SOLIDS LIQUIDS AND GASES KINETIC THEORY explanation of

















- Slides: 33

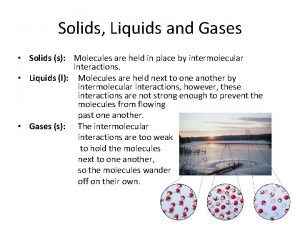
SOLIDS, LIQUIDS, AND GASES

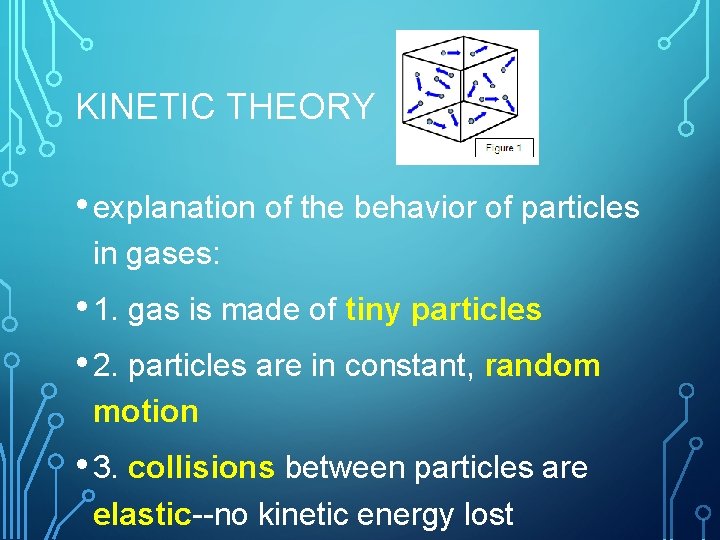
KINETIC THEORY • explanation of the behavior of particles in gases: • 1. gas is made of tiny particles • 2. particles are in constant, random motion • 3. collisions between particles are elastic--no kinetic energy lost

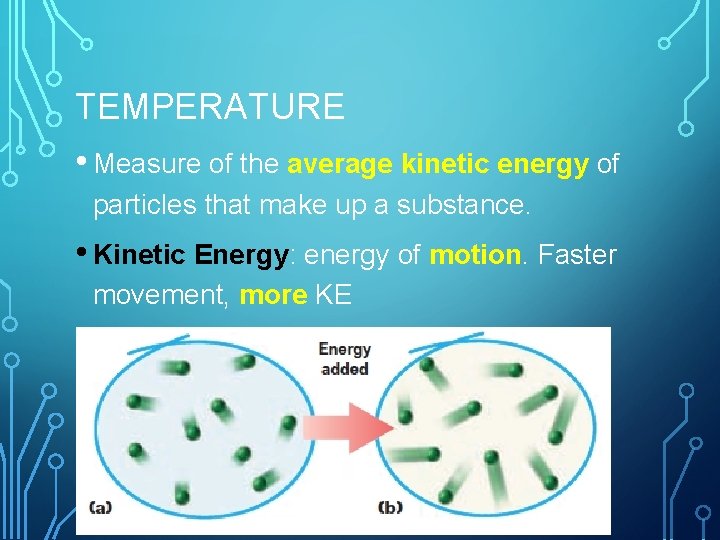
TEMPERATURE • Measure of the average kinetic energy of particles that make up a substance. • Kinetic Energy: energy of motion. Faster movement, more KE


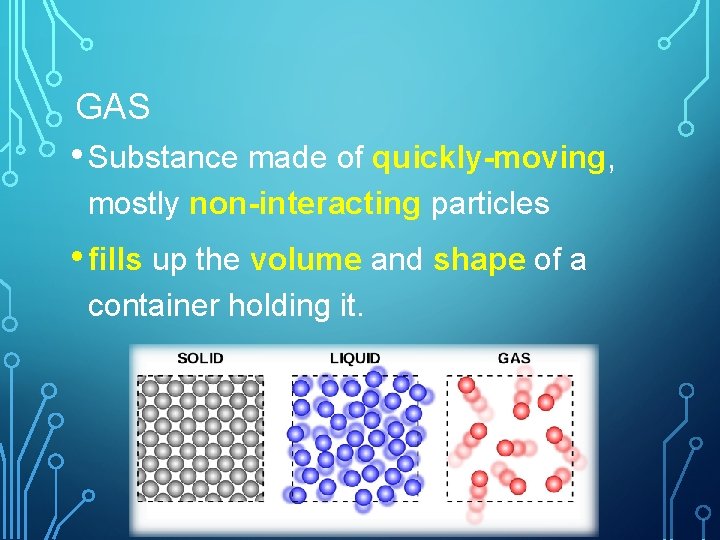
GAS • Substance made of quickly-moving, mostly non-interacting particles • fills up the volume and shape of a container holding it.

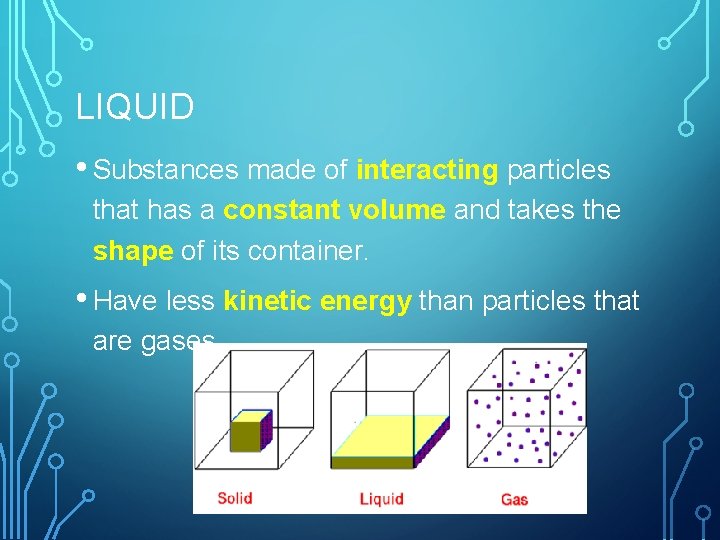
LIQUID • Substances made of interacting particles that has a constant volume and takes the shape of its container. • Have less kinetic energy than particles that are gases.

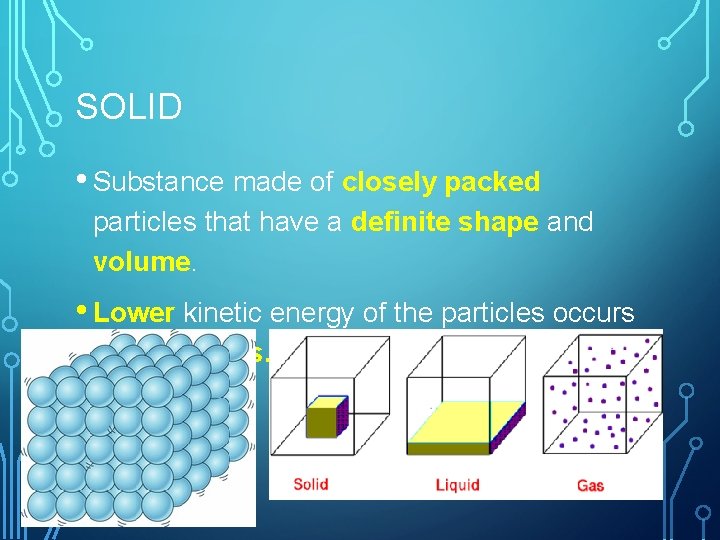
SOLID • Substance made of closely packed particles that have a definite shape and volume. • Lower kinetic energy of the particles occurs as vibrations.

PLASMA • the fourth state of matter consisting of particles with kinetic energy high enough such that electrons are stripped off of atoms • Most of the matter in the universe is in this state.

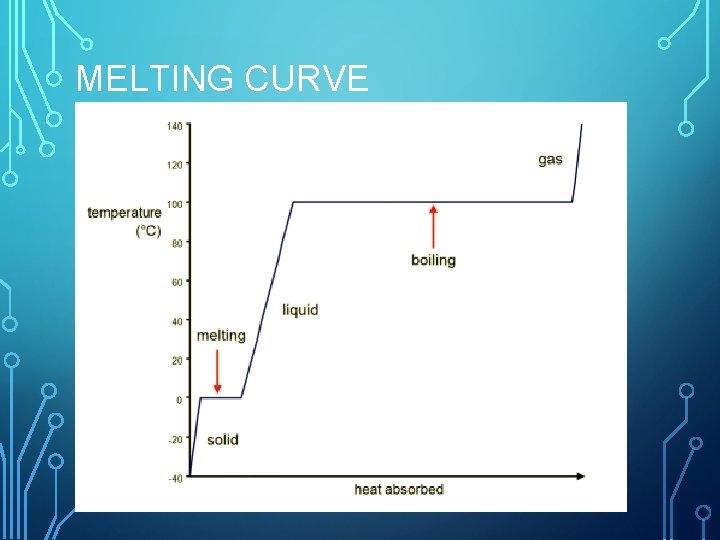
MELTING POINT • temperature at which a solid begins to liquefy. • Water: 0°C • At this temperature particles have the kinetic energy to break free from tighter interactions of the solid state

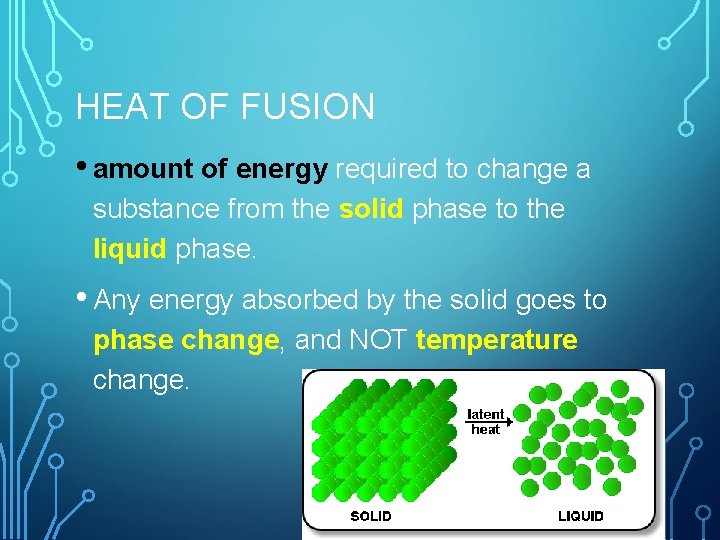
HEAT OF FUSION • amount of energy required to change a substance from the solid phase to the liquid phase. • Any energy absorbed by the solid goes to phase change, and NOT temperature change.

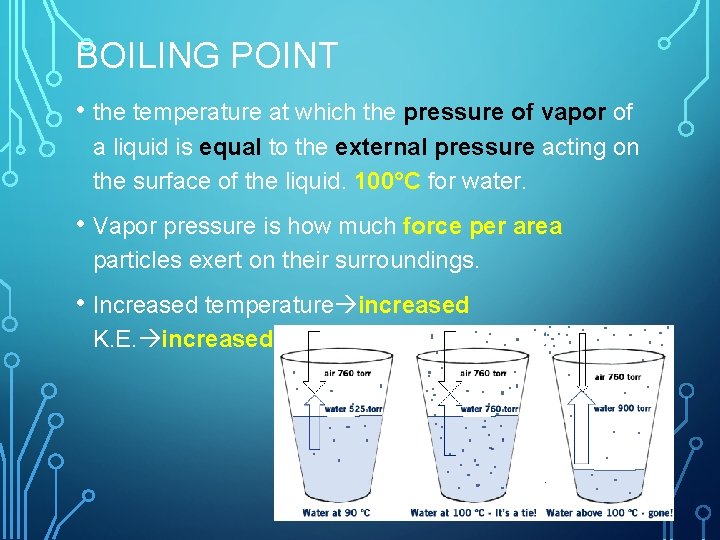
BOILING POINT • the temperature at which the pressure of vapor of a liquid is equal to the external pressure acting on the surface of the liquid. 100°C for water. • Vapor pressure is how much force per area particles exert on their surroundings. • Increased temperature increased K. E. increased vapor pressure

HEAT OF VAPORIZATION • amount of energy required for a liquid at its boiling point to become a gas. • Water may reach 100°C and not boil. • MORE energy is needed to give particles enough K. E. to escape interactions • Boiling fluid will remain at the boiling point temp until all particles are in gas state


MELTING CURVE

• Think: • What would happen to the boiling point of water if the atmospheric pressure were lowered? • Hint: look at boiling point definition • Turn and Talk • Share

SUBLIMATION • the process of a solid changing directly to a vapor without forming a liquid.

DENSITY •

DENSITY • Generally, a solid is more dense than a liquid, which is more dense than a gas • Water is an exception: ice is less dense than liquid water • Density of water = 1 g/m. L (exactly) at 4°C.

THERMAL EXPANSION • increase in the volume of a substance when the temperature is increased • Density decreases

DENSITY PRACTICE PROBLEMS • Pure solid gold has a density of 19. 32 g/cm 3. What is the mass of a cubic meter (or 1, 000 cm 3) of gold? • What is the density of ice if 5. 33 cm 3 of ice has a mass of 4. 886 g?

AMORPHOUS SOLIDS • solids lacking a crystalline structure that do not have a specific melting point • Examples: • Butter, solid grease, petroleum jelly

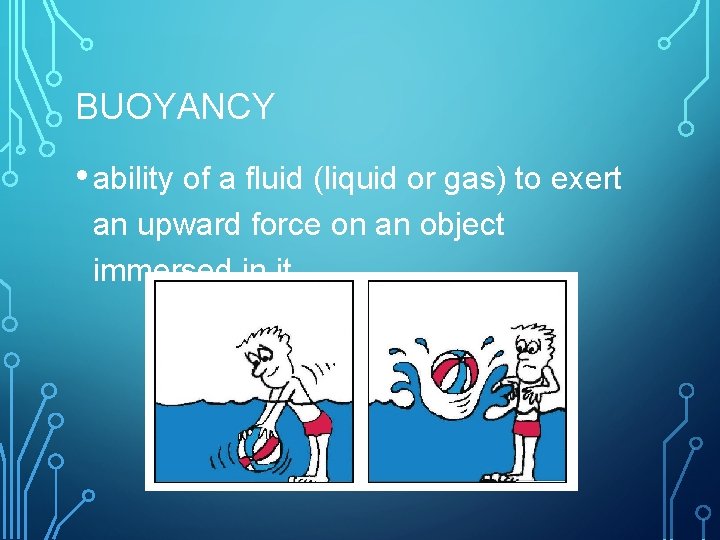
BUOYANCY • ability of a fluid (liquid or gas) to exert an upward force on an object immersed in it.

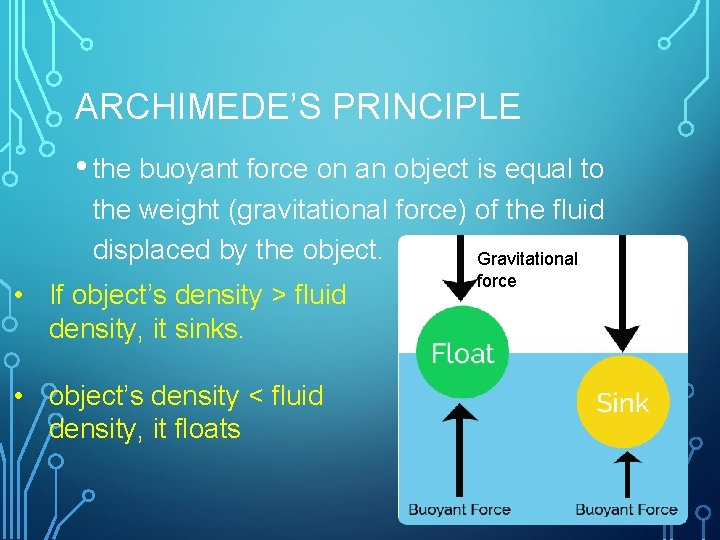
ARCHIMEDE’S PRINCIPLE • the buoyant force on an object is equal to the weight (gravitational force) of the fluid displaced by the object. Gravitational • If object’s density > fluid density, it sinks. • object’s density < fluid density, it floats force

IF AN OBJECT IS FLOATING… • Weight of object (gravitational force) is balanced with the opposing weight of the water displaced (buoyant force) • Weight of object = weight of water displaced • Use the density equation to convert from volume of water displaced to mass/weight of water displaced.

WHY DO WE FLOAT IN THE DEAD SEA?

ARCHIMEDES' PRINCIPLE PRACTICE 1. A man is floating in water. The buoyant force acting on his body is 210 lbs (pounds are a unit of force). What is the man’s weight? 2. A river barge weighs 5, 340 N. What is the weight of the water it displaces when it floats? 3. A floating object displaces 10. 0 cubic feet (ft 3) of water. How much does the object weigh if the weight density of water is 62. 43 lbs/ft 3?

PRESSURE • amount of force exerted per unit area.

UNITS OF FORCE • Newtons (N) and pounds (lbs)

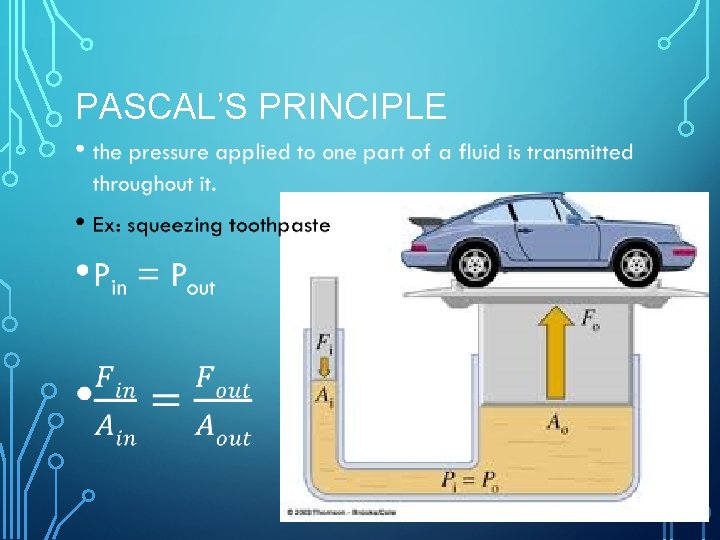
PASCAL’S PRINCIPLE •

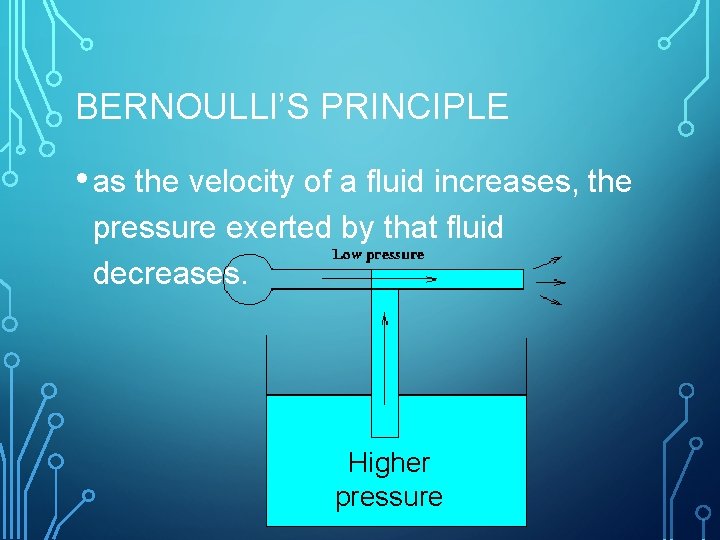
BERNOULLI’S PRINCIPLE • as the velocity of a fluid increases, the pressure exerted by that fluid decreases. Higher pressure

BOYLE’S LAW •

KELVIN (UNITS) • units of temperature equal in magnitude to Celsius, that begin (zero value) at -273. 15 C. • 0 K is called absolute zero. NO kinetic energy.

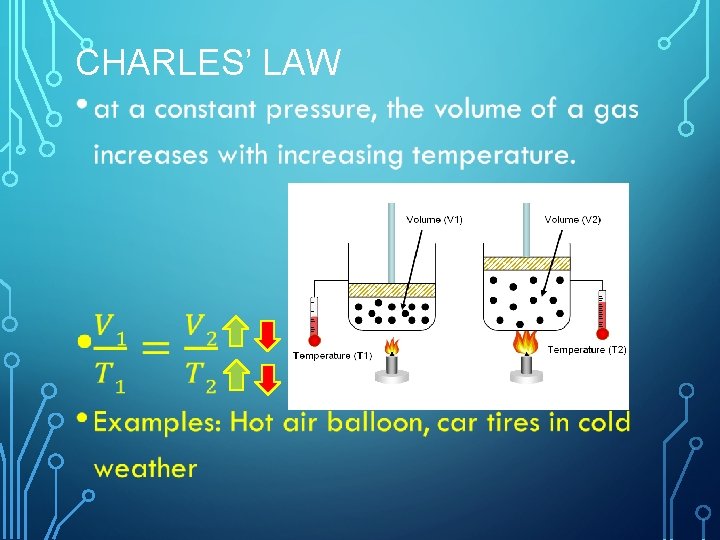
CHARLES’ LAW •
 Chapter 14 solids liquids and gases
Chapter 14 solids liquids and gases Kinetic molecular theory of liquids and solids
Kinetic molecular theory of liquids and solids Expansion of solids liquids and gases examples
Expansion of solids liquids and gases examples Buoyancyability
Buoyancyability Conduction and convection venn diagram
Conduction and convection venn diagram What are the properties of solids
What are the properties of solids Solid liquid gas examples
Solid liquid gas examples Lesson 1 thermal energy and the behavior of matter
Lesson 1 thermal energy and the behavior of matter Chapter 14 solids liquids and gases worksheet answers
Chapter 14 solids liquids and gases worksheet answers Particle movement in solids liquids and gases
Particle movement in solids liquids and gases How does sound travel through solids liquids and gases
How does sound travel through solids liquids and gases Properties of solid liquid and gas
Properties of solid liquid and gas Motion of particles in solids, liquids and gases
Motion of particles in solids, liquids and gases Why is gas easier to compress than a liquid
Why is gas easier to compress than a liquid Molecular theory of gases and liquids
Molecular theory of gases and liquids Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic theory of solids
Kinetic theory of solids Properties of solids and liquids
Properties of solids and liquids Liquids and solids menu
Liquids and solids menu Liquids and solids
Liquids and solids Chapter 11 - states of matter: liquids and solids
Chapter 11 - states of matter: liquids and solids Kinetic theory for ideal gases
Kinetic theory for ideal gases Condensation particle theory
Condensation particle theory Kinetic theory of gases
Kinetic theory of gases Kinetic postulates of gases
Kinetic postulates of gases Kinetic theory
Kinetic theory Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Write the postulates of kinetic theory of gases
Write the postulates of kinetic theory of gases Kinetic theory of gases
Kinetic theory of gases Filter medium resistance formula
Filter medium resistance formula The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter An explanation of how particles in matter behave
An explanation of how particles in matter behave