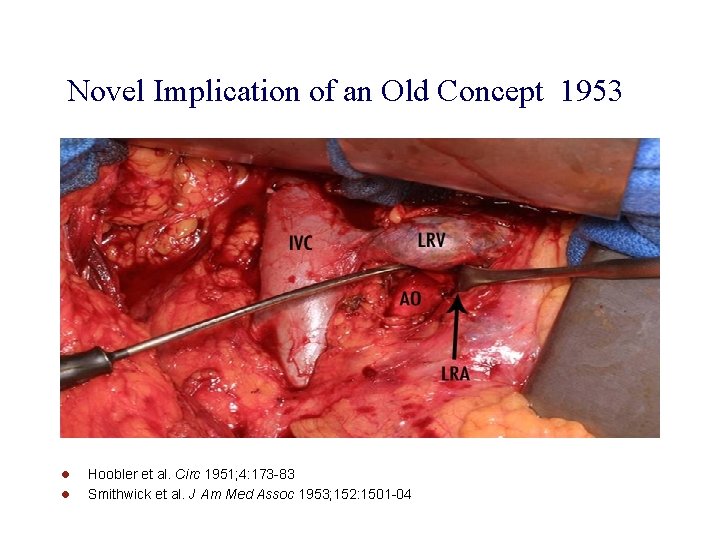
Novel Implication of an Old Concept 1953 l l Hoobler et al. Circ 1951; 4: 173 -83 Smithwick et al. J Am Med Assoc 1953; 152: 1501 -04

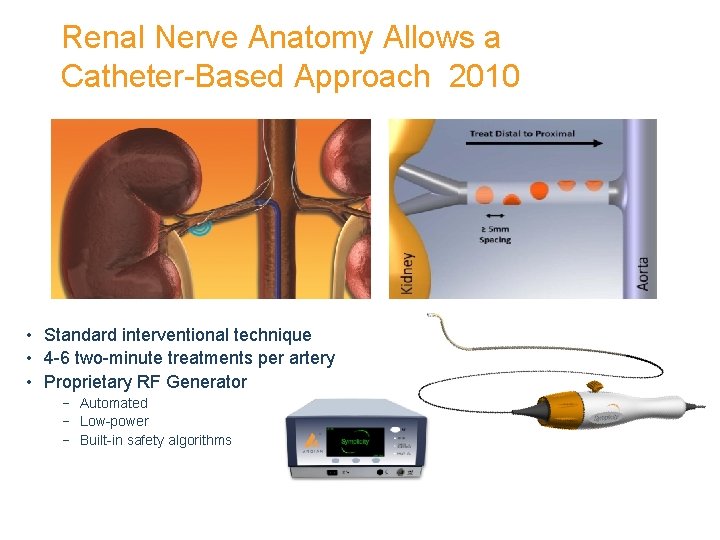
Renal Nerve Anatomy Allows a Catheter-Based Approach 2010 • Standard interventional technique • 4 -6 two-minute treatments per artery • Proprietary RF Generator − Automated − Low-power − Built-in safety algorithms

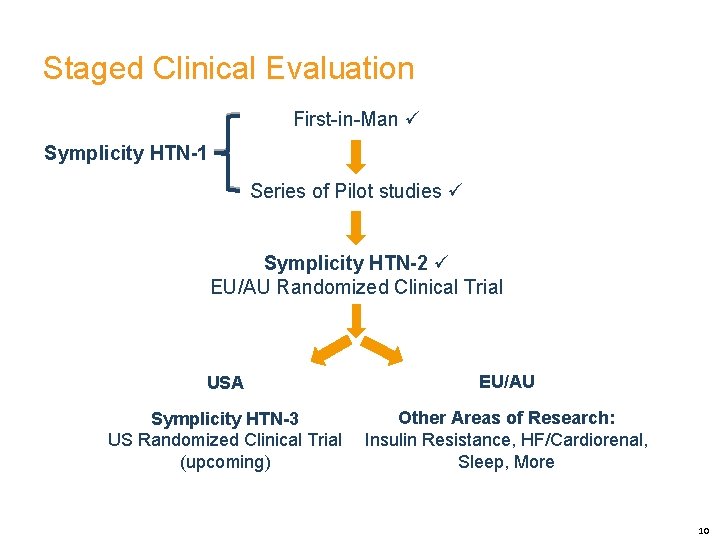
Staged Clinical Evaluation First-in-Man Symplicity HTN-1 Series of Pilot studies Symplicity HTN-2 EU/AU Randomized Clinical Trial USA EU/AU Symplicity HTN-3 US Randomized Clinical Trial (upcoming) Other Areas of Research: Insulin Resistance, HF/Cardiorenal, Sleep, More 10

Symplicity HTN-1 Lancet. 2009; 373: 1275 -1281 Initial Cohort – Reported in the Lancet, 2009: -First-in-man, non-randomized -Cohort of 45 patients with resistant HTN (SBP ≥ 160 mm. Hg on ≥ 3 anti-HTN drugs, including a diuretic; e. GFR ≥ 45 m. L/min) - 12 -month data Expanded Cohort – This Report (Symplicity HTN-1): -Expanded cohort of patients (n=153) -24 -month follow-up Sievert et al. European Society of Cardiology. 2010. 11

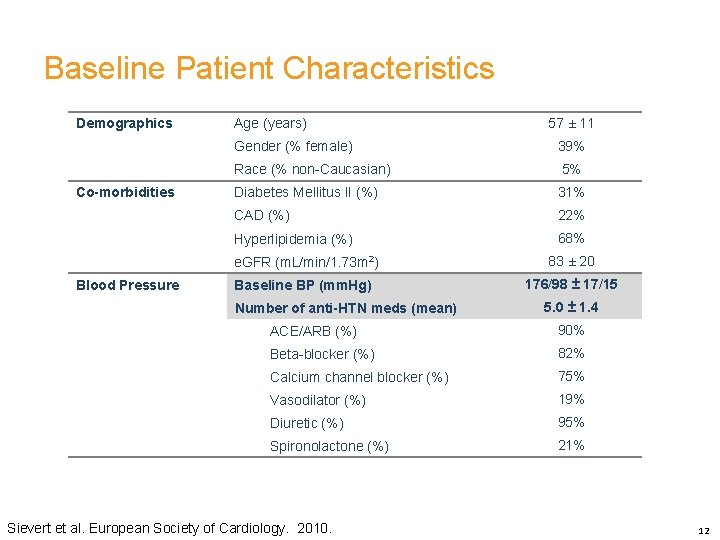
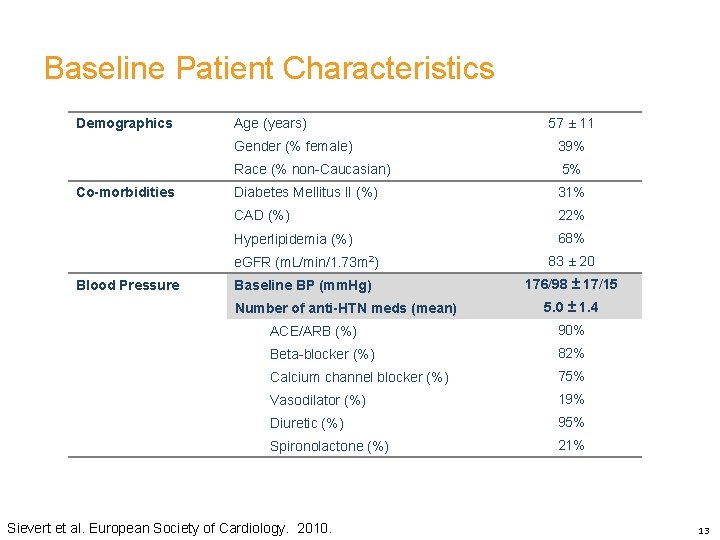
Baseline Patient Characteristics Demographics Co-morbidities Blood Pressure Age (years) 57 ± 11 Gender (% female) 39% Race (% non-Caucasian) 5% Diabetes Mellitus II (%) 31% CAD (%) 22% Hyperlipidemia (%) 68% e. GFR (m. L/min/1. 73 m 2) 83 ± 20 Baseline BP (mm. Hg) 176/98 ± 17/15 Number of anti-HTN meds (mean) 5. 0 ± 1. 4 ACE/ARB (%) 90% Beta-blocker (%) 82% Calcium channel blocker (%) 75% Vasodilator (%) 19% Diuretic (%) 95% Spironolactone (%) 21% Sievert et al. European Society of Cardiology. 2010. 12

Baseline Patient Characteristics Demographics Co-morbidities Blood Pressure Age (years) 57 ± 11 Gender (% female) 39% Race (% non-Caucasian) 5% Diabetes Mellitus II (%) 31% CAD (%) 22% Hyperlipidemia (%) 68% e. GFR (m. L/min/1. 73 m 2) 83 ± 20 Baseline BP (mm. Hg) 176/98 ± 17/15 Number of anti-HTN meds (mean) 5. 0 ± 1. 4 ACE/ARB (%) 90% Beta-blocker (%) 82% Calcium channel blocker (%) 75% Vasodilator (%) 19% Diuretic (%) 95% Spironolactone (%) 21% Sievert et al. European Society of Cardiology. 2010. 13

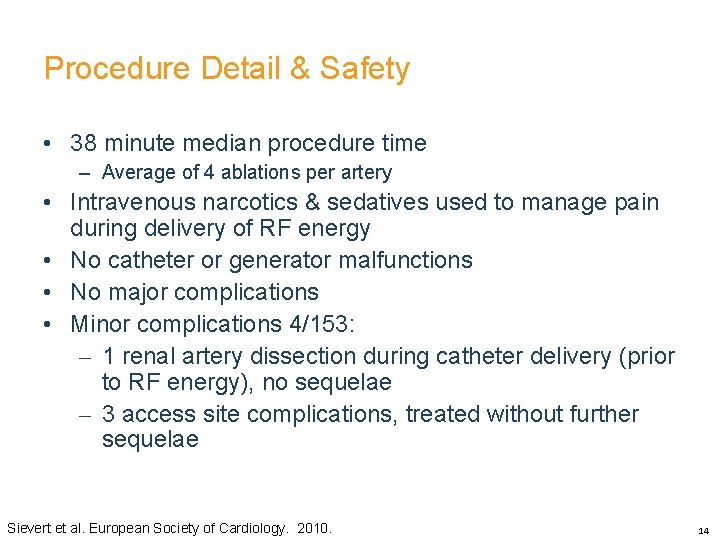
Procedure Detail & Safety • 38 minute median procedure time – Average of 4 ablations per artery • Intravenous narcotics & sedatives used to manage pain during delivery of RF energy • No catheter or generator malfunctions • No major complications • Minor complications 4/153: – 1 renal artery dissection during catheter delivery (prior to RF energy), no sequelae – 3 access site complications, treated without further sequelae Sievert et al. European Society of Cardiology. 2010. 14

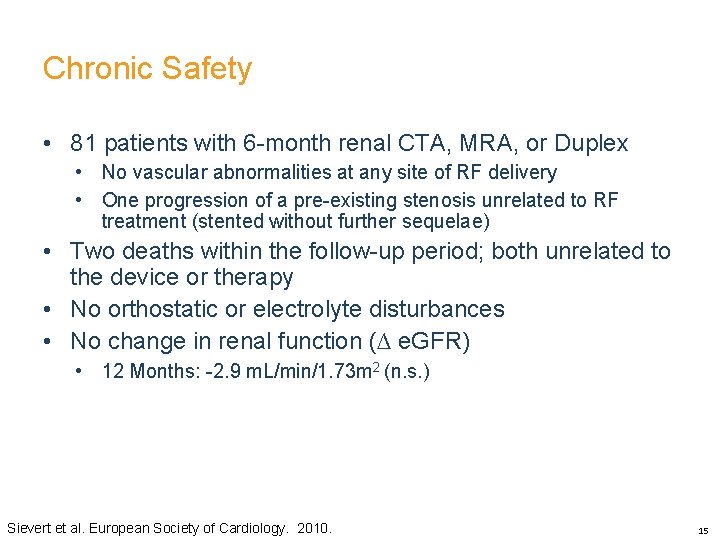
Chronic Safety • 81 patients with 6 -month renal CTA, MRA, or Duplex • No vascular abnormalities at any site of RF delivery • One progression of a pre-existing stenosis unrelated to RF treatment (stented without further sequelae) • Two deaths within the follow-up period; both unrelated to the device or therapy • No orthostatic or electrolyte disturbances • No change in renal function (∆ e. GFR) • 12 Months: -2. 9 m. L/min/1. 73 m 2 (n. s. ) Sievert et al. European Society of Cardiology. 2010. 15

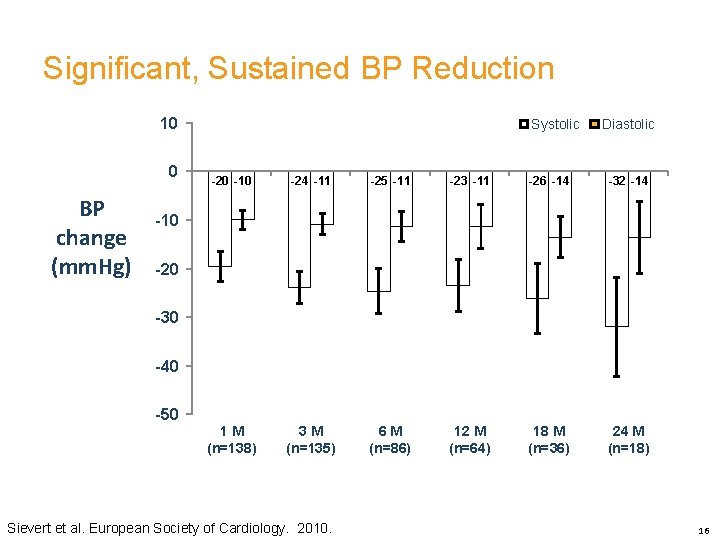
Significant, Sustained BP Reduction 10 0 BP change (mm. Hg) Systolic Diastolic -20 -10 -24 -11 -25 -11 -23 -11 -26 -14 -32 -14 1 M (n=138) 3 M (n=135) 6 M (n=86) 12 M (n=64) 18 M (n=36) 24 M (n=18) -10 -20 -30 -40 -50 Sievert et al. European Society of Cardiology. 2010. 16

Symplicity HTN-2 Lancet. 2010. published electronically on November 17, 2010 • Purpose: To demonstrate the effectiveness of catheter-based renal denervation for reducing blood pressure in patients with uncontrolled hypertension in a prospective, randomized, controlled, clinical trial • Patients: 106 patients randomized 1: 1 to treatment with renal denervation vs. control • Clinical Sites: 24 centers in Europe, Australia, & New Zealand (67% were designated hypertension centers of excellence) Symplicity HTN-2 Investigators. The Lancet. 2010. 17

Symplicity HTN-2 Trial Inclusion Criteria: – Office SBP ≥ 160 mm. Hg (≥ 150 mm. Hg with type II diabetes mellitus) – Stable drug regimen of 3+ more anti-HTN medications – Age 18 -85 years Exclusion Criteria: – Hemodynamically or anatomically significant renal artery abnormalities or prior renal artery intervention – e. GFR < 45 m. L/min/1. 73 m 2 (MDRD formula) – Type 1 diabetes mellitus – Contraindication to MRI – Stenotic valvular heart disease for which reduction of BP would be hazardous – MI, unstable angina, or CVA in the prior 6 months Symplicity HTN-2 Investigators. The Lancet. 2010. 18

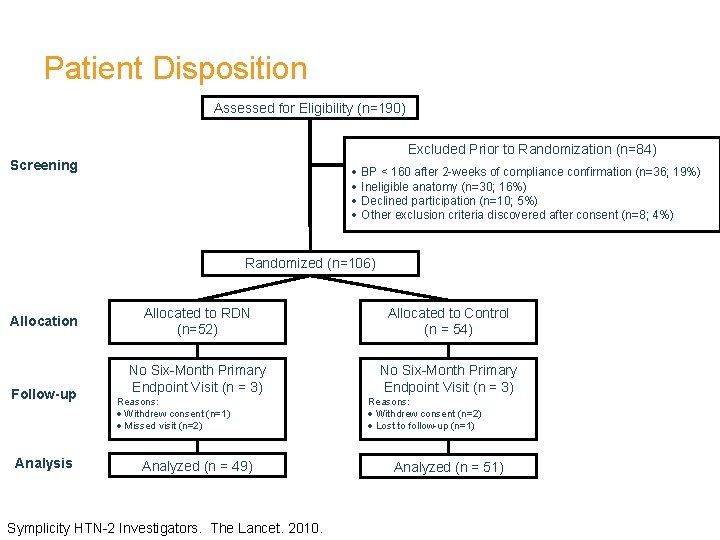
Patient Disposition Assessed for Eligibility (n=190) Excluded Prior to Randomization (n=84) Screening · BP < 160 after 2 -weeks of compliance confirmation (n=36; 19%) · Ineligible anatomy (n=30; 16%) · Declined participation (n=10; 5%) · Other exclusion criteria discovered after consent (n=8; 4%) Randomized (n=106) Allocation Follow-up Analysis Allocated to RDN (n=52) Allocated to Control (n = 54) No Six-Month Primary Endpoint Visit (n = 3) Reasons: · Withdrew consent (n=1) · Missed visit (n=2) Analyzed (n = 49) Symplicity HTN-2 Investigators. The Lancet. 2010. Reasons: · Withdrew consent (n=2) · Lost to follow-up (n=1) Analyzed (n = 51)

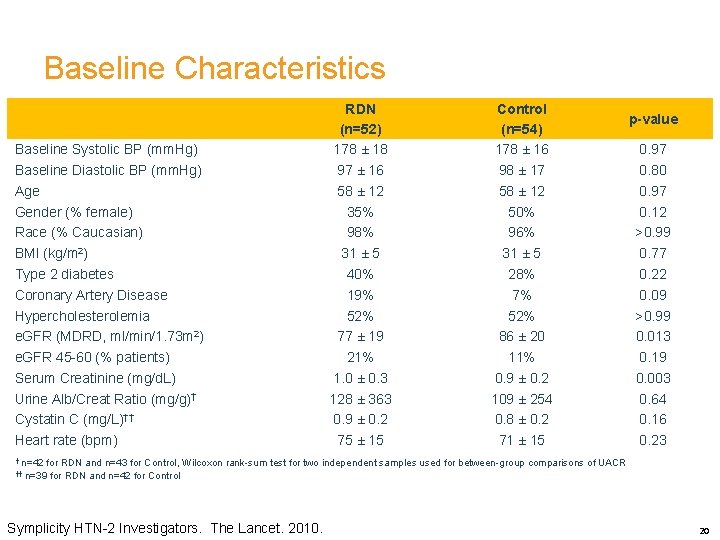
Baseline Characteristics Baseline Systolic BP (mm. Hg) Baseline Diastolic BP (mm. Hg) Age Gender (% female) Race (% Caucasian) BMI (kg/m 2) Type 2 diabetes Coronary Artery Disease Hypercholesterolemia e. GFR (MDRD, ml/min/1. 73 m 2) e. GFR 45 -60 (% patients) Serum Creatinine (mg/d. L) Urine Alb/Creat Ratio (mg/g)† Cystatin C (mg/L)†† Heart rate (bpm) RDN (n=52) 178 ± 18 97 ± 16 58 ± 12 35% 98% 31 ± 5 40% 19% 52% 77 ± 19 21% 1. 0 ± 0. 3 128 ± 363 0. 9 ± 0. 2 75 ± 15 Control (n=54) 178 ± 16 98 ± 17 58 ± 12 50% 96% 31 ± 5 28% 7% 52% 86 ± 20 11% 0. 9 ± 0. 2 109 ± 254 0. 8 ± 0. 2 71 ± 15 p-value 0. 97 0. 80 0. 97 0. 12 >0. 99 0. 77 0. 22 0. 09 >0. 99 0. 013 0. 19 0. 003 0. 64 0. 16 0. 23 † n=42 for RDN and n=43 for Control, Wilcoxon rank-sum test for two independent samples used for between-group comparisons of UACR †† n=39 for RDN and n=42 for Control Symplicity HTN-2 Investigators. The Lancet. 2010. 20

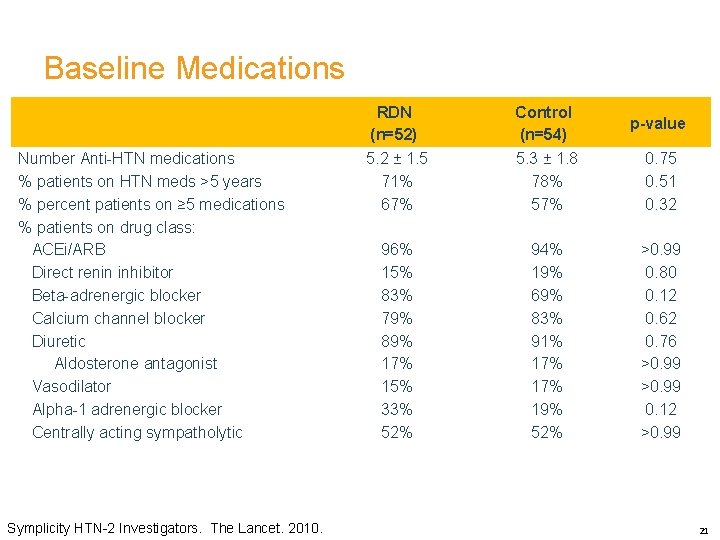
Baseline Medications Number Anti-HTN medications % patients on HTN meds >5 years % percent patients on ≥ 5 medications % patients on drug class: ACEi/ARB Direct renin inhibitor Beta-adrenergic blocker Calcium channel blocker Diuretic Aldosterone antagonist Vasodilator Alpha-1 adrenergic blocker Centrally acting sympatholytic Symplicity HTN-2 Investigators. The Lancet. 2010. RDN (n=52) Control (n=54) p-value 5. 2 ± 1. 5 71% 67% 5. 3 ± 1. 8 78% 57% 0. 75 0. 51 0. 32 96% 15% 83% 79% 89% 17% 15% 33% 52% 94% 19% 69% 83% 91% 17% 19% 52% >0. 99 0. 80 0. 12 0. 62 0. 76 >0. 99 0. 12 >0. 99 21

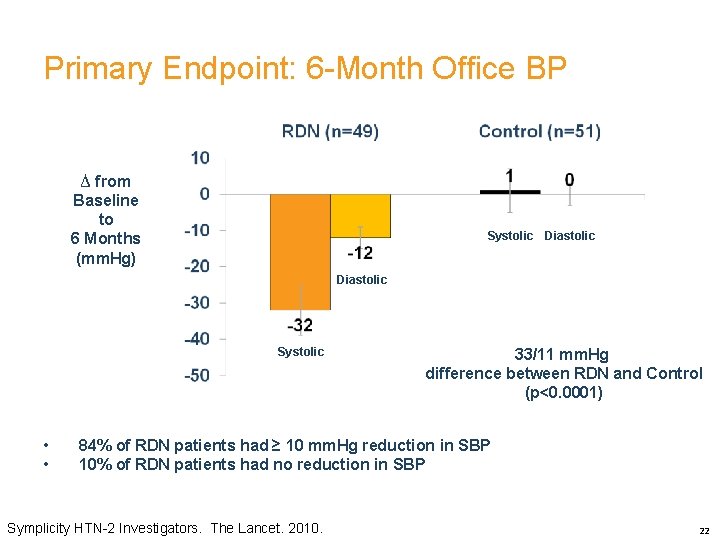
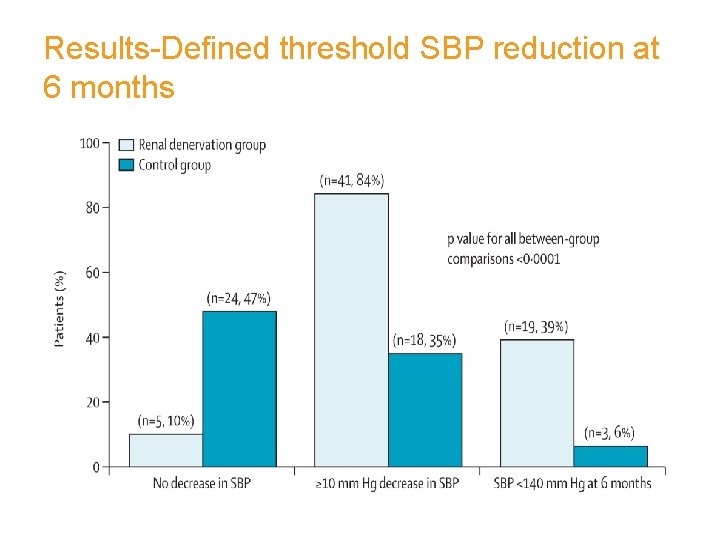
Primary Endpoint: 6 -Month Office BP ∆ from Baseline to 6 Months (mm. Hg) Systolic Diastolic Systolic • • 33/11 mm. Hg difference between RDN and Control (p<0. 0001) 84% of RDN patients had ≥ 10 mm. Hg reduction in SBP 10% of RDN patients had no reduction in SBP Symplicity HTN-2 Investigators. The Lancet. 2010. 22

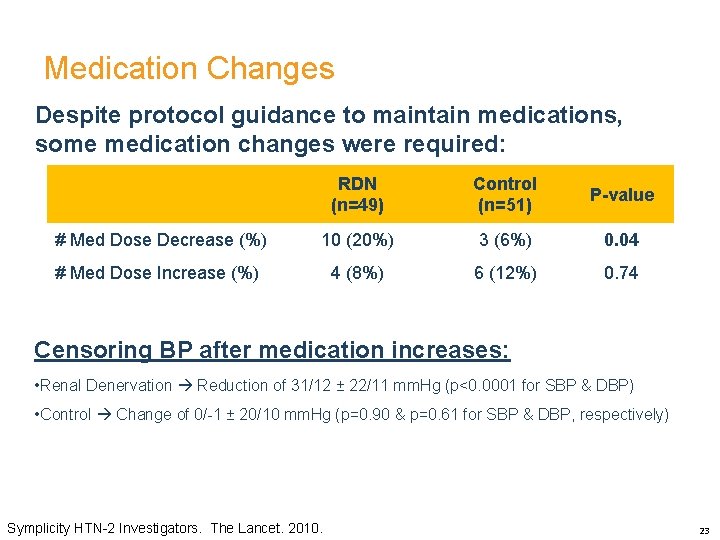
Medication Changes Despite protocol guidance to maintain medications, some medication changes were required: RDN (n=49) Control (n=51) P-value # Med Dose Decrease (%) 10 (20%) 3 (6%) 0. 04 # Med Dose Increase (%) 4 (8%) 6 (12%) 0. 74 Censoring BP after medication increases: • Renal Denervation Reduction of 31/12 ± 22/11 mm. Hg (p<0. 0001 for SBP & DBP) • Control Change of 0/-1 ± 20/10 mm. Hg (p=0. 90 & p=0. 61 for SBP & DBP, respectively) Symplicity HTN-2 Investigators. The Lancet. 2010. 23

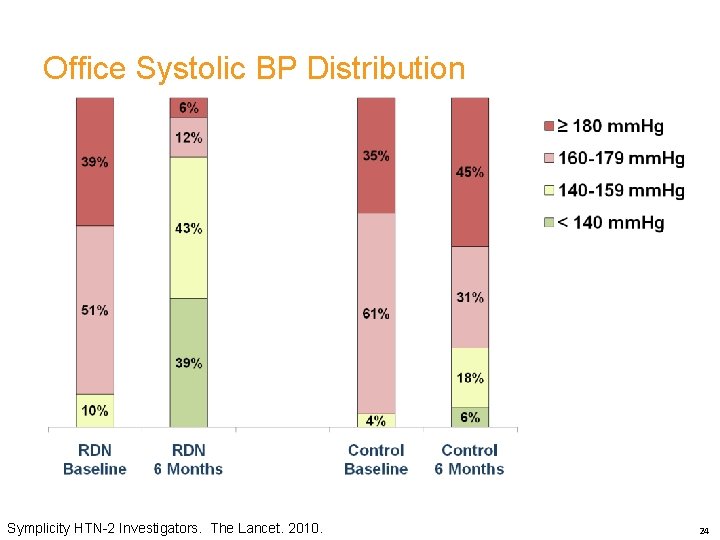
Office Systolic BP Distribution Symplicity HTN-2 Investigators. The Lancet. 2010. 24

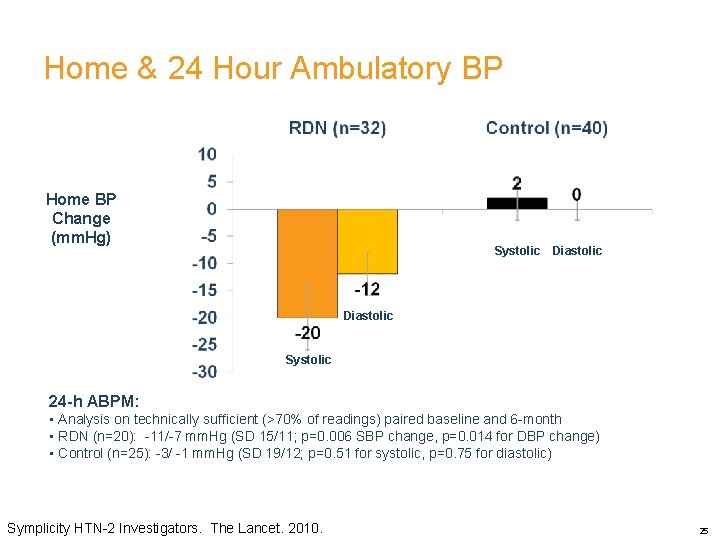
Home & 24 Hour Ambulatory BP Home BP Change BP (mm. Hg) Change (mm. H g) Systolic Diastolic Systolic 24 -h ABPM: • Analysis on technically sufficient (>70% of readings) paired baseline and 6 -month • RDN (n=20): -11/-7 mm. Hg (SD 15/11; p=0. 006 SBP change, p=0. 014 for DBP change) • Control (n=25): -3/ -1 mm. Hg (SD 19/12; p=0. 51 for systolic, p=0. 75 for diastolic) Symplicity HTN-2 Investigators. The Lancet. 2010. 25

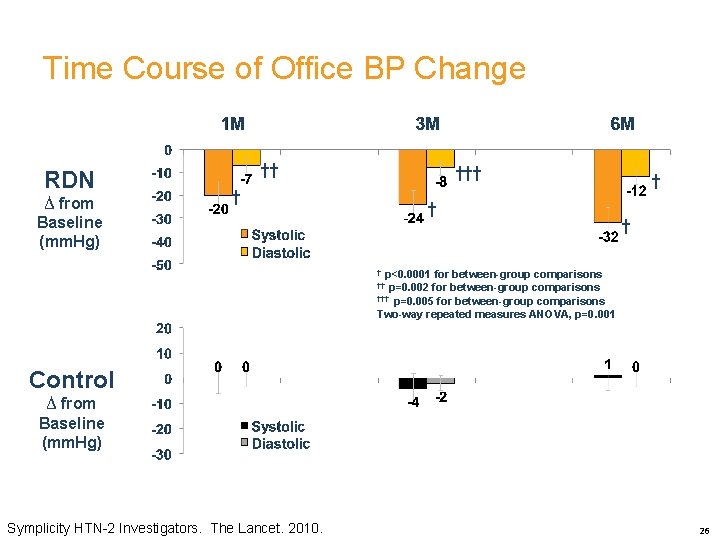
Time Course of Office BP Change RDN ∆ from Baseline (mm. Hg) †† † † † † p<0. 0001 for between-group comparisons †† p=0. 002 for between-group comparisons ††† p=0. 005 for between-group comparisons Two-way repeated measures ANOVA, p=0. 001 Control ∆ from Baseline (mm. Hg) Symplicity HTN-2 Investigators. The Lancet. 2010. 26

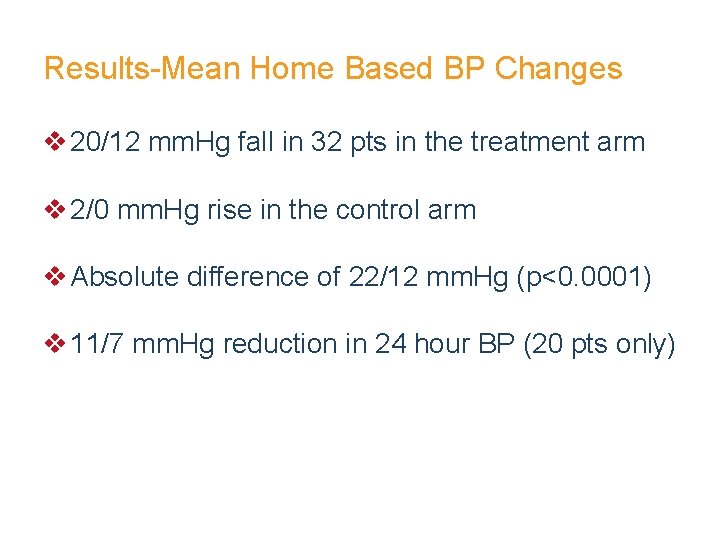
Results-Mean Home Based BP Changes v 20/12 mm. Hg fall in 32 pts in the treatment arm v 2/0 mm. Hg rise in the control arm v Absolute difference of 22/12 mm. Hg (p<0. 0001) v 11/7 mm. Hg reduction in 24 hour BP (20 pts only)

Results-Defined threshold SBP reduction at 6 months

Procedural Safety • No serious device or procedure related adverse events (n=52) • Minor adverse events • 1 femoral artery pseudoaneurysm treated with manual compression • 1 post-procedural drop in BP resulting in a reduction in medication • 1 urinary tract infection • 1 prolonged hospitalization for evaluation of paraesthesias • 1 back pain treated with pain medications & resolved after one month • 6 -month renal imaging (n=43) • • No vascular abnormality at any RF treatment site 1 MRA indicates possible progression of a pre-existing stenosis unrelated to RF treatment (no furtherapy warranted) Symplicity HTN-2 Investigators. The Lancet. 2010. 29

Conclusions v BP reduction can be achieved with catheter based renal denervation v No major adverse effects of renal denervation v Affirms the crucial relevance of the renal sympathetic system in the maintenance of hypertension

Limitations % A safety & efficacy study % Limited Number of patients % Short follow-up period % Up to 10% of patients did not respond to the treatment

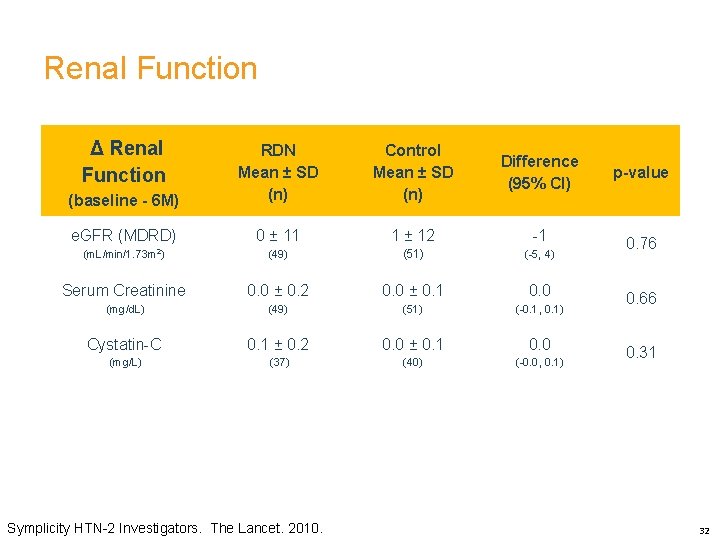
Renal Function Δ Renal Function Control Mean ± SD (n) Difference (95% CI) p-value (baseline - 6 M) RDN Mean ± SD (n) e. GFR (MDRD) 0 ± 11 1 ± 12 -1 (m. L/min/1. 73 m 2) (49) (51) (-5, 4) 0. 76 Serum Creatinine 0. 0 ± 0. 2 0. 0 ± 0. 1 0. 0 (mg/d. L) (49) (51) (-0. 1, 0. 1) Cystatin-C 0. 1 ± 0. 2 0. 0 ± 0. 1 0. 0 (mg/L) (37) (40) (-0. 0, 0. 1) Symplicity HTN-2 Investigators. The Lancet. 2010. 0. 66 0. 31 32

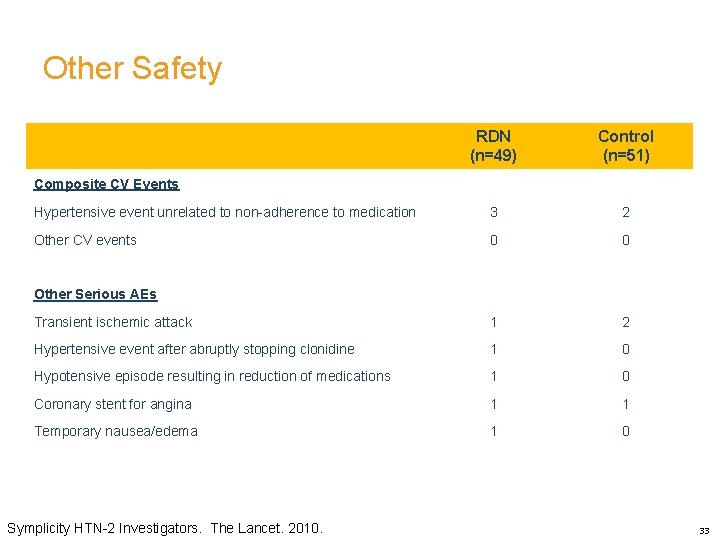
Other Safety RDN (n=49) Control (n=51) Hypertensive event unrelated to non-adherence to medication 3 2 Other CV events 0 0 Transient ischemic attack 1 2 Hypertensive event after abruptly stopping clonidine 1 0 Hypotensive episode resulting in reduction of medications 1 0 Coronary stent for angina 1 1 Temporary nausea/edema 1 0 Composite CV Events Other Serious AEs Symplicity HTN-2 Investigators. The Lancet. 2010. 33

Questions ? ? ?

VALIDATION OF PHYSIOLOGY 35

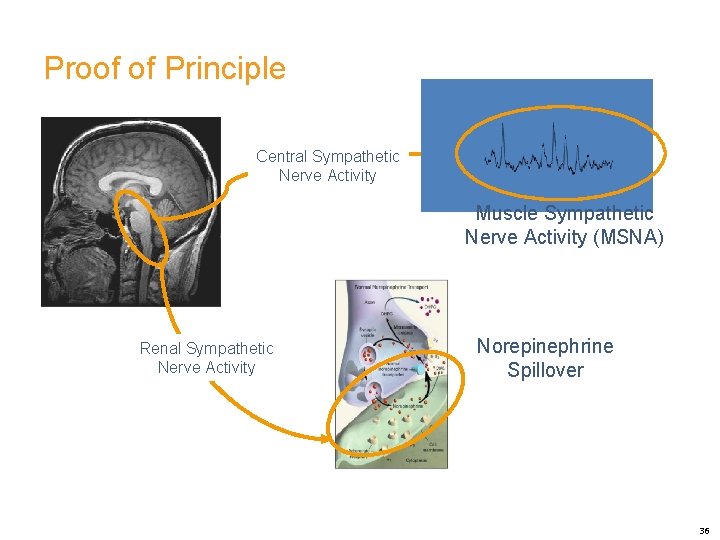
Proof of Principle Central Sympathetic Nerve Activity Muscle Sympathetic Nerve Activity (MSNA) Renal Sympathetic Nerve Activity Norepinephrine Spillover 36

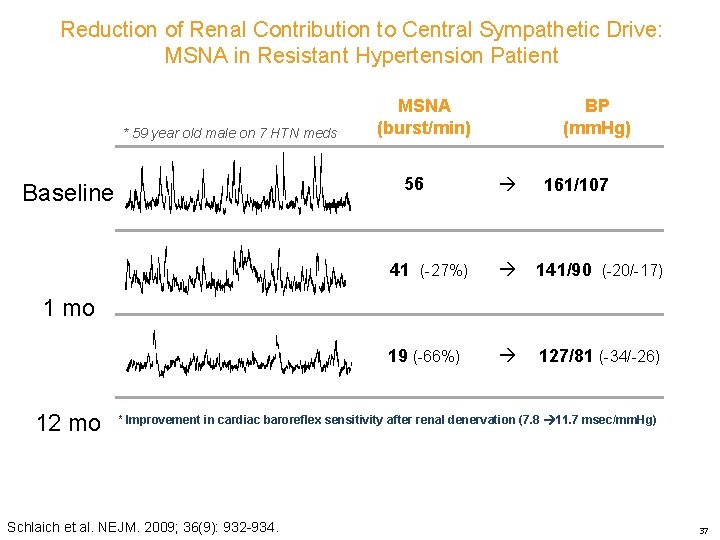
Reduction of Renal Contribution to Central Sympathetic Drive: MSNA in Resistant Hypertension Patient * 59 year old male on 7 HTN meds MSNA (burst/min) 56 Baseline BP (mm. Hg) 161/107 41 (-27%) 141/90 (-20/-17) 19 (-66%) 127/81 (-34/-26) 1 mo 12 mo * Improvement in cardiac baroreflex sensitivity after renal denervation (7. 8 11. 7 msec/mm. Hg) Schlaich et al. NEJM. 2009; 36(9): 932 -934. 37

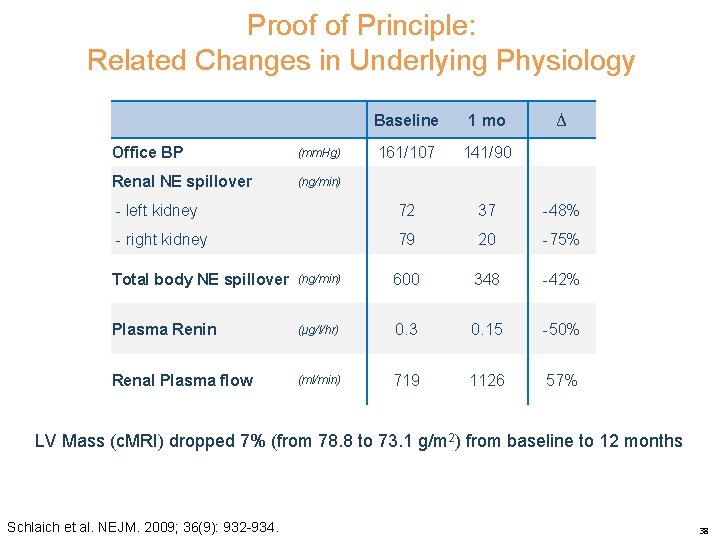
Proof of Principle: Related Changes in Underlying Physiology Baseline 1 mo 161/107 141/90 - left kidney 72 37 -48% - right kidney 79 20 -75% Office BP (mm. Hg) Renal NE spillover (ng/min) ∆ Total body NE spillover (ng/min) 600 348 -42% Plasma Renin (μg/l/hr) 0. 3 0. 15 -50% Renal Plasma flow (ml/min) 719 1126 57% LV Mass (c. MRI) dropped 7% (from 78. 8 to 73. 1 g/m 2) from baseline to 12 months Schlaich et al. NEJM. 2009; 36(9): 932 -934. 38

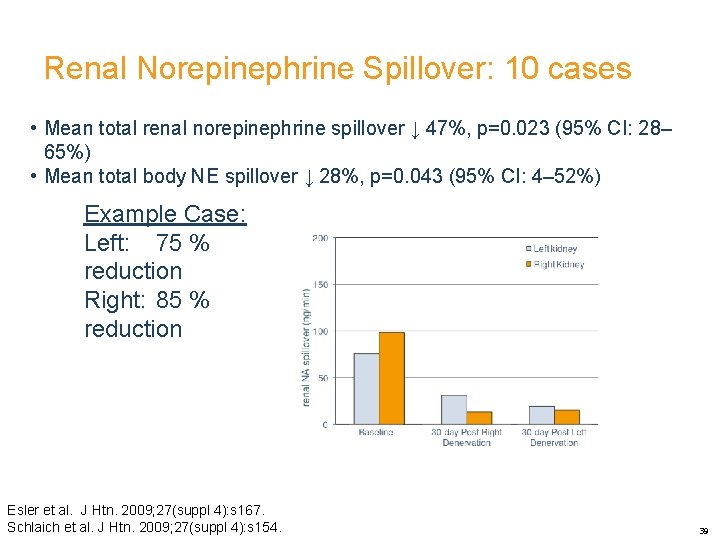
Renal Norepinephrine Spillover: 10 cases • Mean total renal norepinephrine spillover ↓ 47%, p=0. 023 (95% CI: 28– 65%) • Mean total body NE spillover ↓ 28%, p=0. 043 (95% CI: 4– 52%) Example Case: Left: 75 % reduction Right: 85 % reduction Esler et al. J Htn. 2009; 27(suppl 4): s 167. Schlaich et al. J Htn. 2009; 27(suppl 4): s 154. 39
 Renal denervation
Renal denervation Renal denervation
Renal denervation Optic nerve hypoplasia treatment
Optic nerve hypoplasia treatment Wegner grossman theory
Wegner grossman theory Trigeminal nerve which cranial nerve
Trigeminal nerve which cranial nerve Vad är ett minoritetsspråk
Vad är ett minoritetsspråk Typiska novell drag
Typiska novell drag Frgar
Frgar Autokratiskt ledarskap
Autokratiskt ledarskap Indikation för kejsarsnitt på moderns önskan
Indikation för kejsarsnitt på moderns önskan Blomman för dagen drog
Blomman för dagen drog Redogör för vad psykologi är
Redogör för vad psykologi är En lathund för arbete med kontinuitetshantering
En lathund för arbete med kontinuitetshantering Mat för idrottare
Mat för idrottare Gumman cirkel
Gumman cirkel Bästa kameran för astrofoto
Bästa kameran för astrofoto Ledarskapsteorier
Ledarskapsteorier Offentlig förvaltning
Offentlig förvaltning Mantel för kvinnor i antikens rom
Mantel för kvinnor i antikens rom Datorkunskap för nybörjare
Datorkunskap för nybörjare Rita perspektiv
Rita perspektiv Vilken grundregel finns det för tronföljden i sverige?
Vilken grundregel finns det för tronföljden i sverige? Förklara densitet för barn
Förklara densitet för barn Ministerstyre för och nackdelar
Ministerstyre för och nackdelar Bamse för de yngsta
Bamse för de yngsta Tillitsbaserad ledning
Tillitsbaserad ledning Vem räknas som jude
Vem räknas som jude Tack för att ni lyssnade bild
Tack för att ni lyssnade bild Lyrik
Lyrik Nyckelkompetenser för livslångt lärande
Nyckelkompetenser för livslångt lärande Sju för caesar
Sju för caesar Datumr
Datumr Borstål, egenskaper
Borstål, egenskaper Personalliggare bygg undantag
Personalliggare bygg undantag Verktyg för automatisering av utbetalningar
Verktyg för automatisering av utbetalningar Texter för hinduer tantra
Texter för hinduer tantra Kolposkopi px
Kolposkopi px Urban torhamn
Urban torhamn Strategi för svensk viltförvaltning
Strategi för svensk viltförvaltning Tack för att ni lyssnade bild
Tack för att ni lyssnade bild