Stoichiometry and the Mole SOL Review Stoichiometry and

















- Slides: 42

Stoichiometry and the Mole SOL Review

Stoichiometry and the Mole The Mole

The Mole Not everything that counts can be counted, and not everything that can be counted counts. Albert Einstein

What is a mole? n The mole is a counting unit. n Like. . . n n 1 dozen = 12 each 1 yard = 3 feet 1 cup = 8 ounce So then. . . n 1 mol = 6. 022 x 1023 particles That’s Avogadro’s Number!

Where did it come from? Mole (n) is the SI unit for the number of particles n Amedo Avogadro determined the number of particles in a mole n The mole is the measure of the amount of a substance whose number of particles is the same as 12 grams of Carbon - 12 n

Calculations n Using dimensional analysis you can determine the number of particles in a mole 1 mole = 6. 022 x 1023 particles, molecules, etc. n 6. 022 x 1023 particles = 1 mole n

So, let’s count. . . n 1 mol of Ag = n n 1 mol of CO 2 = n n 6. 022 x 1023 atoms Ag 6. 022 x 1023 molecules of CO 2 1 mol of pizza = n 6. 022 x 1023 pizzas!

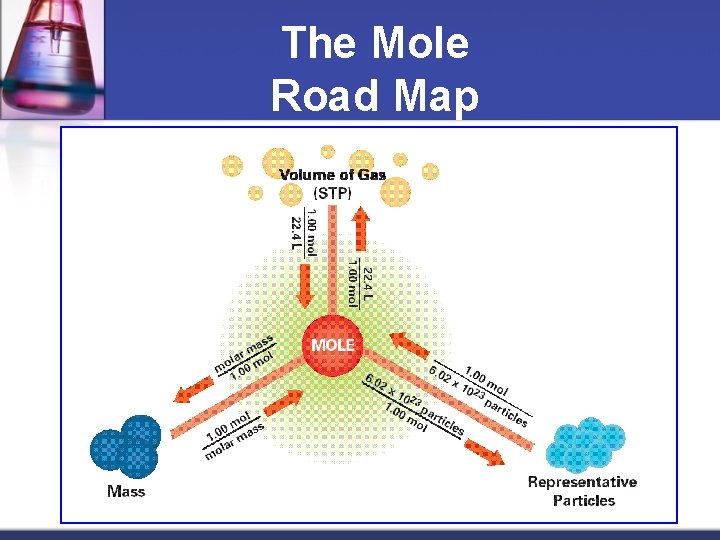
The Mole Road Map

Moles to Mass Conversions n 1 Moles = Molar Mass (g) = Molar mass/Mole or (g/mol) Now let’s apply that knowledge!

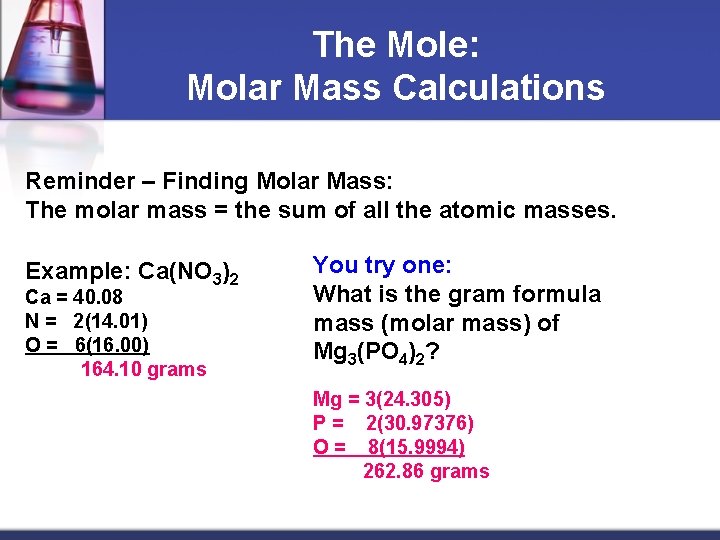
The Mole: Molar Mass Calculations Reminder – Finding Molar Mass: The molar mass = the sum of all the atomic masses. Example: Ca(NO 3)2 Ca = 40. 08 N = 2(14. 01) O = 6(16. 00) 164. 10 grams You try one: What is the gram formula mass (molar mass) of Mg 3(PO 4)2? Mg = 3(24. 305) P = 2(30. 97376) O = 8(15. 9994) 262. 86 grams

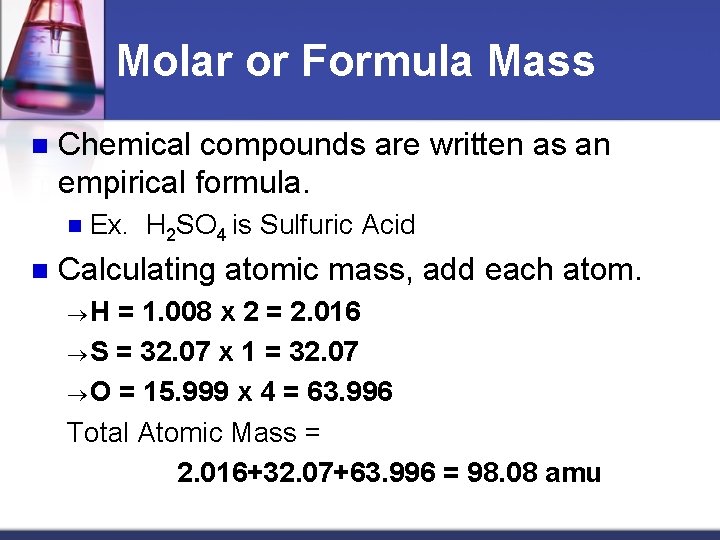
Molar or Formula Mass n Chemical compounds are written as an empirical formula. n n Ex. H 2 SO 4 is Sulfuric Acid Calculating atomic mass, add each atom. H = 1. 008 x 2 = 2. 016 S = 32. 07 x 1 = 32. 07 O = 15. 999 x 4 = 63. 996 Total Atomic Mass = 2. 016+32. 07+63. 996 = 98. 08 amu

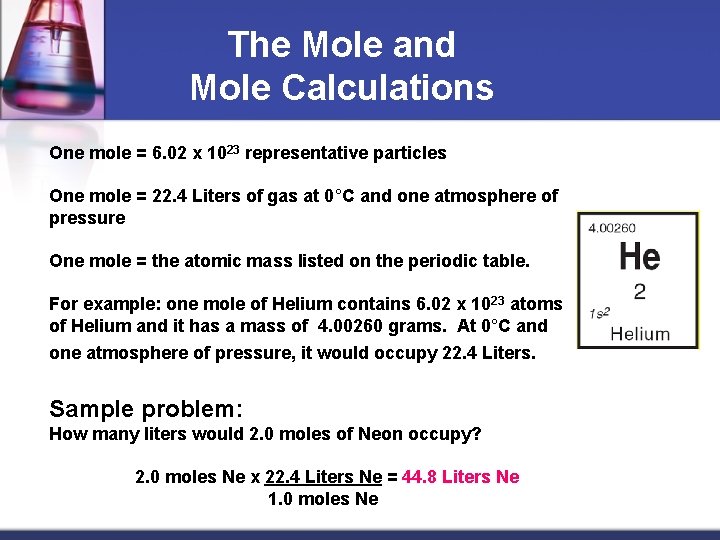
The Mole and Mole Calculations One mole = 6. 02 x 1023 representative particles One mole = 22. 4 Liters of gas at 0°C and one atmosphere of pressure One mole = the atomic mass listed on the periodic table. For example: one mole of Helium contains 6. 02 x 1023 atoms of Helium and it has a mass of 4. 00260 grams. At 0°C and one atmosphere of pressure, it would occupy 22. 4 Liters. Sample problem: How many liters would 2. 0 moles of Neon occupy? 2. 0 moles Ne x 22. 4 Liters Ne = 44. 8 Liters Ne 1. 0 moles Ne

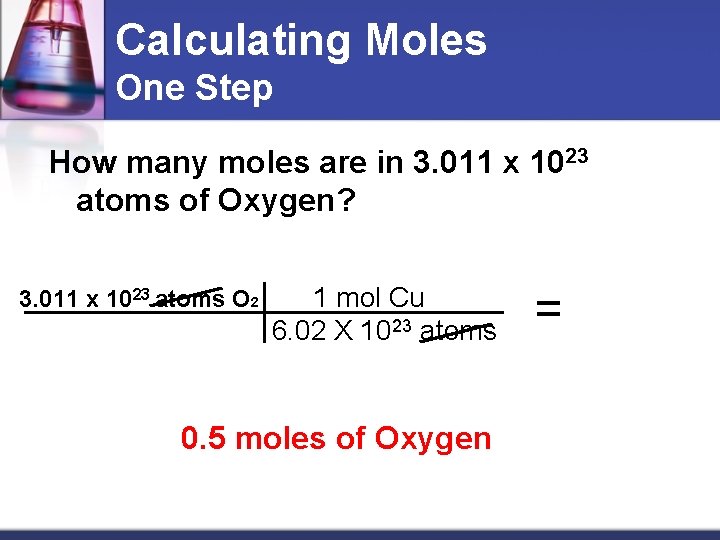
Calculating Moles One Step How many moles are in 3. 011 x 1023 atoms of Oxygen? 3. 011 x 1023 atoms O 2 1 mol Cu 6. 02 X 1023 atoms 0. 5 moles of Oxygen =

More One-Step Conversions Ex 1) Convert 4. 3 grams of Na. Cl to moles. Mass mol 4. 3 g Na. Cl x 1 mol Na. Cl = 7. 4 x 10 -2 mol Na. Cl 58. 45 g Na. Cl Ex 2) Convert 0. 00563 mol NH 3 to grams. Mol -> mass 0. 00563 mol NH 3 x 17 g NH 3 =9. 57 x 10 – 2 g NH 3 1 mol NH 3

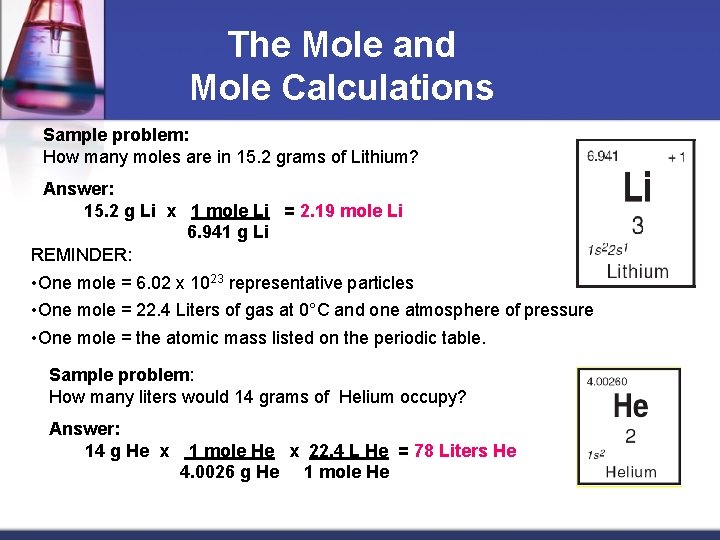
The Mole and Mole Calculations Sample problem: How many moles are in 15. 2 grams of Lithium? Answer: 15. 2 g Li x 1 mole Li = 2. 19 mole Li 6. 941 g Li REMINDER: • One mole = 6. 02 x 1023 representative particles • One mole = 22. 4 Liters of gas at 0°C and one atmosphere of pressure • One mole = the atomic mass listed on the periodic table. Sample problem: How many liters would 14 grams of Helium occupy? Answer: 14 g He x 1 mole He x 22. 4 L He = 78 Liters He 4. 0026 g He 1 mole He

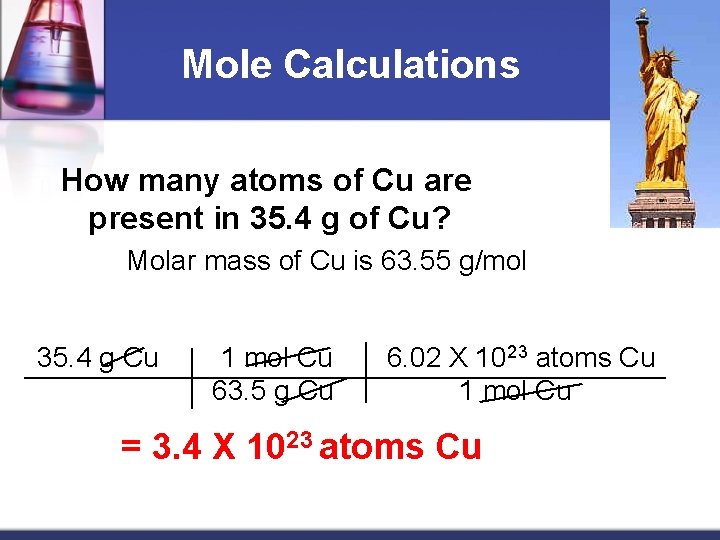
Mole Calculations How many atoms of Cu are present in 35. 4 g of Cu? Molar mass of Cu is 63. 55 g/mol 35. 4 g Cu 1 mol Cu 63. 5 g Cu 6. 02 X 1023 atoms Cu 1 mol Cu = 3. 4 X 1023 atoms Cu

And another. . . n What mass would 4. 52 x 1024 molecules of water have? 4. 52 x 1024 mlcs of H 20 1 mol 6. 02 X 1023 mlcs = 135 g H 2 O 18. 02 g 1 mol

Stoichiometry and the Mole Stoichiometry


Stoichiometry means that if you know one piece of information about ONE compound in an equation, you can determine EVERYTHING else! n If you have 3 L of Nitrogen, how many liters of ammonia will you produce? N 2 + 3 H 2 2 NH 3 n

Stoichiometry n Let’s look at that last reaction again. N 2(g) + 3 H 2(g) 2 NH 3(g) If you start out with 1 mole of Nitrogen gas and 3 moles of Hydrogen gas, you will make 2 moles of Ammonia gas. n It is important in industry to know the exact proportions of your ingredients so that you will not have excess waste in your product. n

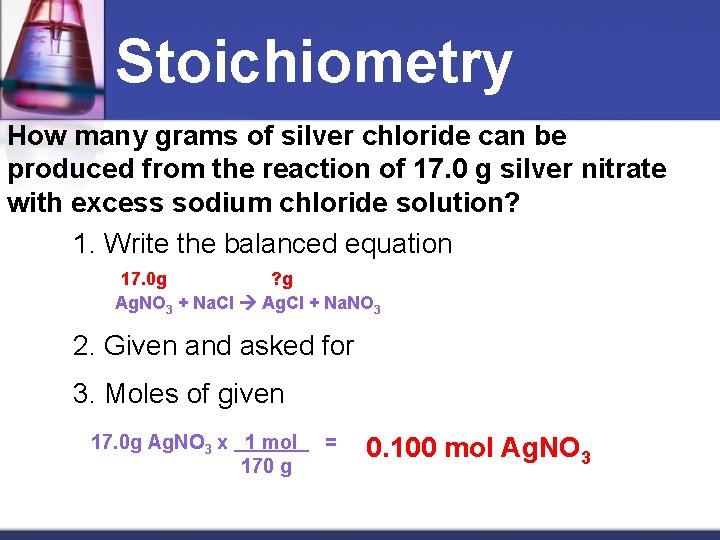
Stoichiometry How many grams of silver chloride can be produced from the reaction of 17. 0 g silver nitrate with excess sodium chloride solution? 1. Write the balanced equation 17. 0 g ? g Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3 2. Given and asked for 3. Moles of given 17. 0 g Ag. NO 3 x 1 mol 170 g = 0. 100 mol Ag. NO 3

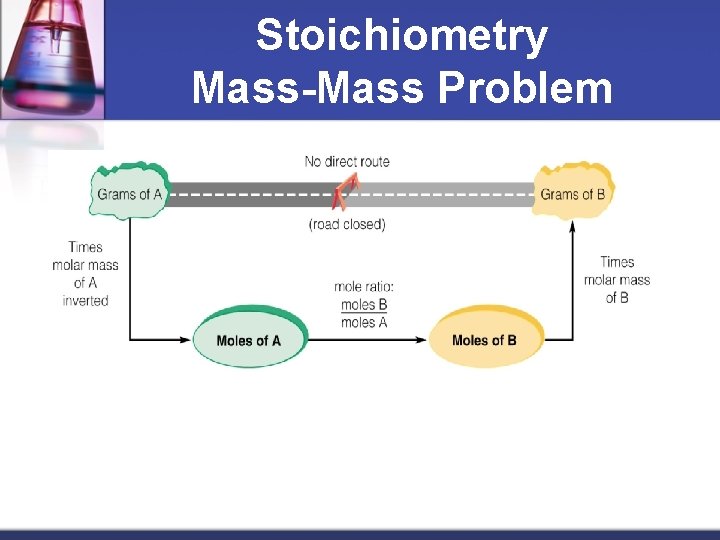
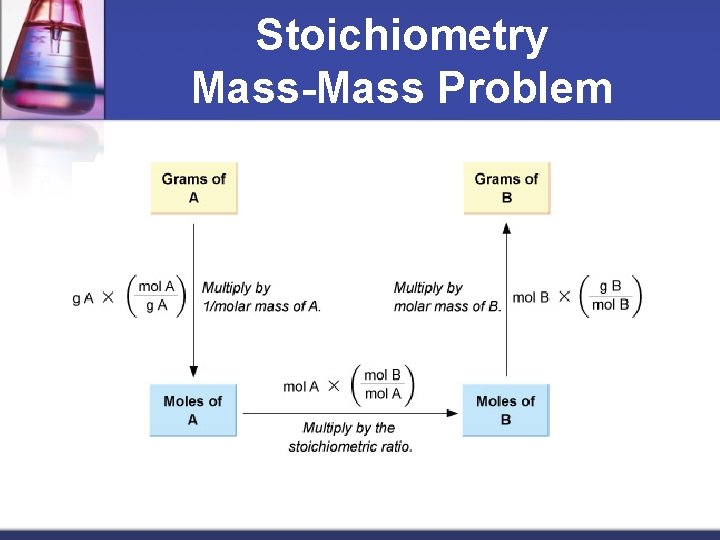
Stoichiometry Mass-Mass Problem

Stoichiometry Mass-Mass Problem

Stoichiometry Mass-Mass Problem Ag. NO 3 + Na. Cl Ag. Cl + Na. NO 3 4. Moles asked for 0. 100 mols Ag. NO 3 x 1 mol Ag. Cl = 1 mol Ag. NO 3 0. 100 mol Ag. Cl 5. Convert your answer 0. 100 mol Ag. Cl x 144 g Ag. Cl = 1 mol Ag. Cl 14. 4 g Ag. Cl

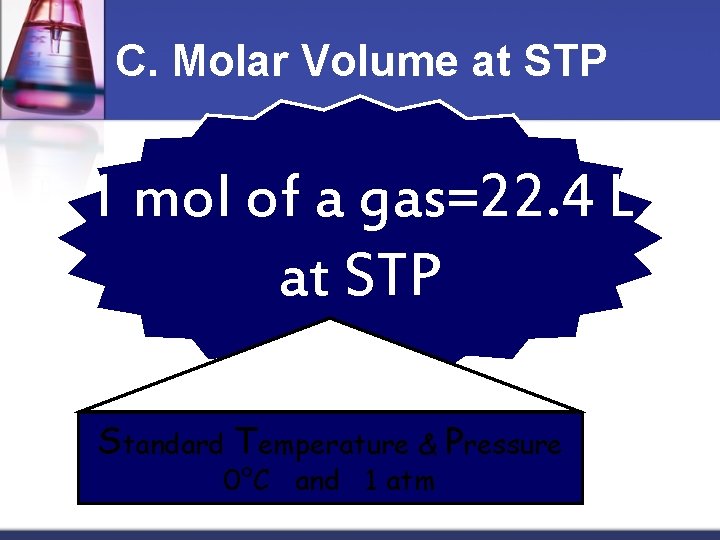
C. Molar Volume at STP 1 mol of a gas=22. 4 L at STP Standard Temperature & Pressure 0°C and 1 atm

Stoichiometry and the Mole Limiting Reactant Problems

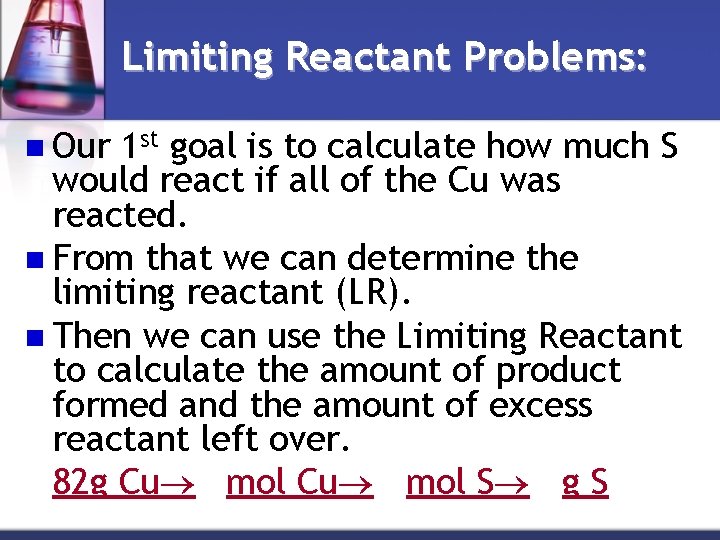
Limiting Reactant Problems: Given the following reaction: 2 Cu + S Cu 2 S • What is the limiting reactant when 82. 0 g of Cu reacts with 25. 0 g S? • What is the maximum amount of Cu 2 S that can be formed? • How much of the other reactant is wasted?

Limiting Reactant Problems: n Our 1 st goal is to calculate how much S would react if all of the Cu was reacted. n From that we can determine the limiting reactant (LR). n Then we can use the Limiting Reactant to calculate the amount of product formed and the amount of excess reactant left over. 82 g Cu mol S g S

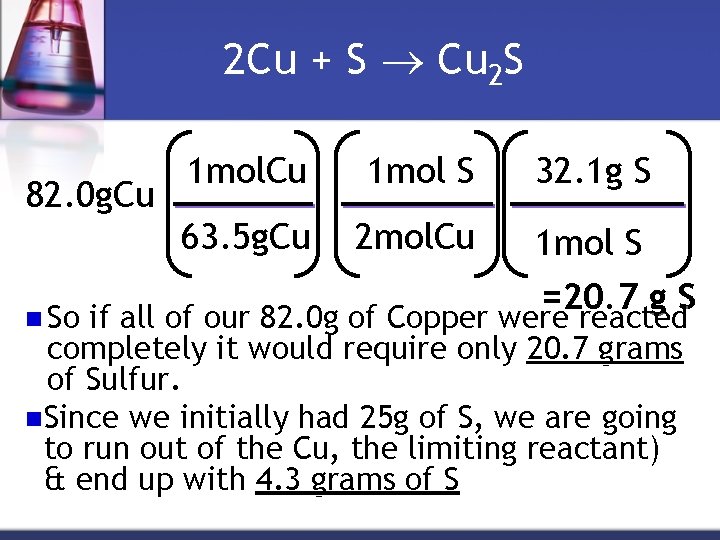
2 Cu + S Cu 2 S 82. 0 g. Cu n So 1 mol. Cu 1 mol S 63. 5 g. Cu 2 mol. Cu 32. 1 g S 1 mol S =20. 7 g S if all of our 82. 0 g of Copper were reacted completely it would require only 20. 7 grams of Sulfur. n. Since we initially had 25 g of S, we are going to run out of the Cu, the limiting reactant) & end up with 4. 3 grams of S

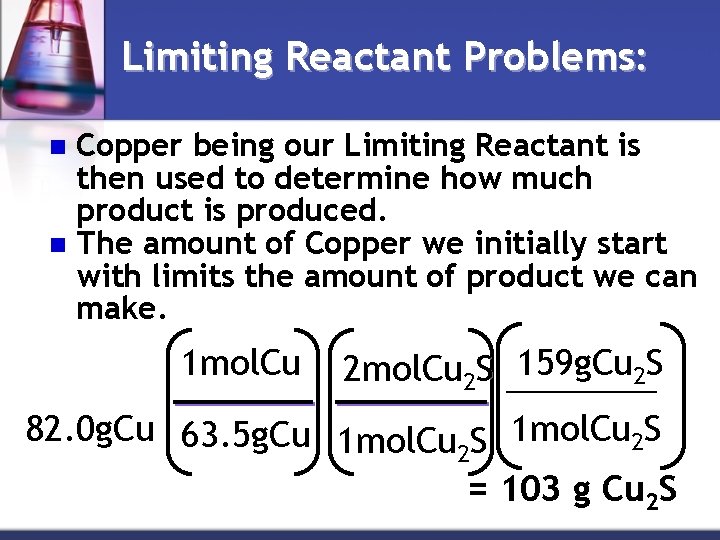
Limiting Reactant Problems: Copper being our Limiting Reactant is then used to determine how much product is produced. n The amount of Copper we initially start with limits the amount of product we can make. n 1 mol. Cu 159 g. Cu 2 S 2 mol. Cu 2 S ____ 82. 0 g. Cu 63. 5 g. Cu 1 mol. Cu S 1 mol. Cu 2 S 2 = 103 g Cu 2 S

Limiting Reactant Problems: n So the reaction between 82. 0 g of Cu and 25. 0 g of S can only produce 103 g of Cu 2 S. n The Cu runs out before the S and we will end up wasting 4. 7 g of the S.

Stoichiometry and the Mole Percent Yield

Calculating Percent Yield n In theory, when a teacher gives an exam to the class, every student should get a grade of 100%. Sadly, this is not always true. The calculation for percent yield is similar. n We already know that we do not get a 100% yield of products in an reaction.

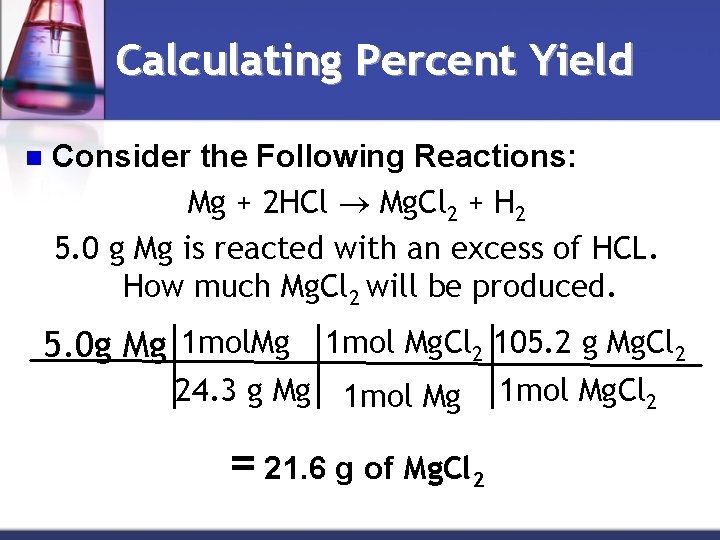
Calculating Percent Yield n Consider the Following Reactions: Mg + 2 HCl Mg. Cl 2 + H 2 5. 0 g Mg is reacted with an excess of HCL. How much Mg. Cl 2 will be produced. 5. 0 g Mg 1 mol Mg. Cl 2 105. 2 g Mg. Cl 2 24. 3 g Mg 1 mol Mg = 21. 6 g of Mg. Cl 2 1 mol Mg. Cl 2

Calculating Percent Yield You might assume that using stoichiometry to calculate that our reaction will produce 21. 6 g of Mg. Cl 2, but we will actually only recover 15. 2 g of Mg. Cl 2 in the lab. n 21. 6 g of Mg. Cl 2 is the value representing theoretical yield(theoretical yield is the maximum amount of product that could be formed). n The 15. 2 g of Mg. Cl 2 is called the actual yield (the actual yield is less than theoretical yield). n

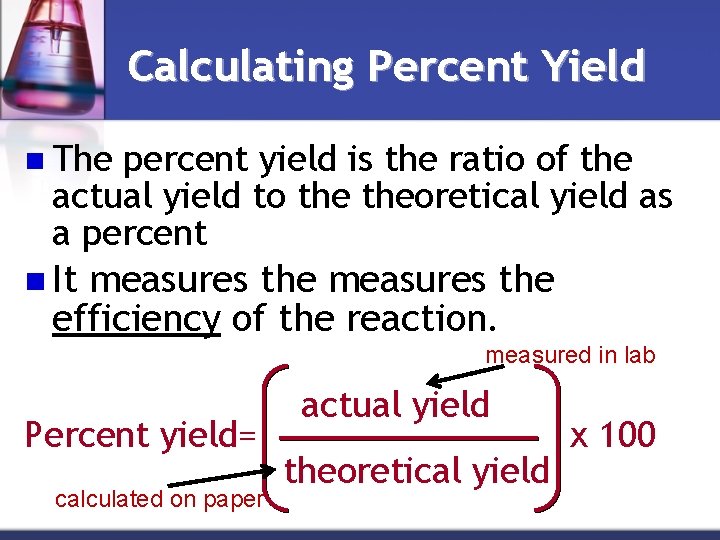
Calculating Percent Yield n The percent yield is the ratio of the actual yield to theoretical yield as a percent n It measures the efficiency of the reaction. measured in lab Percent yield= calculated on paper actual yield theoretical yield x 100

Calculating Percent Yield n Why do reactions not go to completion. n Impure reactants and competing side rxns may cause unwanted products to form. n Actual yield can also be lower than theoretical yield due to a loss of product during filtration or transferring between containers. n If a wet precipitate is recovered it might weigh heavy due to incomplete drying, etc.

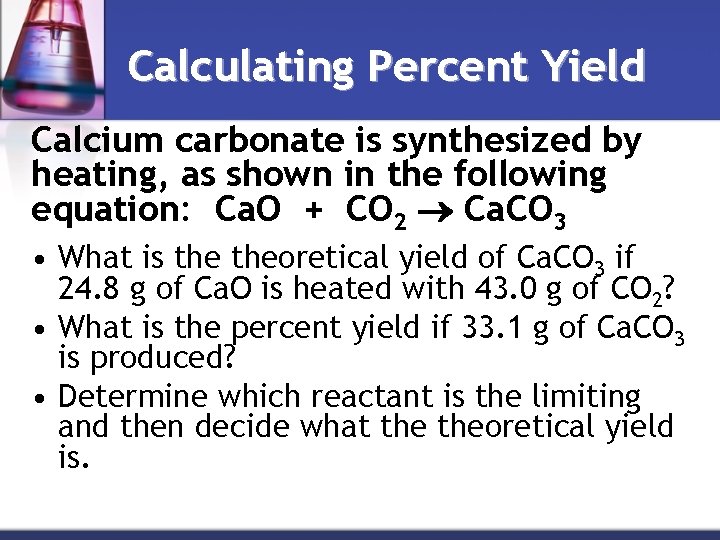
Calculating Percent Yield Calcium carbonate is synthesized by heating, as shown in the following equation: Ca. O + CO 2 Ca. CO 3 • What is theoretical yield of Ca. CO 3 if 24. 8 g of Ca. O is heated with 43. 0 g of CO 2? • What is the percent yield if 33. 1 g of Ca. CO 3 is produced? • Determine which reactant is the limiting and then decide what theoretical yield is.

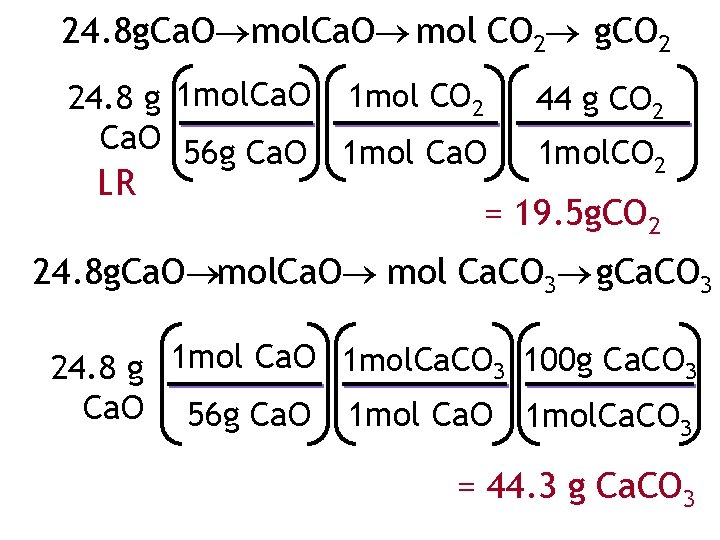
24. 8 g. Ca. O mol CO 2 g. CO 2 24. 8 g 1 mol. Ca. O 1 mol CO 2 Ca. O 56 g Ca. O 1 mol Ca. O LR 44 g CO 2 1 mol. CO 2 = 19. 5 g. CO 2 24. 8 g. Ca. O mol Ca. CO 3 g. Ca. CO 3 24. 8 g 1 mol Ca. O 1 mol. Ca. CO 3 100 g Ca. CO 3 Ca. O 56 g Ca. O 1 mol. Ca. CO 3 = 44. 3 g Ca. CO 3

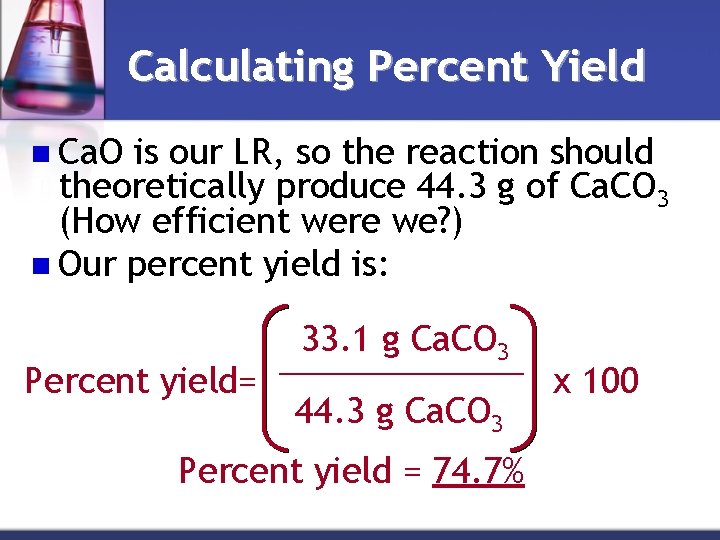
Calculating Percent Yield n Ca. O is our LR, so the reaction should theoretically produce 44. 3 g of Ca. CO 3 (How efficient were we? ) n Our percent yield is: 33. 1 g Ca. CO 3 _______ Percent yield= x 100 44. 3 g Ca. CO 3 Percent yield = 74. 7%

GOOD LUCK!! Mrs. Armani Mr. Buchanan Ms. Nichols Mr. Smith
 Stoichiometry mole-mole problems
Stoichiometry mole-mole problems Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Molar mass of sucrose
Molar mass of sucrose Stoichiometry mole-mole
Stoichiometry mole-mole Mole mass and mole volume relationships
Mole mass and mole volume relationships Mole mole factor
Mole mole factor Mass grams
Mass grams Perbedaan sol liofil dan sol liofob
Perbedaan sol liofil dan sol liofob Stoichiometry island diagram
Stoichiometry island diagram Mole tunnel stoichiometry worksheet answers
Mole tunnel stoichiometry worksheet answers Mole conversion chart
Mole conversion chart Stoichiometry mole island diagram
Stoichiometry mole island diagram 4th grade va studies sol
4th grade va studies sol World history 1 sol review
World history 1 sol review Chemistry sol review packet
Chemistry sol review packet Geometry sol review packet
Geometry sol review packet Earth science sol
Earth science sol Algebra 1 sol review
Algebra 1 sol review Civics sol review
Civics sol review Kuhinjska sol formula
Kuhinjska sol formula Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Chemistry sol review
Chemistry sol review Biology sol review
Biology sol review History sol review
History sol review Chemistry sol practice
Chemistry sol practice Earth science final
Earth science final Chemistry sol review
Chemistry sol review Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Chapter 9 stoichiometry
Chapter 9 stoichiometry Chapter 9 review stoichiometry section 2
Chapter 9 review stoichiometry section 2 A balanced chemical equation allows one to determine the:
A balanced chemical equation allows one to determine the: The calculation of quantities in chemical equations
The calculation of quantities in chemical equations Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Tư thế worms-breton
Tư thế worms-breton Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Môn thể thao bắt đầu bằng từ chạy
Môn thể thao bắt đầu bằng từ chạy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới