STATES OF MATTER The Four States of Matter
















- Slides: 28


STATES OF MATTER • The Four States of Matter • Solid • Liquid • Gas • Plasma • Four States

STATES OF MATTER Ø Based upon particle arrangement Ø Based upon energy of particles Ø Based upon distance between particles

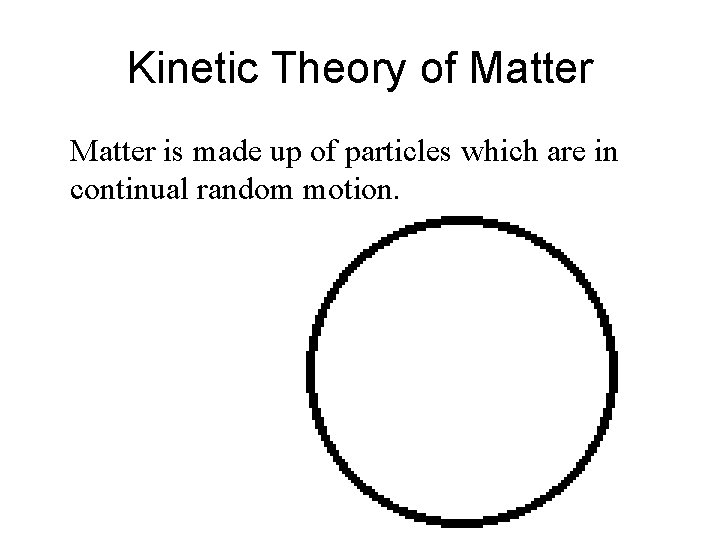
Kinetic Theory of Matter is made up of particles which are in continual random motion.

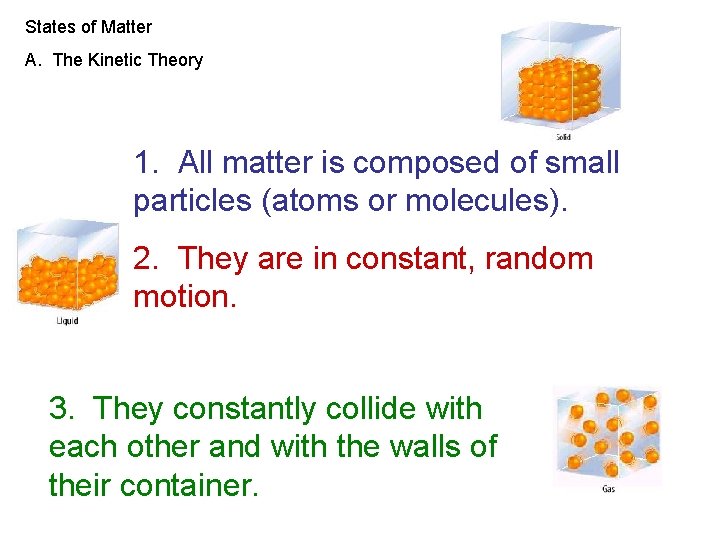
States of Matter A. The Kinetic Theory 1. All matter is composed of small particles (atoms or molecules). 2. They are in constant, random motion. 3. They constantly collide with each other and with the walls of their container.

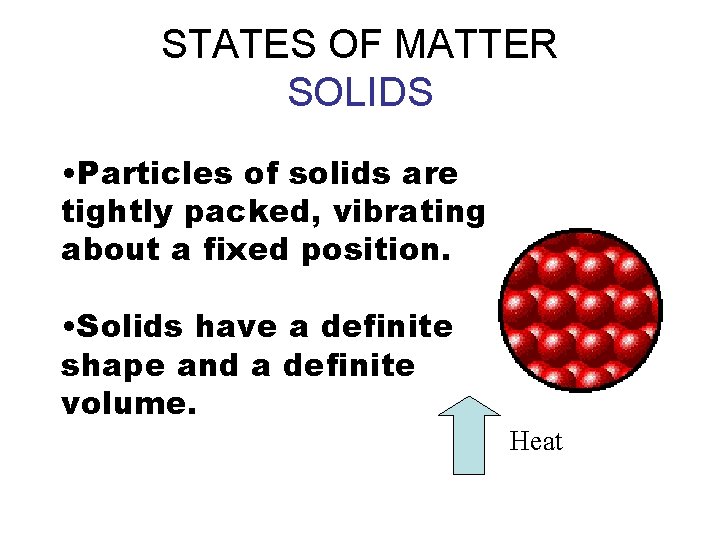
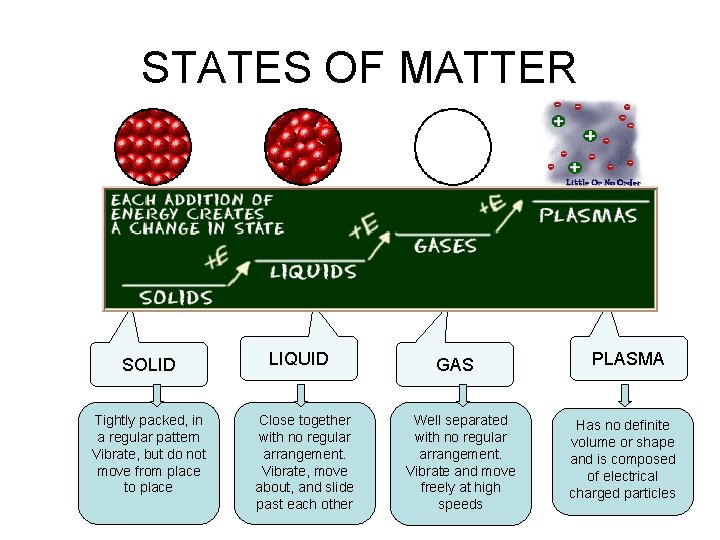
STATES OF MATTER SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. • Solids have a definite shape and a definite volume. Heat

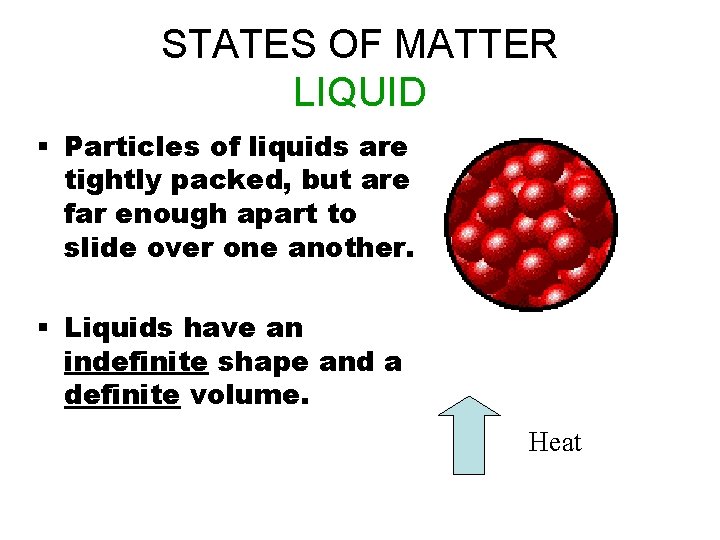
STATES OF MATTER LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume. Heat

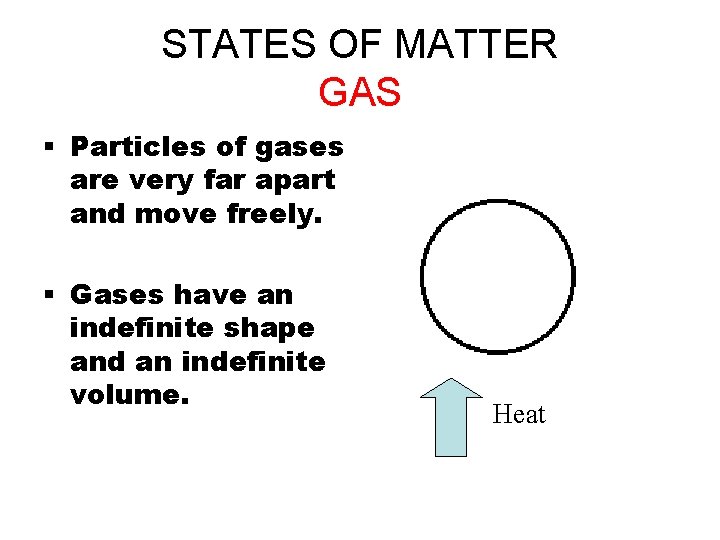
STATES OF MATTER GAS § Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume. Heat

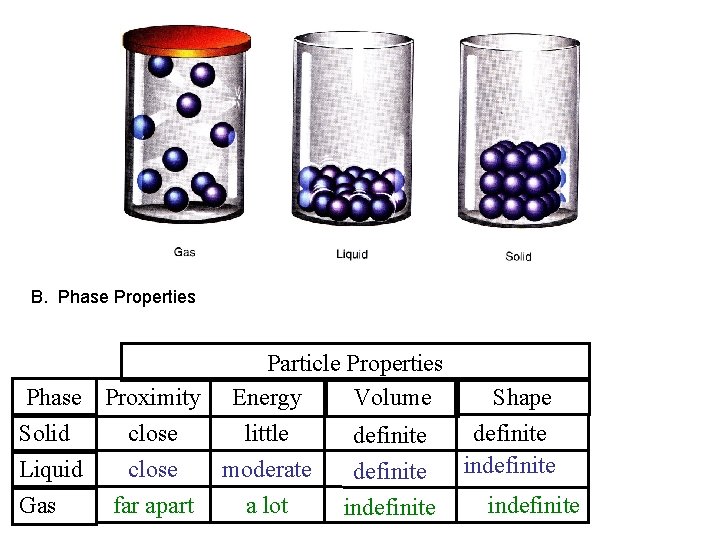
B. Phase Properties Particle Properties Phase Proximity Energy Volume Shape Solid close little definite indefinite Liquid close moderate definite Gas far apart a lot indefinite

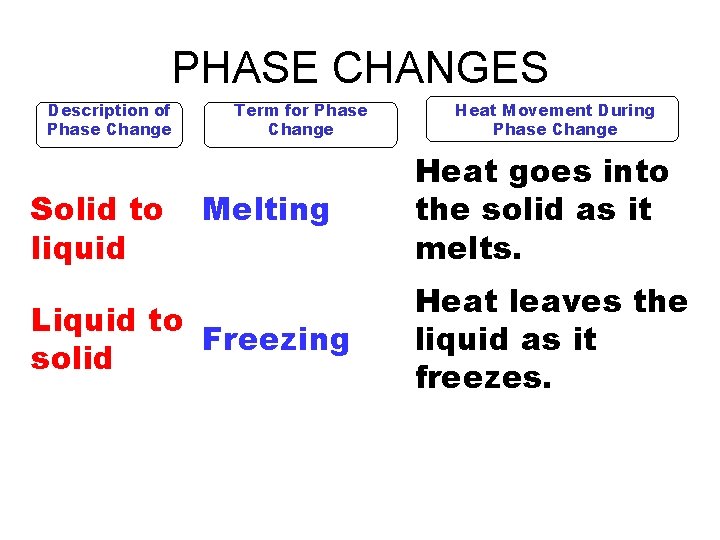
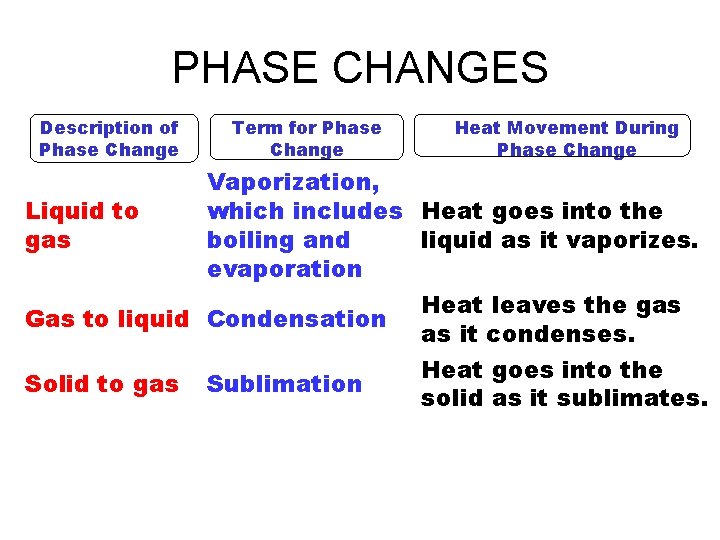
PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Vaporization, which includes Heat goes into the Liquid to gas boiling and liquid as it vaporizes. evaporation Heat leaves the gas Gas to liquid Condensation as it condenses. Heat goes into the Solid to gas Sublimation solid as it sublimates.

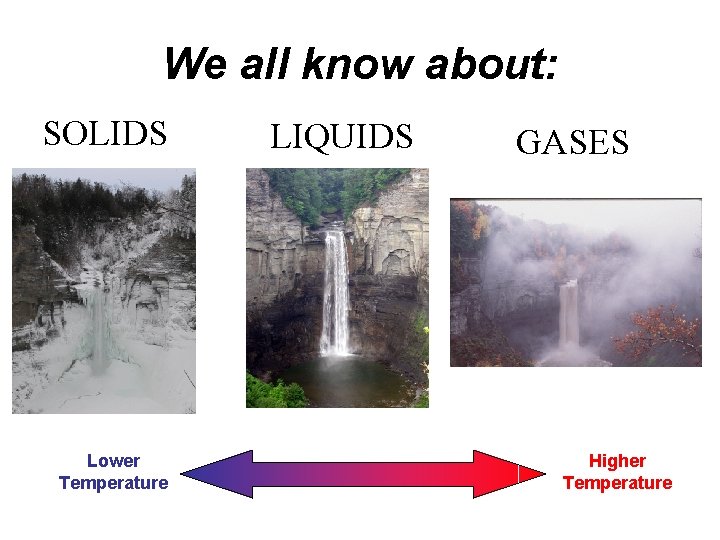
We all know about: SOLIDS Lower Temperature LIQUIDS GASES Higher Temperature

But what happens if you raise the temperature to super-high levels… between 1000°C and 1, 000, 000°C ? Will everything just be a gas?

STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles

NO! If the gas is made up of particles which carry an electric charge (“ionized particles”), but the entire gas as a whole has no electric charge, and if the density is not too high, then we can get The 4 th state of matter: PLASMA

Some places where plasmas are found… 1. Flames

2. Lightning

3. Aurora (Northern Lights)

5. Stars make up 99% of the total matter in the Universe. Therefore, 99% of everything that exists in the entire Universe is in the plasma state.

The Sun is an example of a star in its plasma state

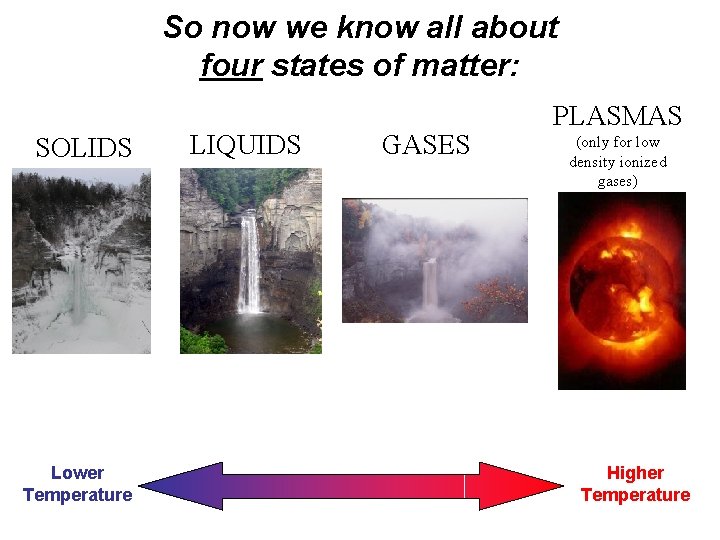
So now we know all about four states of matter: SOLIDS Lower Temperature LIQUIDS GASES PLASMAS (only for low density ionized gases) Higher Temperature

But now what happens if you lower the temperature way, down to 100 nano degrees above “Absolute Zero” (-273°C) Will everything just be a frozen solid?



Not Necessarily! In 1924 (82 years ago), two scientists, Albert Einstein and Satyendra Bose predicted a 5 th state of matter which would occur at very low temperatures. Einstein Bose +

Finally, in 1995, Wolfgang Ketterle and his team of graduate students discovered the 5 th state of matter for the first time. Ketterle and his students The 5 th state of matter: Bose-Einstein Condensate

To really understand Bose -Einstein condensate you need to know Quantum Physics

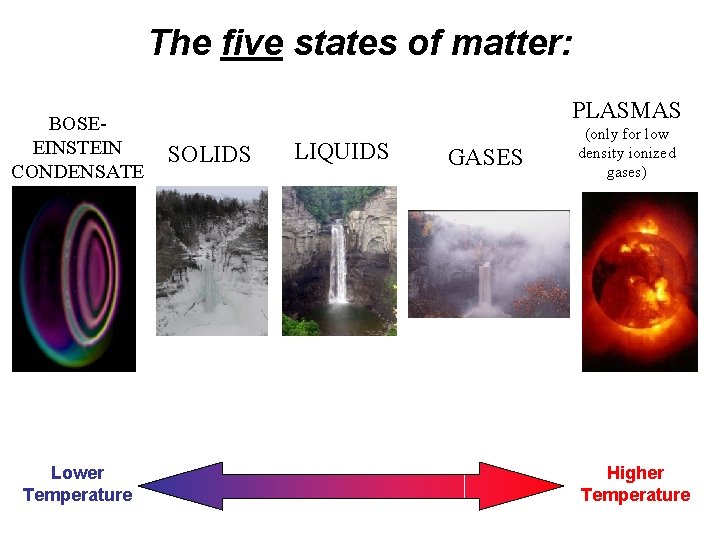
The five states of matter: BOSEEINSTEIN CONDENSATE Lower Temperature PLASMAS SOLIDS LIQUIDS GASES (only for low density ionized gases) Higher Temperature
 4 phases of matter
4 phases of matter Four states of matter
Four states of matter Whats the four states of matter
Whats the four states of matter Four states of matter
Four states of matter Solid liquid gas plasma
Solid liquid gas plasma Lời thề hippocrates
Lời thề hippocrates Tư thế worm breton là gì
Tư thế worm breton là gì đại từ thay thế
đại từ thay thế Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tiính động năng
Công thức tiính động năng Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dot
Dot Biện pháp chống mỏi cơ
Biện pháp chống mỏi cơ Bổ thể
Bổ thể độ dài liên kết
độ dài liên kết Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia điện thế nghỉ
điện thế nghỉ Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Sơ đồ cơ thể người
Sơ đồ cơ thể người Số.nguyên tố
Số.nguyên tố đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới