States of Matter Chemistry The Four States of























- Slides: 26

States of Matter Chemistry The Four States of Matter Chumbler - Properties of Matter 1

States of Matter The Four States of Matter Four States u. Solid u. Liquid u. Gas u. Plasma Chumbler - Properties of Matter 2

States of Matter The Four States of Matter Basis of Classification of the Four Types ØBased upon particle arrangement ØBased upon energy of particles Chumbler - Properties of Matter 3

States of Matter Solids §Particles of solids are tightly packed, vibrating about a fixed position. §Solids have a definite shape and a definite volume. §Solids have an infinite number of free surfaces. Chumbler - Properties of Matter 4

States of Matter § § § Two Types of Solids Crystalline and Amorphous Crystalline have a very orderly, 3 -D arrangement of particles. Amorphous solids are made of particles that are in no special order. Chumbler - Properties of Matter 5

States of Matter Solids Particle Movement Examples Chumbler - Properties of Matter 6

States of Matter Liquids §Particles of liquids are tightly packed, but are far enough apart to slide over one another. §Liquids have an indefinite shape and a definite volume. §Liquids take the shape of any container it is put in. Chumbler - Properties of Matter 7

States of Matter Liquids cont. The particles in liquids move fast enough to overcome some of the attractions between them. Realize that the particles in a liquid state are still very close together; they just slide past each other. u Liquids have two properties Surface Tension – is a force that acts on the particles at the surface of a liquid, which cause spherical drops u Viscosity – is a liquids resistance to flow, the stronger attraction between liquid particles the more Chumbler - Properties of Matter 8

States of Matter Liquids Particle Movement Examples Chumbler - Properties of Matter 9

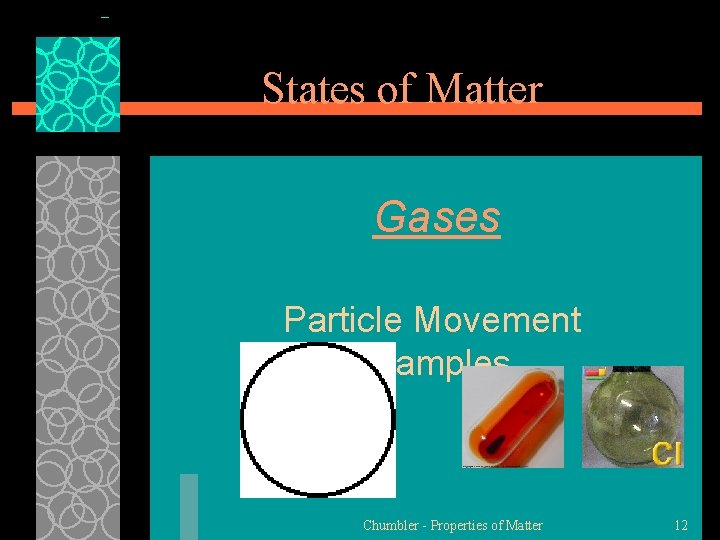
States of Matter Gases §Gas is the state in which matter changes in both shape and volume §Particles in a gas move very fast and are very far apart and move freely. §Gases have an indefinite Chumbler - Properties of Matter 10

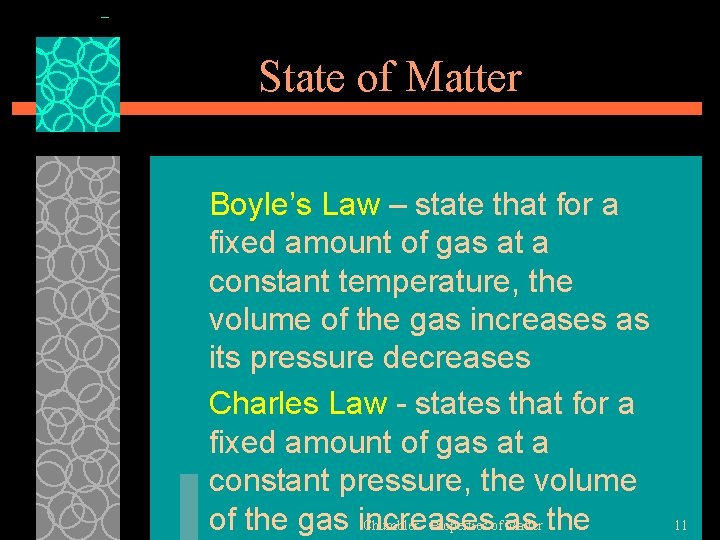
State of Matter Boyle’s Law – state that for a fixed amount of gas at a constant temperature, the volume of the gas increases as its pressure decreases Charles Law - states that for a fixed amount of gas at a constant pressure, the volume of the gas increases as the Chumbler - Properties of Matter 11

States of Matter Gases Particle Movement Examples Chumbler - Properties of Matter 12

States of Matter Plasma §Plasma is the state of matter that does not have a definite shape or volume. §A plasma is a very good conductor of electricity and is affected by magnetic fields. §Plasmas conduct electric current, Chumbler - Properties of Matter 13

States of Matter Plasma Particles The negatively charged electrons (yellow) are freely streaming through the positively charged ions (blue). Chumbler - Properties of Matter 14

States of Matter Plasma Examples Chumbler - Properties of Matter 15

States of Matter Microscopic Explanation for Properties of Solids §Solids have a definite shape and a definite volume because the particles are locked into place §Solids are not easily compressible because there is little free space between particles §Solids do not flow easily because the particles cannot move/slide past one Chumbler - Properties of Matter 16

States of Matter Microscopic Explanation for Properties of Liquids §Liquids have an indefinite shape because the particles can slide past one another. §Liquids are not easily compressible and have a definite volume because there is little free space between particles. §Liquids flow easily because the particles can move/slide past one another. Chumbler - Properties of Matter 17

States of Matter Microscopic Explanation for Properties of Gases §Gases have an indefinite shape and an indefinite volume because the particles can move past one another. §Gases are easily compressible because there is a great deal of free space between particles. §Gases flow very easily because the particles randomly move past one another. Chumbler - Properties of Matter 18

States of Matter Microscopic Explanation for Properties of Plasmas §Plasmas have an indefinite shape and an indefinite volume because the particles can move past one another. §Plasmas are easily compressible because there is a great deal of free space between particles. §Plasmas are good conductors of electricity and are affected by magnetic fields because they are composed of ions Chumbler - Properties of Matter (negatively charged electrons and 19

States of Matter The Four States of Matter The Classification and Properties of Matter Depend Upon Microscopic Structure ØParticle arrangement ØParticle energy Chumbler - Properties of Matter 20

Change of State ØIs the change of a substance from one physical form to another ØAll changes of state are physical changes ØParticles of a substance have different amount of energy Chumbler - Properties of Matter 21

Melting ØIs the change of state from a solid to a liquid ØThe temperature at which a substance changes from a solid to a liquid is the melting point ØMelting is an endothermic Chumbler - Properties of Matter 22

Freezing ØIs the change of state from a liquid to a solid ØThe temperature at which a substance changes into a solid is the freezing a point ØFreezing is an exothermic change because energy is removed from or taken out of… Chumbler - Properties of Matter o 23

Vaporization ØIs the change of state from a liquid to a gas Ø 2 types of Vaporization Øi. Boiling Point – occurs when a boiling point is reached Ø 1. BP for water is 100 o. C Øii. Evaporation- occurs below the boiling point 24

Condensation ØIs the change of state from a gas to a liquid Example: beads of ice on a glass of water Chumbler - Properties of Matter 25

Sublimation ØIs the change of state from a solid directly to a gas Example: Dry ice Chumbler - Properties of Matter 26
 Four states of matter
Four states of matter Four states of matter
Four states of matter Plasma to gas
Plasma to gas 4 phases of matter
4 phases of matter Whats the four states of matter
Whats the four states of matter Section 1 composition of matter
Section 1 composition of matter Grey matter of nervous system
Grey matter of nervous system Composition of matter section 1
Composition of matter section 1 Chapter 2 section 1 classifying matter answer key
Chapter 2 section 1 classifying matter answer key Median and lateral apertures
Median and lateral apertures Section 1 composition of matter chapter 15 answer key
Section 1 composition of matter chapter 15 answer key Gray matter and white matter
Gray matter and white matter What is gray matter in the brain
What is gray matter in the brain Flow of energy vs flow of matter
Flow of energy vs flow of matter Chemistry matter and its changes
Chemistry matter and its changes Chemistry matter and change chapter 7
Chemistry matter and change chapter 7 Chemistry matter and change chapter 10
Chemistry matter and change chapter 10 Examples of matter in chemistry
Examples of matter in chemistry Flowchart of how matter is classified
Flowchart of how matter is classified 1s 22 s22 p63 s23 p64 s2
1s 22 s22 p63 s23 p64 s2 Chemistry matter and change chapter 6
Chemistry matter and change chapter 6 Chapter 10 study guide the mole
Chapter 10 study guide the mole Chemistry matter and change answer key chapter 2
Chemistry matter and change answer key chapter 2 Matter flowchart chemistry
Matter flowchart chemistry Classification graphic organizer
Classification graphic organizer Non examples of homogeneous mixture
Non examples of homogeneous mixture Chapter 4 basic food chemistry the nature of matter
Chapter 4 basic food chemistry the nature of matter