Renal Sympathetic Denervation for Treatment of Resistant Hypertension






- Slides: 20

Renal Sympathetic Denervation for Treatment of Resistant Hypertension: One-Year Results from the Symplicity HTN-2 Randomized Controlled Trial Prof Murray Esler Baker IDI Heart and Diabetes Institute, Melbourne On behalf of Henry Krum, Markus Schlaich, Roland Schmieder, Michael Böhm, Paul Sobotka and the Symplicity HTN-2 Investigators

Disclaimer Speaker is the Chief Investigator of the multicentre randomized international trial of therapeutic endovascular renal denervation with the Symplicity catheter in drug-resistant hypertension (HTN-2 trial), and is a recipient of research grant, travel and consultancy funding from Medtronic and Ardian Inc. He holds no stock or shares in either company, or patent rights for renal denervation.

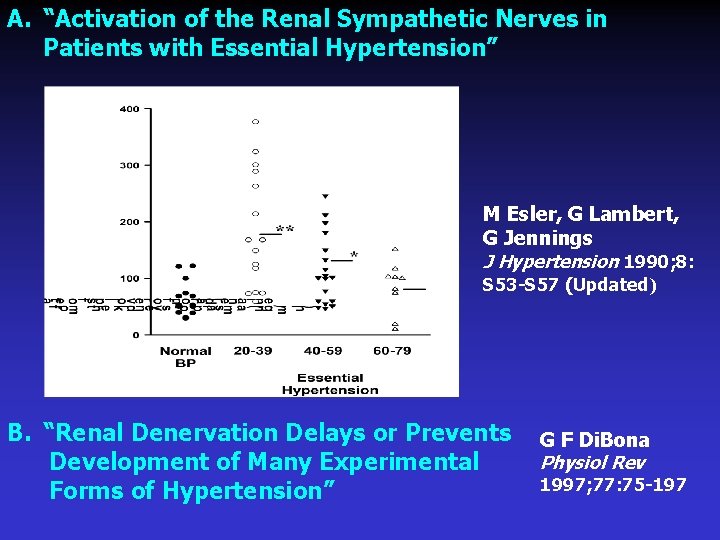
A. “Activation of the Renal Sympathetic Nerves in Patients with Essential Hypertension” M Esler, G Lambert, G Jennings J Hypertension 1990; 8: S 53 -S 57 (Updated) B. “Renal Denervation Delays or Prevents G F Di. Bona Physiol Rev Development of Many Experimental 1997; 77: 75 -197 Forms of Hypertension”

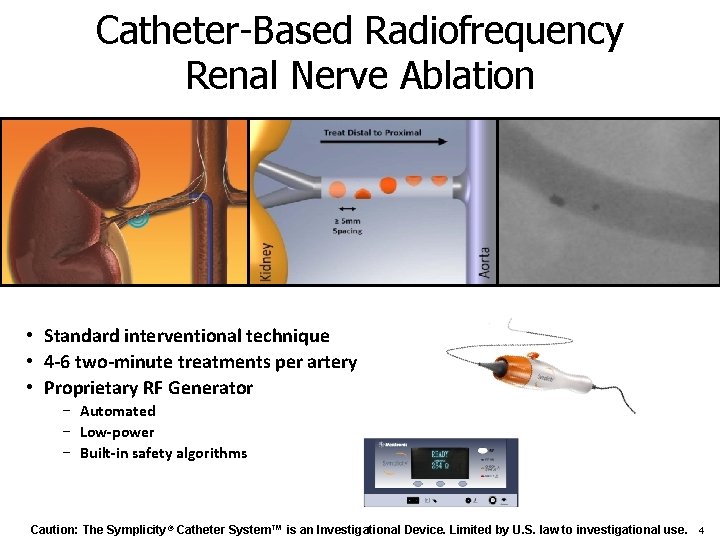
Catheter-Based Radiofrequency Renal Nerve Ablation • Standard interventional technique • 4 -6 two-minute treatments per artery • Proprietary RF Generator − Automated − Low-power − Built-in safety algorithms Caution: The Symplicity® Catheter System™ is an Investigational Device. Limited by U. S. law to investigational use. 4

Symplicity HTN-2 Design • Purpose: To demonstrate the effectiveness of catheter-based renal denervation (RDN) for reducing blood pressure in patients with uncontrolled hypertension in a prospective, randomized, controlled, clinical trial • Patients: 106 patients with drug-resistant hypertension randomized 1: 1 to treatment with RDN vs. control • Clinical Sites: 24 centers in Europe, Australia, & New Zealand • 67% were designated hypertension centers of excellence • Primary Endpoint: Office systolic BP change from baseline at 6 months Symplicity HTN-2 Investigators. Lancet. 2010; 376: 1903 -1909. 5

Participating Centers: HTN-2 Randomized Controlled Trial • • • Universitätsklinikum des Saarlandes Homburg, Germany (Michael Böhm) Cardio. Vascular Center Frankfurt, Germany (Horst Sievert) Universitätsklinikum Düsseldorf, Germany (Lars Christian Rump) Universität Erlangen-Nürnberg Erlangen, Germany (Roland Schmieder) Barts and The London, UK (Mel Lobo) Pauls Stradins Clinical University Hospital Riga, Latvia (Andrejs Erglis) L'Hôpital Européen Georges Pompidou Paris, France (Guillaume Bobrie) John Hunter Hospital Newcastle, Australia (Suku Thambar) Cliniques Universitaires Saint-Luc Brussels, Belgium (Alexandre Persu) Universitaetsklinikum Schleswig-Holstein Lübeck, Germany (Heribert Schunkert) Universität zu Köln, Germany (Uta Hoppe) The Alfred Hospital Melbourne, Australia (Henry Krum) Symplicity HTN-2 Investigators. Lancet. 2010; 376: 1903 -1909. • • • Universität Leipzig – Herzzentrum Leipzig, Germany (Dierk Scheinert) Allgemeines Krankenhaus der Stadt Wien Vienna, Austria (Thomas Binder) Samodzielna Pracownia Hemodynamiczna Warsaw, Poland (Andrzej Januszewicz & Adam Witkowski) Hospital 12 de Octubre Madrid, Spain (Luis Ruilope) St. Vincent’s Hospital Melbourne, Australia (Robert Whitbourn) Universitätsklinikum Essen, Germany (Heike Bruck) Kent and Canterbury Hospital Canterbury, UK (Mark Downes) University Hospital Zurich, Switzerland (Thomas Lüscher) University of Glasgow, UK (Alan Jardine) Auckland City Hospital Auckland, New Zealand (Mark Webster) Herz-Zentrum Bad Krozingen, Germany (Thomas Zeller) The John Paul II Hospital Krakow, Poland (Jerzy Sadowski)

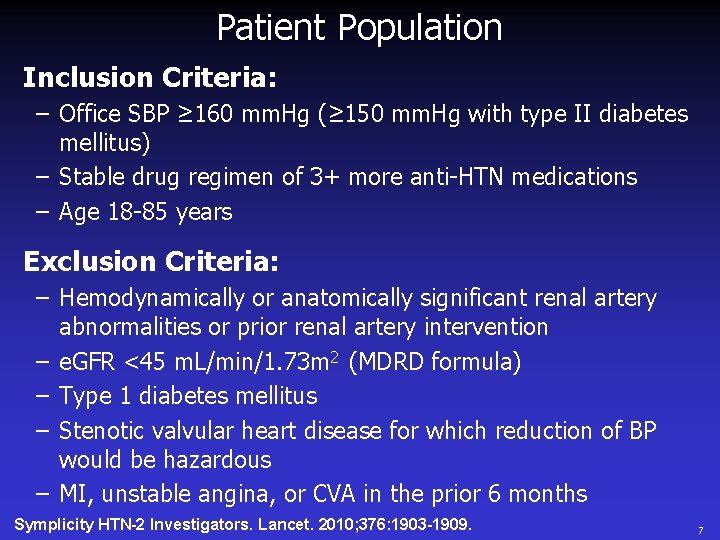
Patient Population Inclusion Criteria: – Office SBP ≥ 160 mm. Hg (≥ 150 mm. Hg with type II diabetes mellitus) – Stable drug regimen of 3+ more anti-HTN medications – Age 18 -85 years Exclusion Criteria: – Hemodynamically or anatomically significant renal artery abnormalities or prior renal artery intervention – e. GFR <45 m. L/min/1. 73 m 2 (MDRD formula) – Type 1 diabetes mellitus – Stenotic valvular heart disease for which reduction of BP would be hazardous – MI, unstable angina, or CVA in the prior 6 months Symplicity HTN-2 Investigators. Lancet. 2010; 376: 1903 -1909. 7

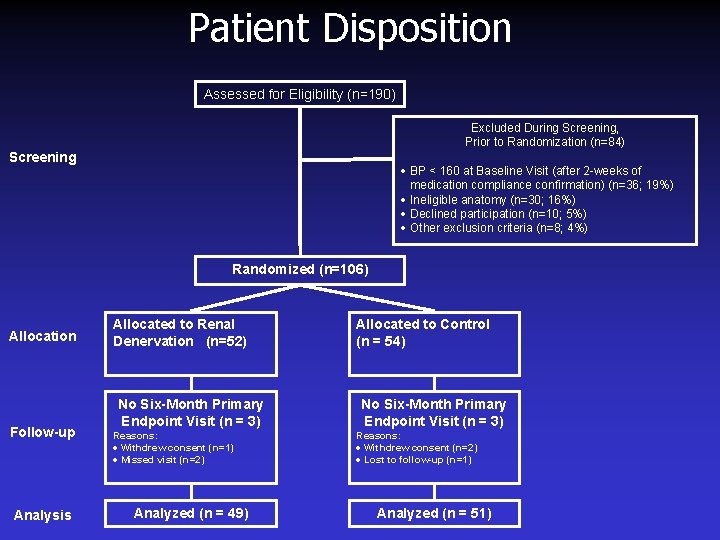
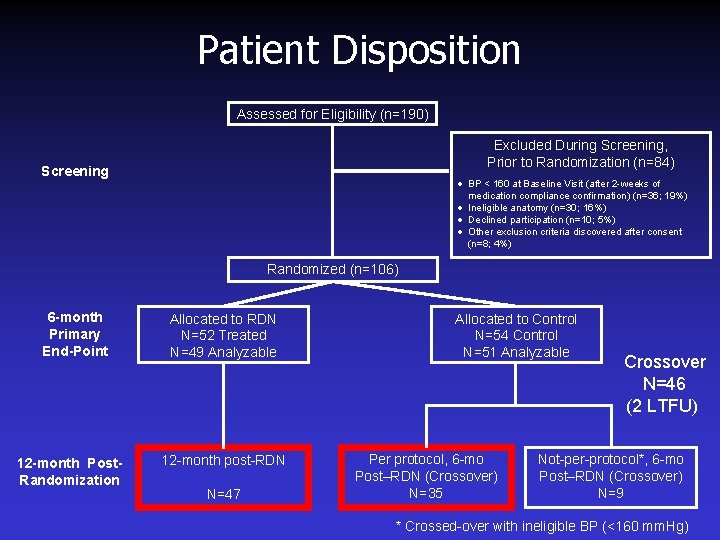
Patient Disposition Assessed for Eligibility (n=190) Excluded During Screening, Prior to Randomization (n=84) Screening · BP < 160 at Baseline Visit (after 2 -weeks of medication compliance confirmation) (n=36; 19%) · Ineligible anatomy (n=30; 16%) · Declined participation (n=10; 5%) · Other exclusion criteria (n=8; 4%) Randomized (n=106) Allocation Follow-up Analysis Allocated to Renal Denervation (n=52) No Six-Month Primary Endpoint Visit (n = 3) Reasons: · Withdrew consent (n=1) · Missed visit (n=2) Analyzed (n = 49) Allocated to Control (n = 54) No Six-Month Primary Endpoint Visit (n = 3) Reasons: · Withdrew consent (n=2) · Lost to follow-up (n=1) Analyzed (n = 51)

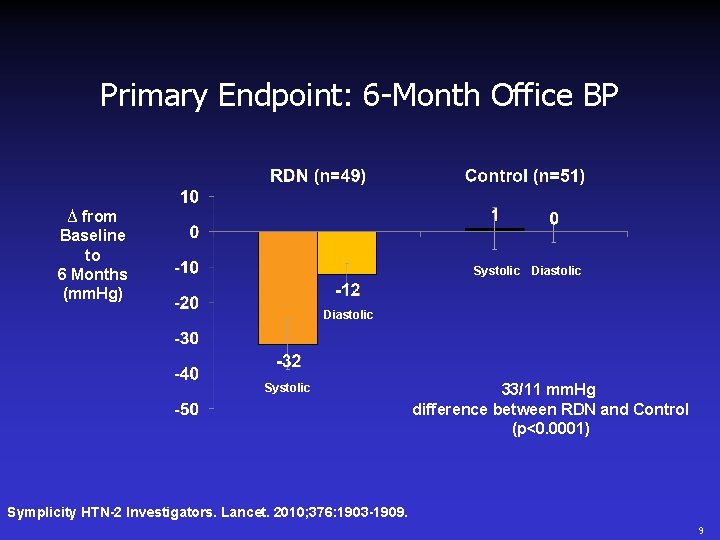
Primary Endpoint: 6 -Month Office BP ∆ from Baseline to 6 Months (mm. Hg) Systolic Diastolic Systolic 33/11 mm. Hg difference between RDN and Control (p<0. 0001) Symplicity HTN-2 Investigators. Lancet. 2010; 376: 1903 -1909. 9

Patient Disposition Assessed for Eligibility (n=190) Excluded During Screening, Prior to Randomization (n=84) Screening · BP < 160 at Baseline Visit (after 2 -weeks of medication compliance confirmation) (n=36; 19%) · Ineligible anatomy (n=30; 16%) · Declined participation (n=10; 5%) · Other exclusion criteria discovered after consent (n=8; 4%) Randomized (n=106) 6 -month Primary End-Point 12 -month Post. Randomization Allocated to RDN N=52 Treated N=49 Analyzable 12 -month post-RDN N=47 Allocated to Control N=54 Control N=51 Analyzable Per protocol, 6 -mo Post–RDN (Crossover) N=35 Crossover N=46 (2 LTFU) Not-per-protocol*, 6 -mo Post–RDN (Crossover) N=9 * Crossed-over with ineligible BP (<160 mm. Hg)

HTN-2 Baseline Demographics RDN (n=49) Cross-Over (n=35) Office SBP 178. 3 ± 18. 2 182. 8 ± 16. 3 Office DBP 96. 1 ± 15. 5 99. 1 ± 17. 0 Age 59. 0 ± 11. 5 58. 1 ± 13. 0 Gender (% female) 32. 7% 60. 0% Race (% Caucasian) 98. 0% 97. 1% 30. 8 ± 5. 2 31. 5 ± 5. 3 Type II DM 42. 9% 28. 6% CAD 18. 4% 5. 7% Hypercholesterolemia 53. 1% 45. 7% Heart Rate 75 ± 15 73 ± 15 e. GFR (ml/min/1. 73 m 2)* 76. 9 ± 19. 3 88. 8 ± 20. 7 Serum Creatinine (mg/d. L) 1. 03 ± 0. 29 0. 84 ± 0. 21 Cystatin C (mg/L) 0. 91 ± 0. 25 0. 78 ± 0. 17 BMI Standard deviations are presented unless otherwise specified *e. GFR is calculated per MDRD formula throughout

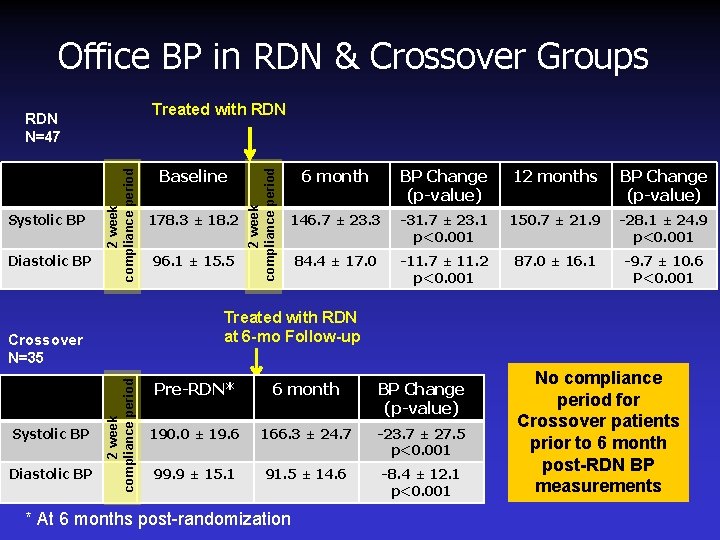
Office BP in RDN & Crossover Groups Diastolic BP 2 week compliance period Systolic BP 2 week compliance period Diastolic BP 178. 3 ± 18. 2 96. 1 ± 15. 5 6 month BP Change (p-value) 12 months BP Change (p-value) 146. 7 ± 23. 3 -31. 7 ± 23. 1 p<0. 001 150. 7 ± 21. 9 -28. 1 ± 24. 9 p<0. 001 84. 4 ± 17. 0 -11. 7 ± 11. 2 p<0. 001 87. 0 ± 16. 1 -9. 7 ± 10. 6 P<0. 001 Treated with RDN at 6 -mo Follow-up Crossover N=35 Systolic BP Baseline 2 week compliance period Treated with RDN N=47 Pre-RDN* 6 month BP Change (p-value) 190. 0 ± 19. 6 166. 3 ± 24. 7 -23. 7 ± 27. 5 p<0. 001 99. 9 ± 15. 1 91. 5 ± 14. 6 -8. 4 ± 12. 1 p<0. 001 * At 6 months post-randomization No compliance period for Crossover patients prior to 6 month post-RDN BP measurements

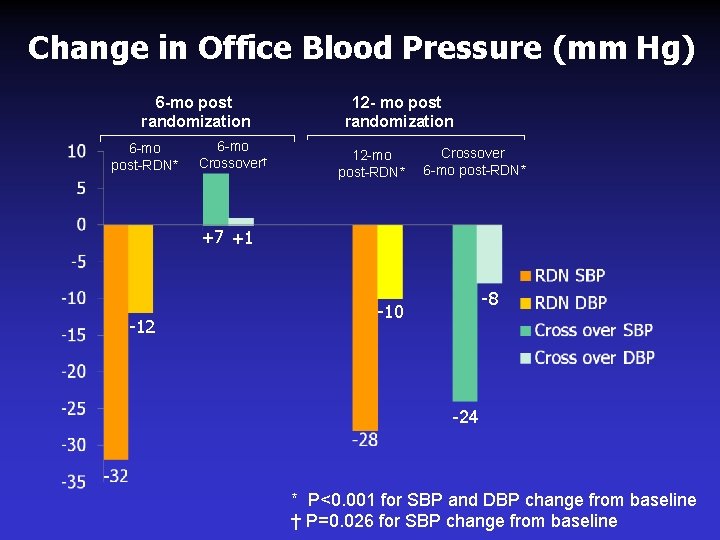
Change in Office Blood Pressure (mm Hg) 6 -mo post randomization 6 -mo post-RDN* 6 -mo Crossover† 12 - mo post randomization 12 -mo post-RDN* Crossover 6 -mo post-RDN* +7 +1 -12 -8 -10 -24 * P<0. 001 for SBP and DBP change from baseline † P=0. 026 for SBP change from baseline

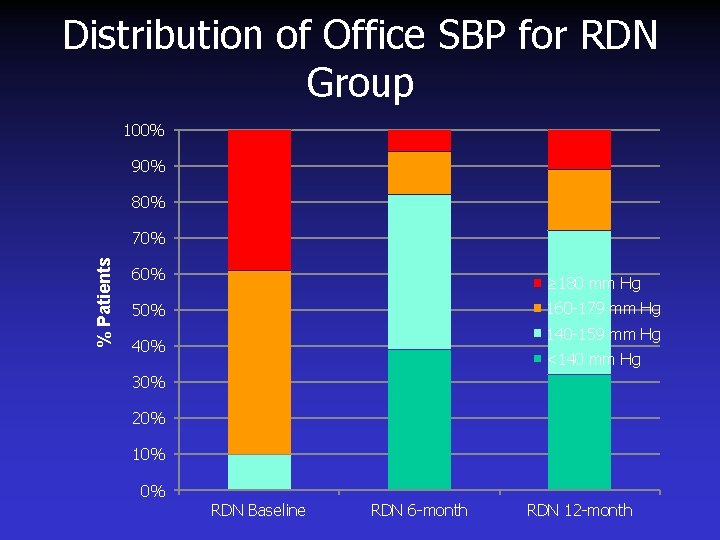
Distribution of Office SBP for RDN Group 100% 90% 80% % Patients 70% 60% ≥ 180 mm Hg 160 -179 mm Hg 50% 140 -159 mm Hg 40% <140 mm Hg 30% 20% 10% 0% RDN Baseline RDN 6 -month RDN 12 -month

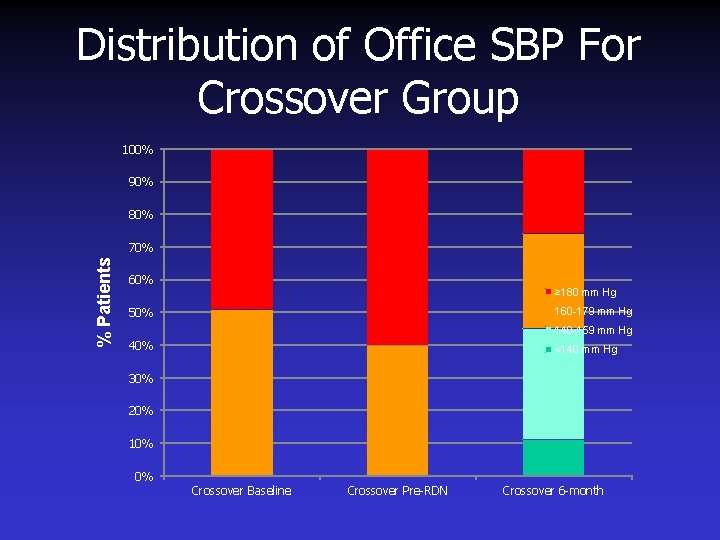
Distribution of Office SBP For Crossover Group 100% 90% 80% % Patients 70% 60% ≥ 180 mm Hg 160 -179 mm Hg 50% 140 -159 mm Hg 40% <140 mm Hg 30% 20% 10% 0% Crossover Baseline Crossover Pre-RDN Crossover 6 -month

Crossover Procedural Safety • One renal artery dissection following guide catheter insertion during angiography. The lesion was stented without further • One hospitalization prolonged in a crossover patient due to hypotension following the RDN procedure. IV fluids administered, antihypertensive medications decreased and patient discharge without further incident • No radiofrequency-related renal artery stenosis or aneurysm occurred in any patient

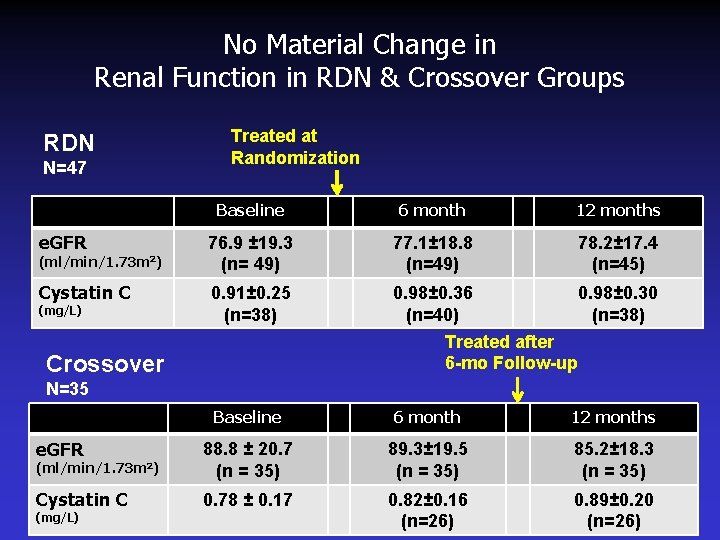
No Material Change in Renal Function in RDN & Crossover Groups RDN N=47 Treated at Randomization Baseline 6 month 12 months e. GFR 76. 9 ± 19. 3 (n= 49) 77. 1± 18. 8 (n=49) 78. 2± 17. 4 (n=45) Cystatin C 0. 91± 0. 25 (n=38) 0. 98± 0. 36 (n=40) 0. 98± 0. 30 (n=38) (ml/min/1. 73 m 2) (mg/L) Treated after 6 -mo Follow-up Crossover N=35 Baseline 6 month 12 months e. GFR (ml/min/1. 73 m 2) 88. 8 ± 20. 7 (n = 35) 89. 3± 19. 5 (n = 35) 85. 2± 18. 3 (n = 35) Cystatin C 0. 78 ± 0. 17 0. 82± 0. 16 (n=26) 0. 89± 0. 20 (n=26) (mg/L)

Other Safety 6 to 12 Months Post Randomization • Three hypertensive events occurred in 2 patients in the crossover group requiring hospitalization • No deaths occurred at any time point during the follow up period for either group • No observed changes in e. GFR

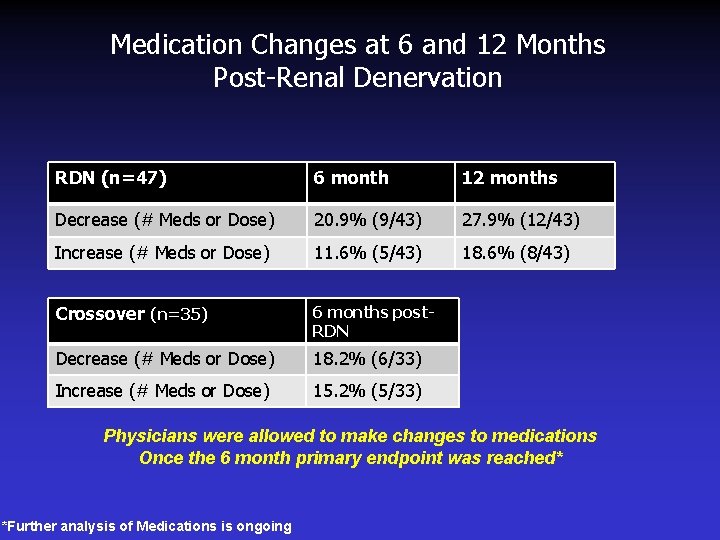
Medication Changes at 6 and 12 Months Post-Renal Denervation RDN (n=47) 6 month 12 months Decrease (# Meds or Dose) 20. 9% (9/43) 27. 9% (12/43) Increase (# Meds or Dose) 11. 6% (5/43) 18. 6% (8/43) Crossover (n=35) 6 months post. RDN Decrease (# Meds or Dose) 18. 2% (6/33) Increase (# Meds or Dose) 15. 2% (5/33) Physicians were allowed to make changes to medications Once the 6 month primary endpoint was reached* *Further analysis of Medications is ongoing

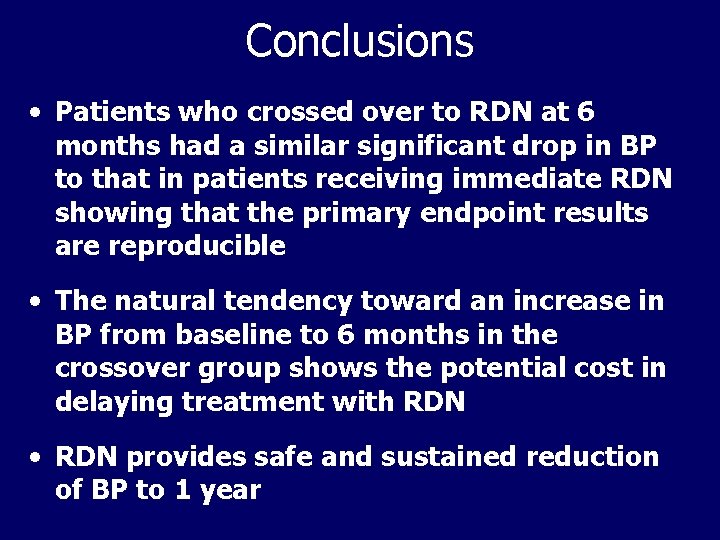
Conclusions • Patients who crossed over to RDN at 6 months had a similar significant drop in BP to that in patients receiving immediate RDN showing that the primary endpoint results are reproducible • The natural tendency toward an increase in BP from baseline to 6 months in the crossover group shows the potential cost in delaying treatment with RDN • RDN provides safe and sustained reduction of BP to 1 year
 Barostim
Barostim Renal denervation
Renal denervation Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Teoria do nefron intacto
Teoria do nefron intacto Peritubular capillaries and vasa recta difference
Peritubular capillaries and vasa recta difference Malignant hypertention
Malignant hypertention Hypokalemia
Hypokalemia Resistant materials tools
Resistant materials tools Iatrogenic withdrawal syndrome
Iatrogenic withdrawal syndrome Fire resistant environmental ensemble
Fire resistant environmental ensemble Insect-resistant packaging solutions
Insect-resistant packaging solutions Collision resistant hash function
Collision resistant hash function Earthquake resistant
Earthquake resistant Design of seismic-resistant steel building structures
Design of seismic-resistant steel building structures Local buckling
Local buckling Spill resistant vacuum breaker
Spill resistant vacuum breaker Fire resistant cable thai yazaki
Fire resistant cable thai yazaki Collision resistant hash function
Collision resistant hash function Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Glycopeptide
Glycopeptide Cyanide-resistant respiration slideshare
Cyanide-resistant respiration slideshare