Renal Sympathetic Denervation for Treatment of Resistant Hypertension







- Slides: 24

Renal Sympathetic Denervation for Treatment of Resistant Hypertension: 24 -Month Results from the Symplicity HTN -2 Randomized Controlled Trial Henry Krum MBBS Ph. D FRACP FESC Monash University, Melbourn, Australia On behalf of Murray Esler, Markus Schlaich, Roland Schmieder, Michael Böhm, Paul Sobotka and the Symplicity HTN-2 Investigators For OMA distribution only. Not for distribution in the USA or Japan. Trademarks may be registered and are the property of their respective owners. © 2013 Medtronic, Inc. All rights reserved. 10093676 DOC_1 A. 03/2013

Disclaimer Henry Krum MBBS Ph. D FRACP FESC Research grants, speakers bureau, advisory board, honorarium: Medtronic For OMA distribution only. Not for distribution in the USA or Japan. Trademarks may be registered and are the property of their respective owners. © 2013 Medtronic, Inc. All rights reserved. 10093676 DOC_1 A. 03/2013

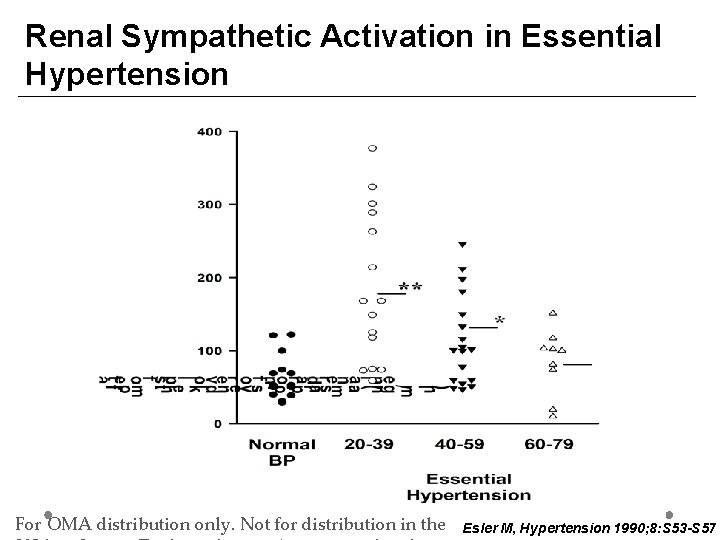
Renal Sympathetic Activation in Essential Hypertension Age (yrs) For OMA distribution only. Not for distribution in the Esler M, Hypertension 1990; 8: S 53 -S 57

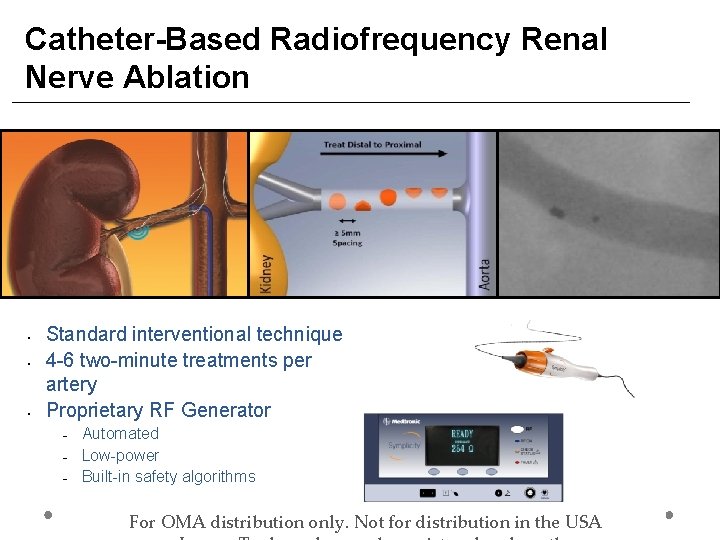
Catheter-Based Radiofrequency Renal Nerve Ablation • • • Standard interventional technique 4 -6 two-minute treatments per artery Proprietary RF Generator − − − Automated Low-power Built-in safety algorithms For OMA distribution only. Not for distribution in the USA

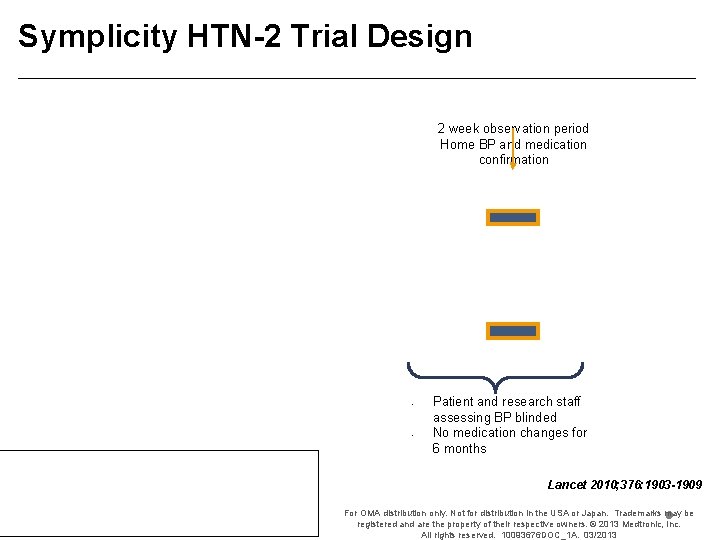
Symplicity HTN-2 Trial Design 2 week observation period Home BP and medication confirmation • • Patient and research staff assessing BP blinded No medication changes for 6 months Lancet 2010; 376: 1903 -1909 For OMA distribution only. Not for distribution in the USA or Japan. Trademarks may be registered and are the property of their respective owners. © 2013 Medtronic, Inc. All rights reserved. 10093676 DOC_1 A. 03/2013

Participating Centers • • • • Universitätsklinikum des Saarlandes Homburg, Germany (Michael Böhm) Cardio. Vascular Center Frankfurt, Germany (Horst Sievert) Universitätsklinikum Düsseldorf, Germany (Lars Christian Rump) Universität Erlangen-Nürnberg Erlangen, Germany (Roland Schmieder) Barts and The London, UK (Mel Lobo) Pauls Stradins Clinical University Hospital Riga, Latvia (Andrejs Erglis) L'Hôpital Européen Georges Pompidou Paris, France (Guillaume Bobrie) John Hunter Hospital Newcastle, Australia (Suku Thambar) Cliniques Universitaires Saint-Luc Brussels, Belgium (Alexandre Persu) Universitaetsklinikum Schleswig-Holstein Lübeck, Germany (Heribert Schunkert) Universität zu Köln, Germany (Hannes Reuter) The Alfred Hospital Melbourne, Australia (Henry Krum) The John Paul II Hospital Krakow, Poland (Jerzy Sadowski) University Hospital Zurich Universität Leipzig – Herzzentrum Leipzig, Germany (Dierk Scheinert) Allgemeines Krankenhaus der Stadt Wien Vienna, Austria (Thomas Binder) Samodzielna Pracownia Hemodynamiczna Warsaw, Poland (Adam Witkowski) Hospital 12 de Octubre Madrid, Spain (Luis Ruilope) St. Vincent’s Hospital Melbourne, Australia (Robert Whitbourn) Universitätsklinikum Essen, Germany (Heike Bruck) Kent and Canterbury Hospital Canterbury, UK (Robert Kaikini) University of Glasgow, UK (Alan Jardine) Auckland City Hospital Auckland, New Zealand (Mark Webster) Herz-Zentrum Bad Krozingen, Germany (Thomas Zeller) For OMA distri butio n only. Not for distri butio n in the USA or Japa n. Trad emar ks may be regist ered and are the prop erty of their resp ectiv e owne rs. © 2013 Medt ronic, Inc. All rights reser ved.

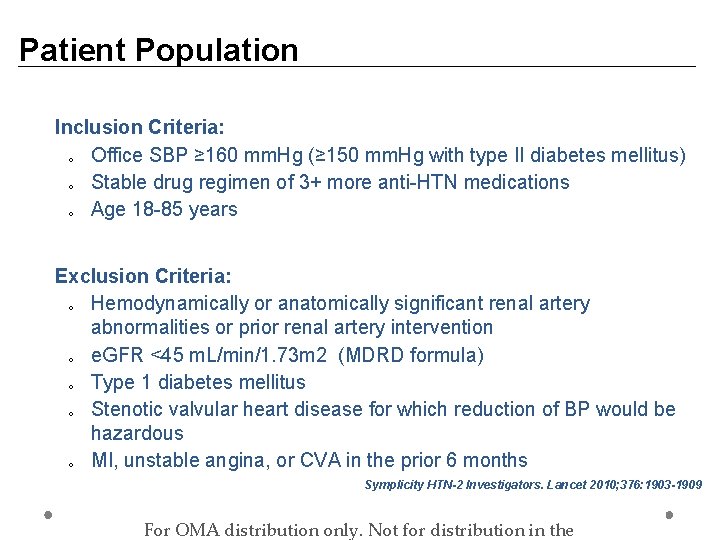
Patient Population Inclusion Criteria: o Office SBP ≥ 160 mm. Hg (≥ 150 mm. Hg with type II diabetes mellitus) o Stable drug regimen of 3+ more anti-HTN medications o Age 18 -85 years Exclusion Criteria: o Hemodynamically or anatomically significant renal artery abnormalities or prior renal artery intervention o e. GFR <45 m. L/min/1. 73 m 2 (MDRD formula) o Type 1 diabetes mellitus o Stenotic valvular heart disease for which reduction of BP would be hazardous o MI, unstable angina, or CVA in the prior 6 months Symplicity HTN-2 Investigators. Lancet 2010; 376: 1903 -1909 For OMA distribution only. Not for distribution in the

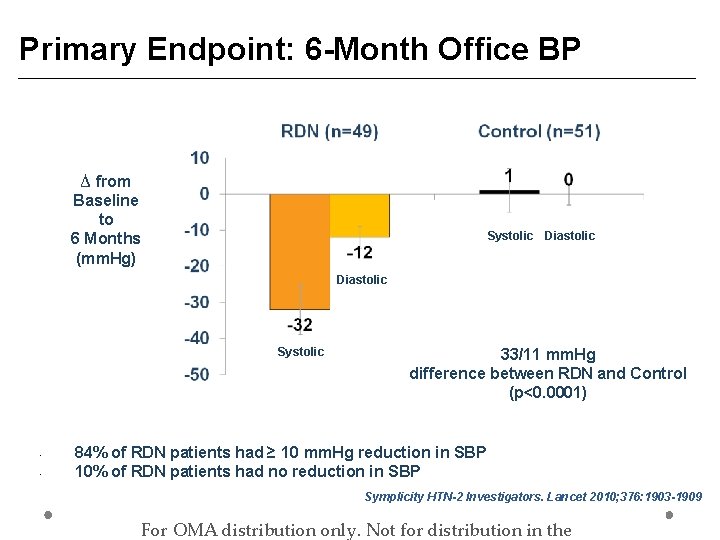
Primary Endpoint: 6 -Month Office BP ∆ from Baseline to 6 Months (mm. Hg) Systolic Diastolic Systolic • • 33/11 mm. Hg difference between RDN and Control (p<0. 0001) 84% of RDN patients had ≥ 10 mm. Hg reduction in SBP 10% of RDN patients had no reduction in SBP Symplicity HTN-2 Investigators. Lancet 2010; 376: 1903 -1909 For OMA distribution only. Not for distribution in the

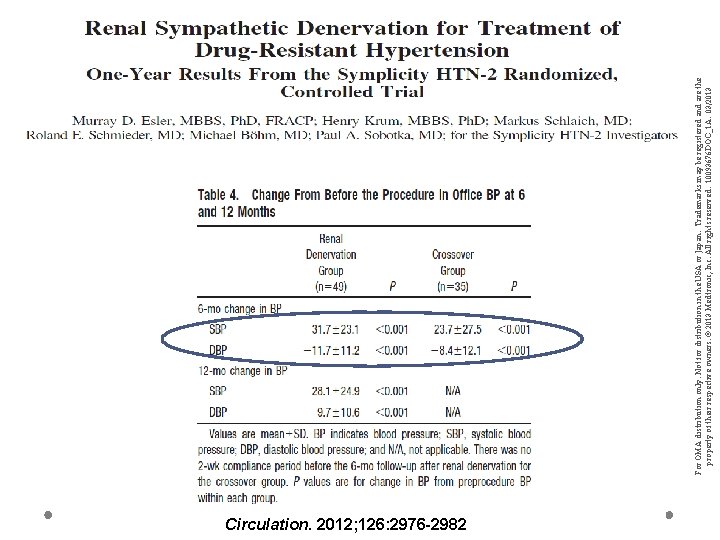
Circulation. 2012; 126: 2976 -2982 For OMA distribution only. Not for distribution in the USA or Japan. Trademarks may be registered and are the property of their respective owners. © 2013 Medtronic, Inc. All rights reserved. 10093676 DOC_1 A. 03/2013

Assessed for Eligibility (n=190) Excluded During Screening, (n=84) Scr ee nin g Randomized (n=106) 6 mont h Prim ary End- Allocated to RDN n=52 Treated; n=49 Analyzable BP < 160 at Baseline Visit (after 2 -weeks of medication compliance confirmation) (n=36; 19%) Ineligible anatomy (n=30; 16%) Declined participation (n=10; 5%) Other exclusion criteria discovered after consent (n=8; 4%) Allocated to Control n=54 Control; n=51 Analyzable Crossover n=46 6 -month Per Protocol Post RDN (Crossover) n=35 12 mo nth Po st RD N 12 -month Post RDN n=47 12 -month Post RDN (Crossover) n=33 24 -month Post RDN n= 40 24 -month Post RDN (Crossover) n= 26 Not-Per Protocol*, (Crossover) n=9 For OM A dist ribu tion only. Not for dist ribu tion in the US A or Japa n. Tra dem arks may be regi ster ed and are the pro pert y of thei r resp ecti ve own ers. © 2013 Me dtro nic, Inc. All righ ts rese rved. 1009 3676 Patient Disposition *Crossed-over with ineligible BP (<160 mm. Hg)

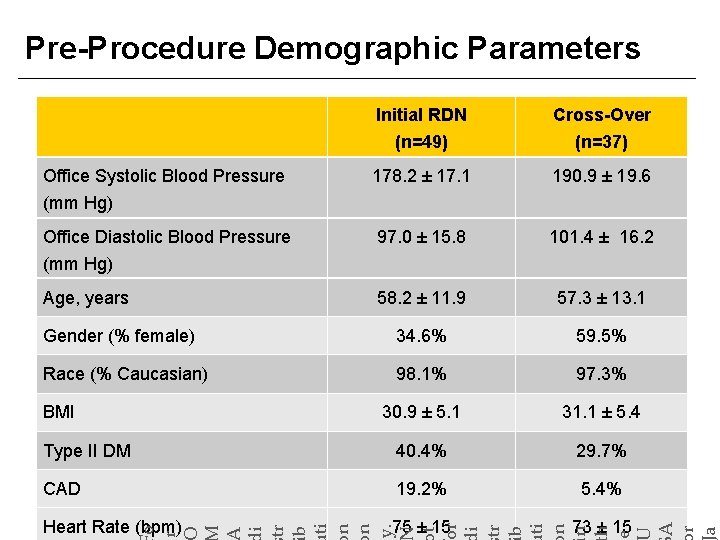
Pre-Procedure Demographic Parameters 178. 2 ± 17. 1 190. 9 ± 19. 6 Office Diastolic Blood Pressure (mm Hg) 97. 0 ± 15. 8 101. 4 ± 16. 2 Age, years 58. 2 ± 11. 9 57. 3 ± 13. 1 Gender (% female) 34. 6% 59. 5% Race (% Caucasian) 98. 1% 97. 3% 30. 9 ± 5. 1 31. 1 ± 5. 4 Type II DM 40. 4% 29. 7% CAD 19. 2% 5. 4% Heart Rate (bpm) 75 ± 15 73 ± 15 o r BMI A r a Office Systolic Blood Pressure (mm Hg) t or i tr b ti n n h e Cross-Over (n=37) i tr b ti n n y. Initial RDN (n=49)

Office SBP through 24 Months 6 m Primary Endpoint Control Crossover* Randomization 12 m post procedure 18 m post procedure 24 m post procedure mm Hg 6 m post procedure 6 m post randomization 12 m post randomization 18 m post randomization 24 m post randomization For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 *Patients randomized to control were offered RDN following the primary endpoint assessment. Only patients still meeting entry criteria (SBP ≥ 160 mm. Hg) were included in this analysis (n=37)

mm Hg Office SBP through 24 Months Pre Procedure 6 m Post Procedure 12 m Post Procedure 18 m Post Procedure 24 m Post Procedure For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 *Patients randomized to control were offered RDN following the primary endpoint assessment. Only patients still meeting entry criteria (SBP ≥ 160 mm. Hg) were included in this analysis (n=37)

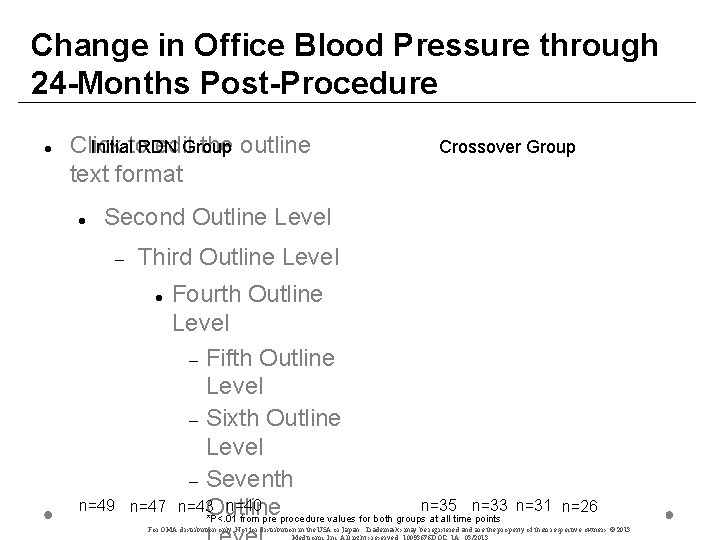
Change in Office Blood Pressure through 24 -Months Post-Procedure Click edit. Group the outline Initialto RDN text format Crossover Group Second Outline Level Third Outline Level Fourth Outline Level Fifth Outline Level Sixth Outline Level Seventh n=40 n=35 n=33 n=47 n=43 Outline *P<. 01 from pre procedure values for both groups at all time points n=49 n=31 n=26 For OMA distribution only. Not for distribution in the USA or Japan. Trademarks may be registered and are the property of their respective owners. © 2013

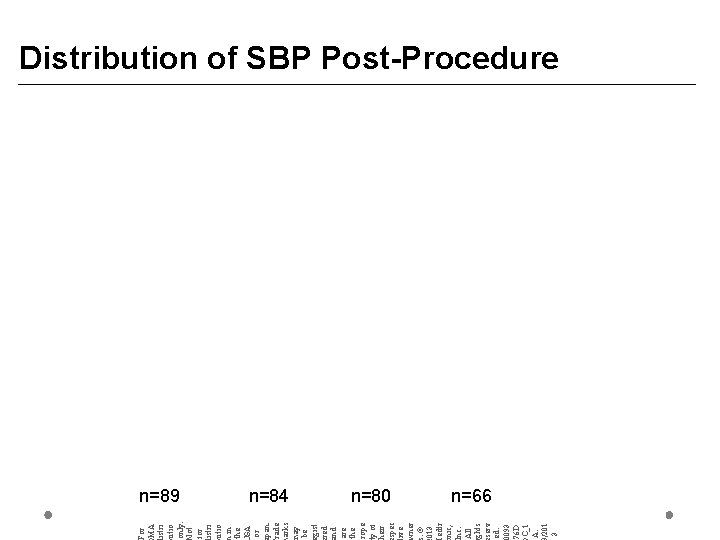
For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 Distribution of SBP Post-Procedure n=89 n=84 n=80 n=66

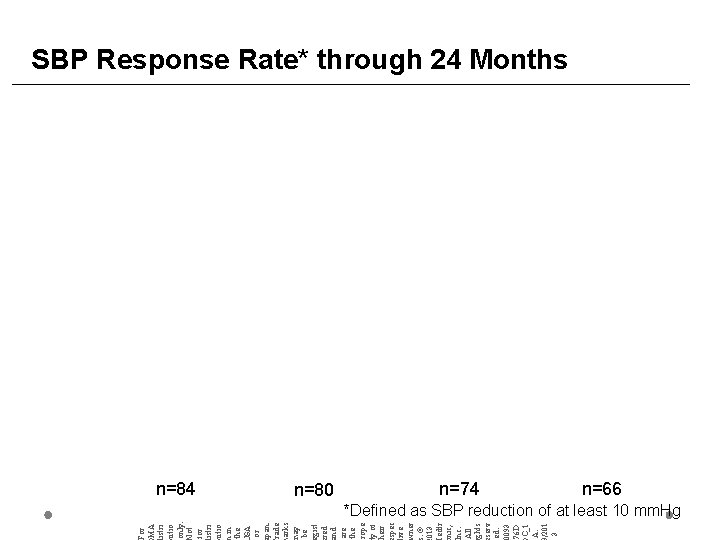
For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 SBP Response Rate* through 24 Months n=84 n=80 n=74 n=66 *Defined as SBP reduction of at least 10 mm. Hg

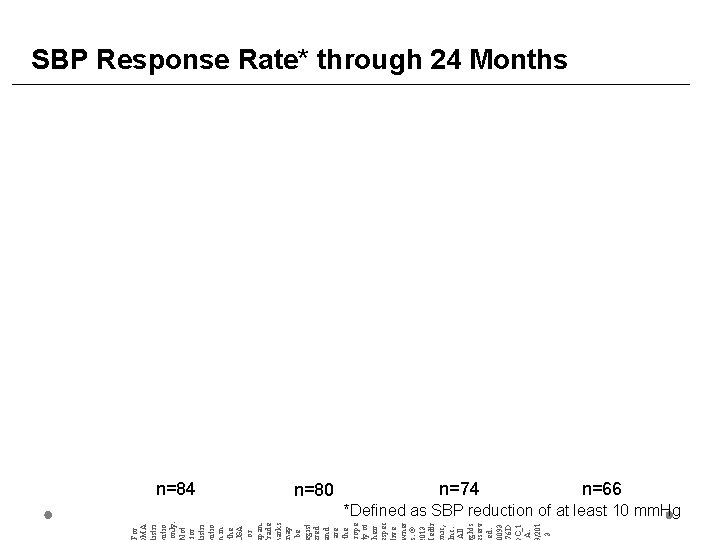
For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 SBP Response Rate* through 24 Months n=84 n=80 n=74 n=66 *Defined as SBP reduction of at least 10 mm. Hg

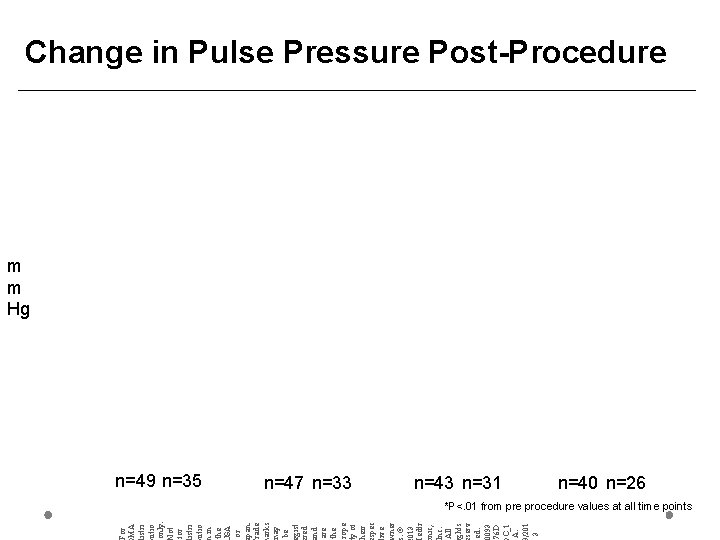
For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 Change in Pulse Pressure Post-Procedure m m Hg n=49 n=35 n=47 n=33 n=43 n=31 n=40 n=26 *P<. 01 from pre procedure values at all time points

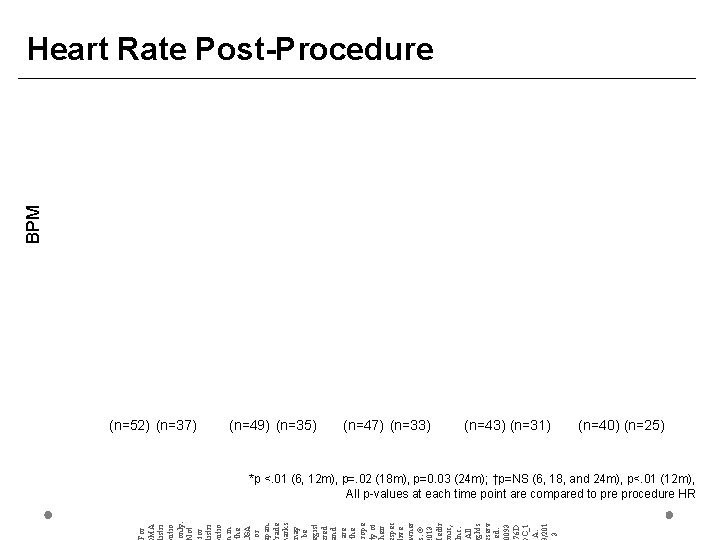
BPM Heart Rate Post-Procedure (n=52) (n=37) (n=49) (n=35) (n=47) (n=33) (n=43) (n=31) (n=40) (n=25) For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 *p <. 01 (6, 12 m), p=. 02 (18 m), p=0. 03 (24 m); †p=NS (6, 18, and 24 m), p<. 01 (12 m), All p-values at each time point are compared to pre procedure HR

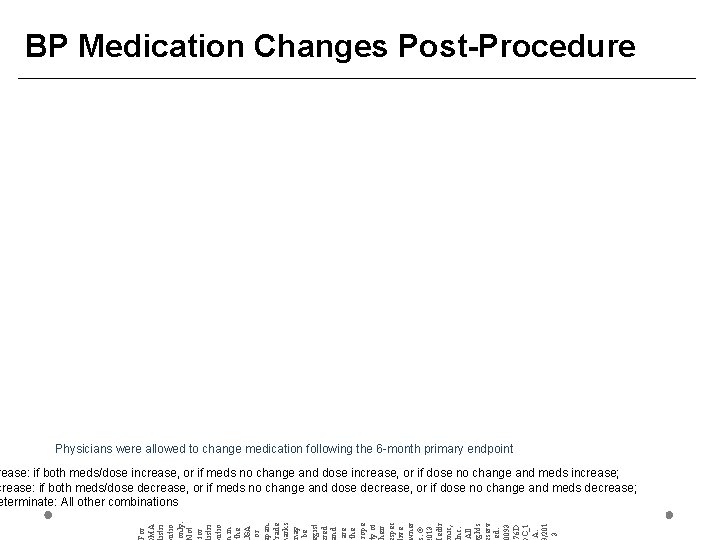
BP Medication Changes Post-Procedure Physicians were allowed to change medication following the 6 -month primary endpoint For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 rease: if both meds/dose increase, or if meds no change and dose increase, or if dose no change and meds increase; crease: if both meds/dose decrease, or if meds no change and dose decrease, or if dose no change and meds decrease; eterminate: All other combinations

Safety Post-Procedure • 3 hypertensive events requiring hospitalization in the initially treated group • 1 hypotensive event which required hospitalization • 1 mild transient acute renal failure o 2 deaths unrelated to the device or therapy For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 • Admitted with elevated K+, meds temporarily d/c (Co-Amilofruse, perindopril, metformin). IV fluids administered; K+ decreased. Patient discharged and remained off perindopril.

Conclusions • • • Subjects in both renal denervation groups had BP reductions which were significant compared to baseline and sustained through 24 -months of follow-up BP reductions were achieved in absence of increases in BP medications Heart rate was stable or was reduced following renal denervation After 24 -months of follow-up there were no device-related serious adverse effects and no detrimental effects on the renal vasculature following treatment For MA istri utio only. ot for istri utio in the SA or pan. rade arks ay be gist red nd are the rope y of heir spec ive ner. © 013 edtr nic, nc. All ghts serv ed. 0093 76 D C_1 A. /201 3 •

F or O M A d is tr ib u ti o nl y. N ot fo r d is tr

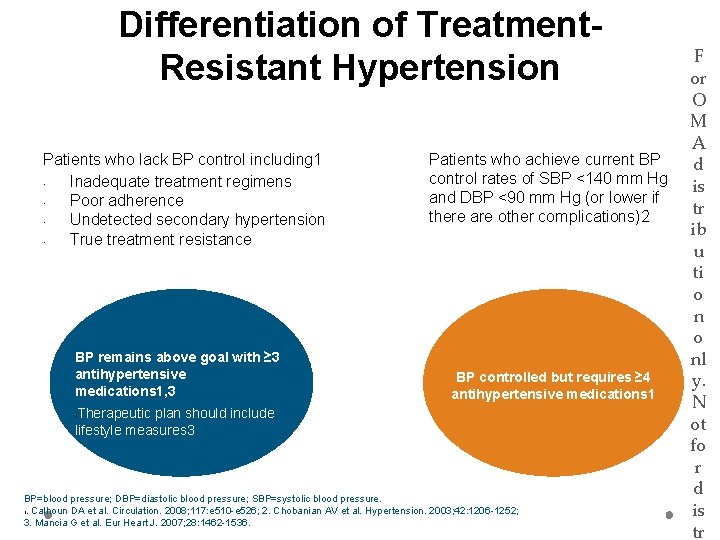
Differentiation of Treatment. Resistant Hypertension Patients who lack BP control including 1 • Inadequate treatment regimens • Poor adherence • Undetected secondary hypertension • True treatment resistance BP remains above goal with ≥ 3 antihypertensive medications 1, 3 Patients who achieve current BP control rates of SBP <140 mm Hg and DBP <90 mm Hg (or lower if there are other complications)2 BP controlled but requires ≥ 4 antihypertensive medications 1 Therapeutic plan should include lifestyle measures 3 • BP=blood pressure; DBP=diastolic blood pressure; SBP=systolic blood pressure. 1. Calhoun DA et al. Circulation. 2008; 117: e 510 -e 526; 2. Chobanian AV et al. Hypertension. 2003; 42: 1206 -1252; 3. Mancia G et al. Eur Heart J. 2007; 28: 1462 -1536. F or O M A d is tr ib u ti o nl y. N ot fo r d is tr
 Renal denervation
Renal denervation Mark wholey md
Mark wholey md Ira pré renal renal e pós renal
Ira pré renal renal e pós renal Sindrome nefrótica
Sindrome nefrótica Renal corpuscle
Renal corpuscle Hypertensive encephalopathy
Hypertensive encephalopathy Hypertensive crisis
Hypertensive crisis Fiber optic cable rodent protection
Fiber optic cable rodent protection Arc resistant switchgear
Arc resistant switchgear Tamper resistant packaging definition
Tamper resistant packaging definition Resistant materials tools
Resistant materials tools Savage syndrome
Savage syndrome Fire resistant environmental ensemble
Fire resistant environmental ensemble Insect-resistant packaging solutions
Insect-resistant packaging solutions Collision resistant hash function
Collision resistant hash function Earthquake resistant
Earthquake resistant Design of seismic-resistant steel building structures
Design of seismic-resistant steel building structures Smrf
Smrf Spill resistant vacuum breaker
Spill resistant vacuum breaker Fire resistant cable thai yazaki
Fire resistant cable thai yazaki Collision resistant hash function
Collision resistant hash function Chapter 19 disease transmission and infection prevention
Chapter 19 disease transmission and infection prevention Does metronidazole contain penicillin
Does metronidazole contain penicillin Cyanide-resistant respiration slideshare
Cyanide-resistant respiration slideshare Flame resistant lab coat amazon
Flame resistant lab coat amazon