STATES OF MATTER The Four States of Matter









- Slides: 12


STATES OF MATTER • The Four States of Matter • Solid • Liquid • Gas • Plasma • Four States

STATES OF MATTER Ø Based upon particle arrangement Ø Based upon energy of particles Ø Based upon distance between particles

Kinetic Theory of Matter is made up of particles which are in continual random motion.

STATES OF MATTER SOLIDS • Particles of solids are tightly packed, vibrating about a fixed position. • Solids have a definite shape and a definite volume.

Types of Solids • Crystalline Solid – made up of crystals with a regular, repeating pattern – Salt, Sugar, and snow – Melts at a distinct temperature • Amorphous Solid – particles are not in a regular pattern - Glass, Plastic, and Rubber - Does NOT melt at a distinct temperature

STATES OF MATTER LIQUID § Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume.

Properties of Liquids • Surface Tension – An inward force or pull, among the molecules in a liquid that brings the molecules on the surface closer together. – Example: Water on leaves • Viscosity – A liquids resistance to flowing. – High viscosity = slow flow – Low viscosity = fast flow

STATES OF MATTER GAS § Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume.

PHASE CHANGES Description of Phase Change Solid to liquid Term for Phase Change Melting Liquid to Freezing solid Heat Movement During Phase Change Heat goes into the solid as it melts. Heat leaves the liquid as it freezes.

PHASE CHANGES Description of Phase Change Term for Phase Change Heat Movement During Phase Change Vaporization, which includes Heat goes into the Liquid to gas boiling and liquid as it vaporizes. evaporation Heat leaves the gas Gas to liquid Condensation as it condenses. Heat goes into the Solid to gas Sublimation solid as it sublimates.

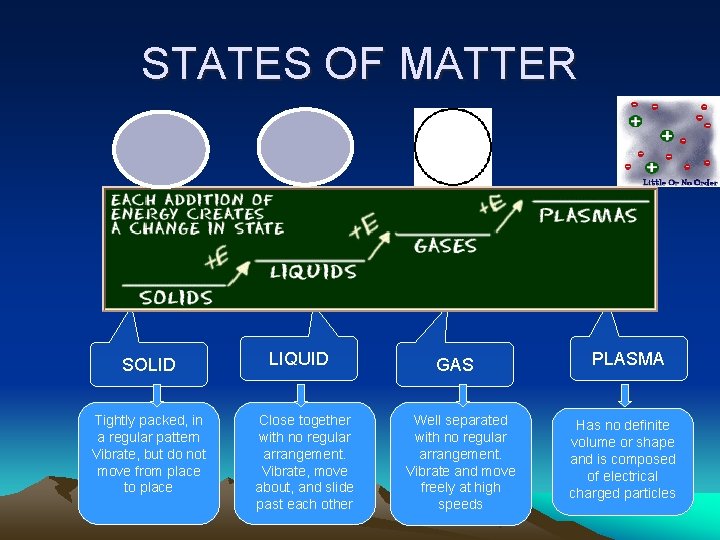
STATES OF MATTER SOLID Tightly packed, in a regular pattern Vibrate, but do not move from place to place LIQUID Close together with no regular arrangement. Vibrate, move about, and slide past each other GAS Well separated with no regular arrangement. Vibrate and move freely at high speeds PLASMA Has no definite volume or shape and is composed of electrical charged particles
 Four states of matter
Four states of matter Four states of matter
Four states of matter Whats states of matter
Whats states of matter Four states of matter
Four states of matter Solid to plasma
Solid to plasma Chụp tư thế worms-breton
Chụp tư thế worms-breton đại từ thay thế
đại từ thay thế Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công của trọng lực
Công của trọng lực Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới