Chapter 7 Gases Liquids and Solids The Kinetic









- Slides: 37

Chapter 7 Gases, Liquids, and Solids The Kinetic Molecular Theory of Matter

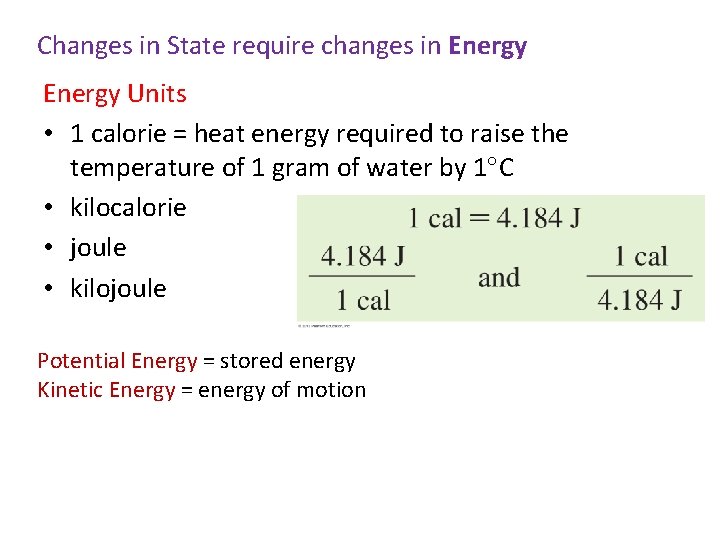
Changes in State require changes in Energy Units • 1 calorie = heat energy required to raise the temperature of 1 gram of water by 1 C • kilocalorie • joule • kilojoule Potential Energy = stored energy Kinetic Energy = energy of motion

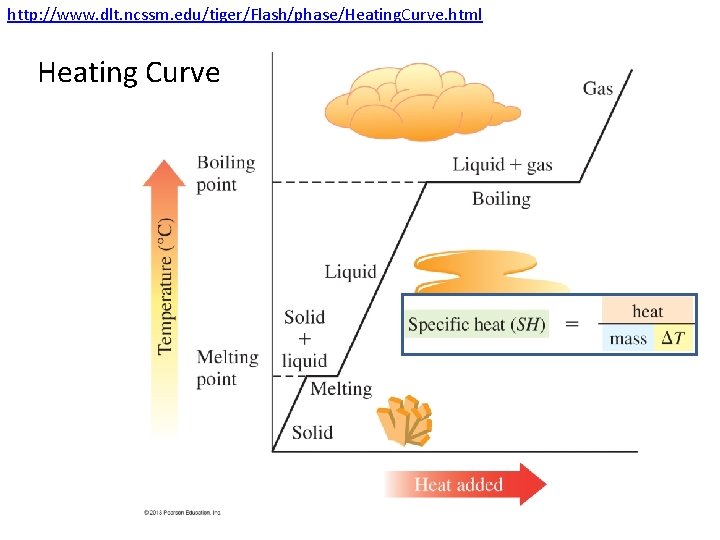
http: //www. dlt. ncssm. edu/tiger/Flash/phase/Heating. Curve. html Heating Curve

Example: Calculations using Specific Heat How many calories of heat are required to heat 250 grams of water from 20 C to 95 C? How many joules?

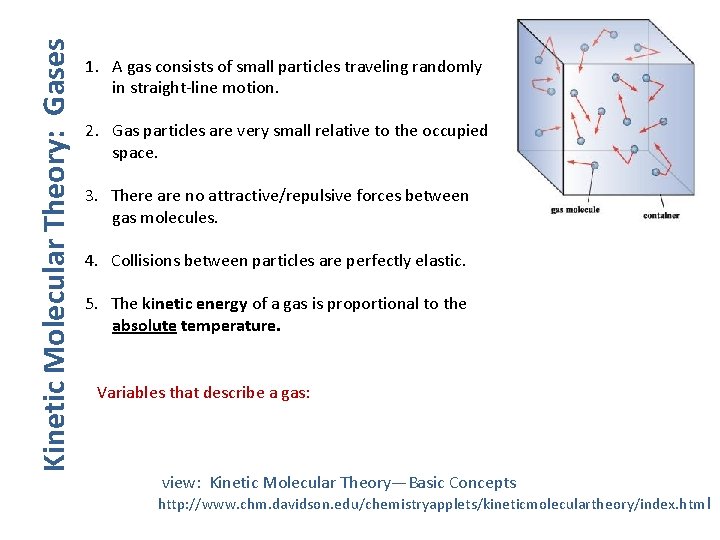
Kinetic Molecular Theory: Gases 1. A gas consists of small particles traveling randomly in straight-line motion. 2. Gas particles are very small relative to the occupied space. 3. There are no attractive/repulsive forces between gas molecules. 4. Collisions between particles are perfectly elastic. 5. The kinetic energy of a gas is proportional to the absolute temperature. Variables that describe a gas: view: Kinetic Molecular Theory—Basic Concepts http: //www. chm. davidson. edu/chemistryapplets/kineticmoleculartheory/index. htm l

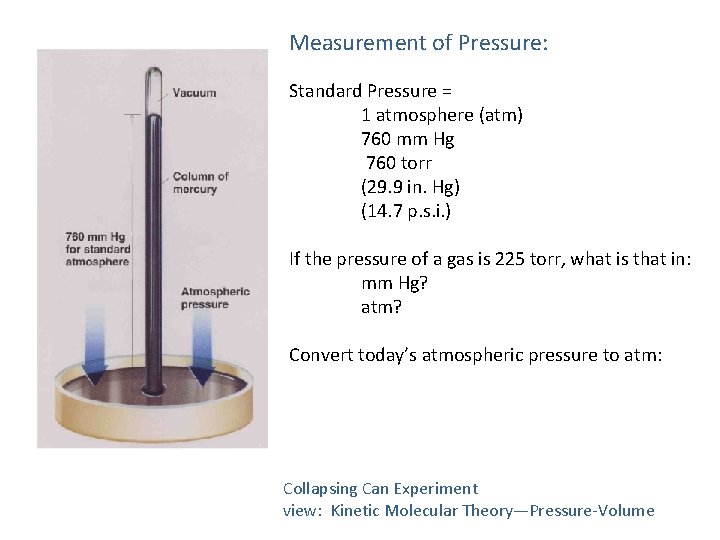
Measurement of Pressure: Standard Pressure = 1 atmosphere (atm) 760 mm Hg 760 torr (29. 9 in. Hg) (14. 7 p. s. i. ) If the pressure of a gas is 225 torr, what is that in: mm Hg? atm? Convert today’s atmospheric pressure to atm: Collapsing Can Experiment view: Kinetic Molecular Theory—Pressure-Volume

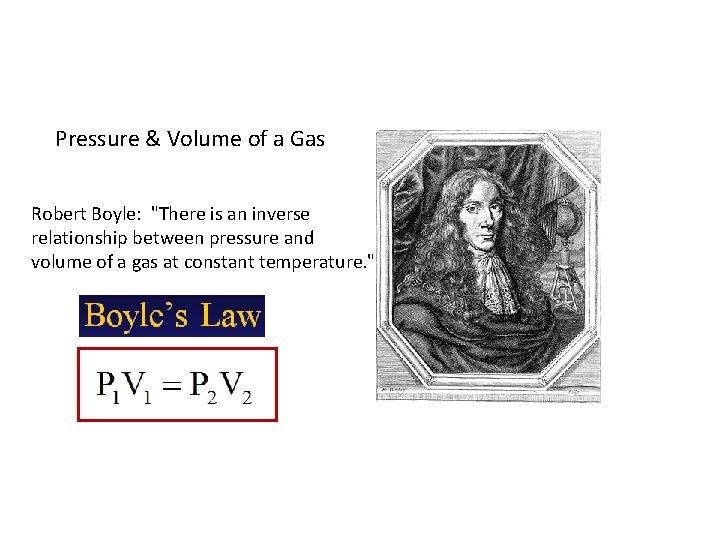
Pressure & Volume of a Gas Robert Boyle: "There is an inverse relationship between pressure and volume of a gas at constant temperature. "

Sample Problems: 1. A gas occupies 2. 65 Liters at 655 mm Hg. At what pressure will it occupy 75. 0 Liters? 2. A sample of hydrogen gas occupies a volume of 6. 52 L at a pressure of 634 mm Hg and a temperature of 35 C. What volume will it occupy if the pressure is decreased to 475 mm Hg with no change in temperature?

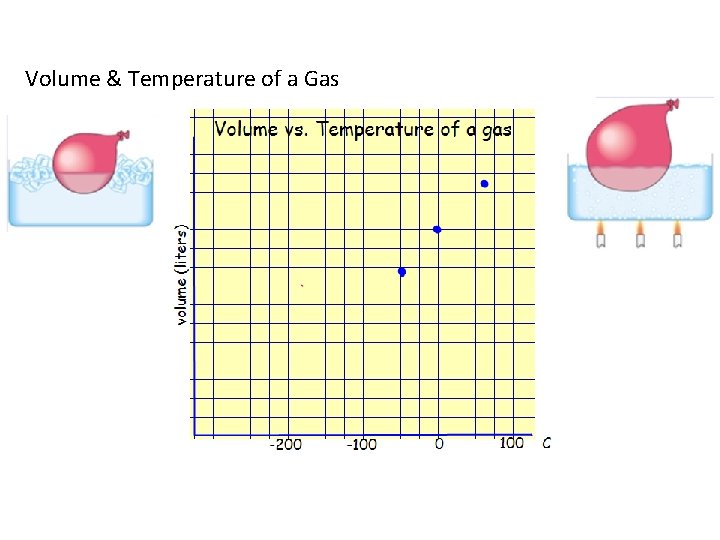
Volume & Temperature of a Gas

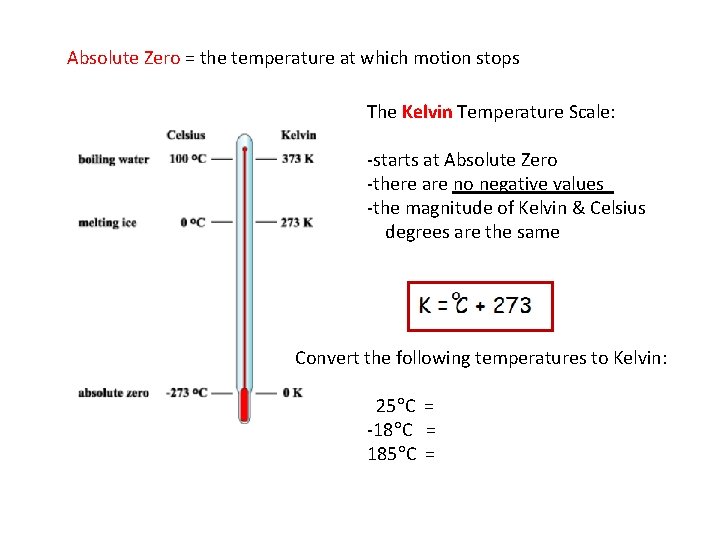
Absolute Zero = the temperature at which motion stops The Kelvin Temperature Scale: -starts at Absolute Zero -there are no negative values -the magnitude of Kelvin & Celsius degrees are the same Convert the following temperatures to Kelvin: 25 C = -18 C = 185 C =

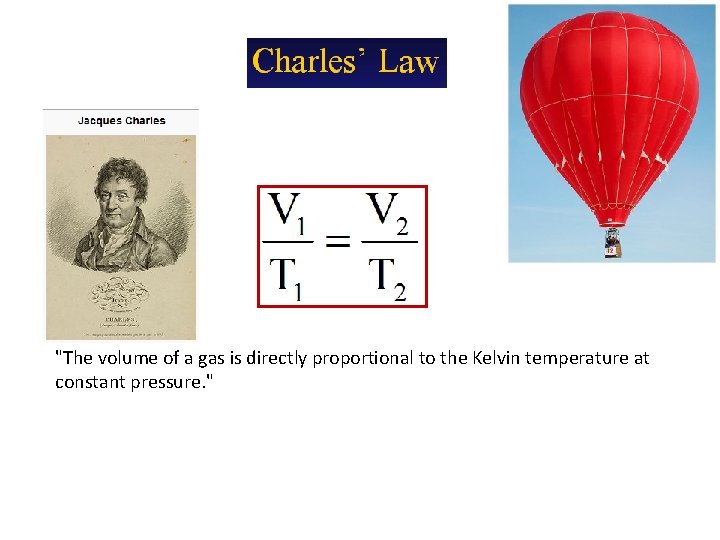
"The volume of a gas is directly proportional to the Kelvin temperature at constant pressure. "

Sample Problems: 1. Find the volume of a gas at -25 C if the volume is 259 ml at 110 C. 2. A sample of dry air, with a volume of 252 m. L at 25 C, is heated at constant pressure to 55 C. What is the new volume? view: Charles’ Law Experiment http: //www. youtube. com/watch? v=Ik. RIKGN 3 i 0 k

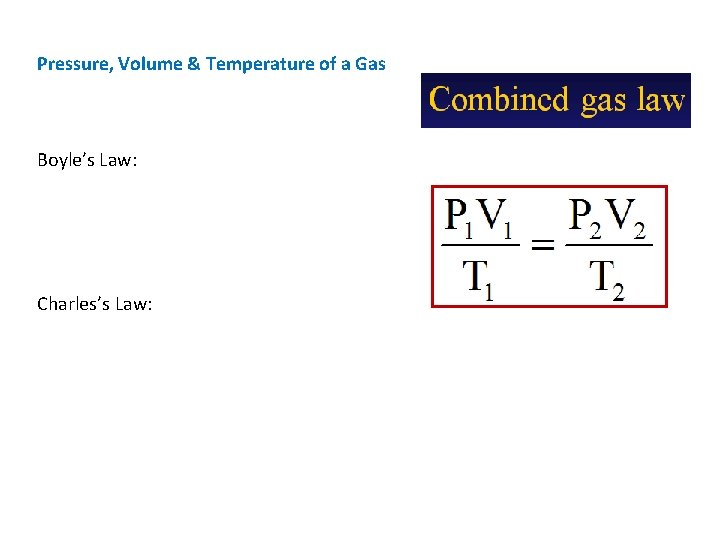
Pressure, Volume & Temperature of a Gas Boyle’s Law: Charles’s Law:

Sample Problems: 1. A gas has a volume of 425 ml at -12 C and 682 torr. What volume will it have at 78 C and 528 torr?

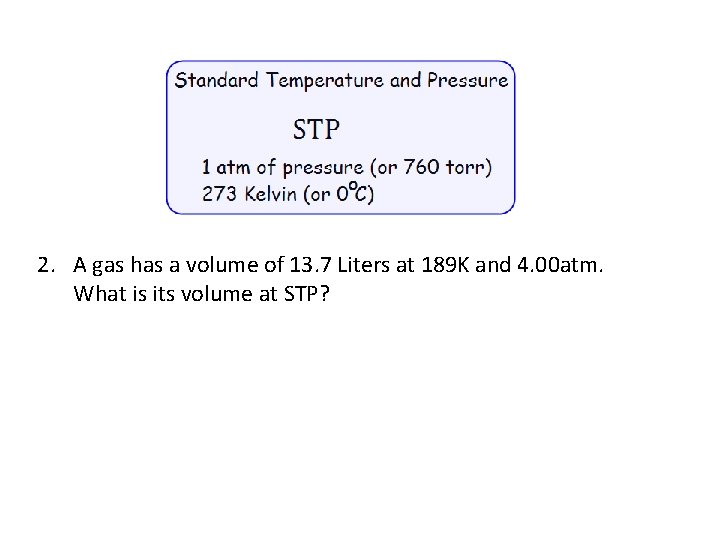
2. A gas has a volume of 13. 7 Liters at 189 K and 4. 00 atm. What is its volume at STP?

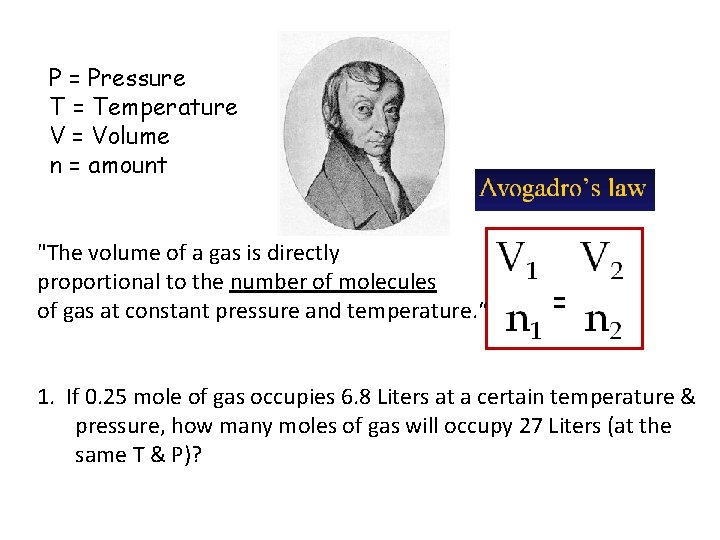
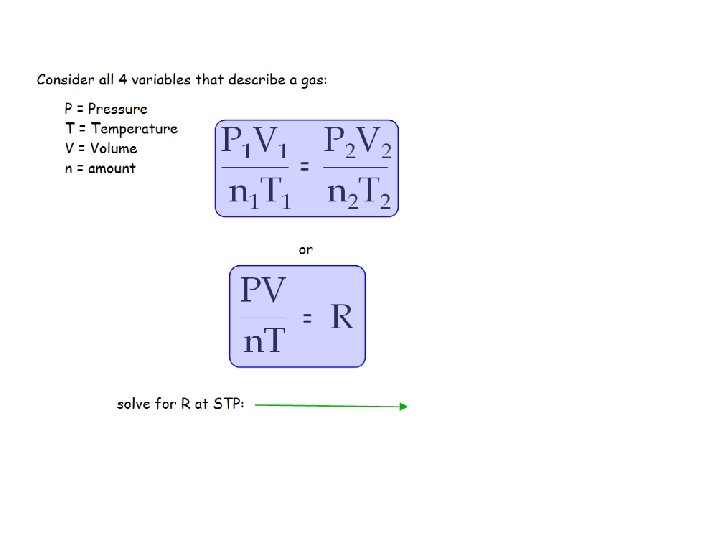
P = Pressure T = Temperature V = Volume n = amount "The volume of a gas is directly proportional to the number of molecules of gas at constant pressure and temperature. “ = 1. If 0. 25 mole of gas occupies 6. 8 Liters at a certain temperature & pressure, how many moles of gas will occupy 27 Liters (at the same T & P)?

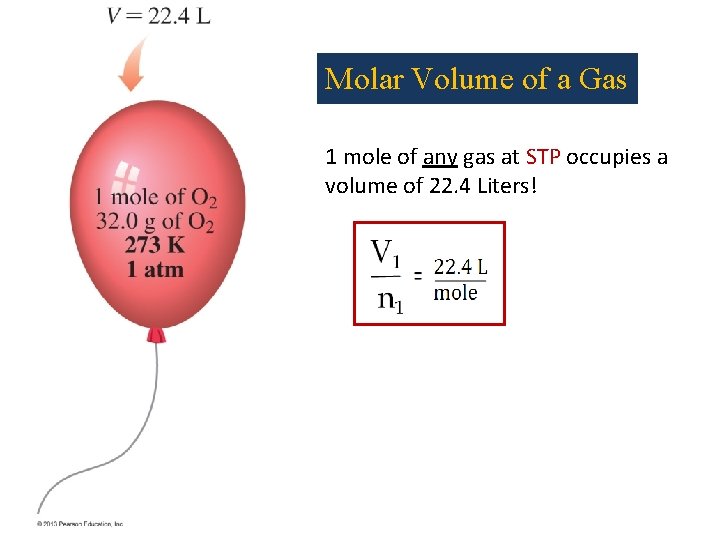
Molar Volume of a Gas 1 mole of any gas at STP occupies a volume of 22. 4 Liters!


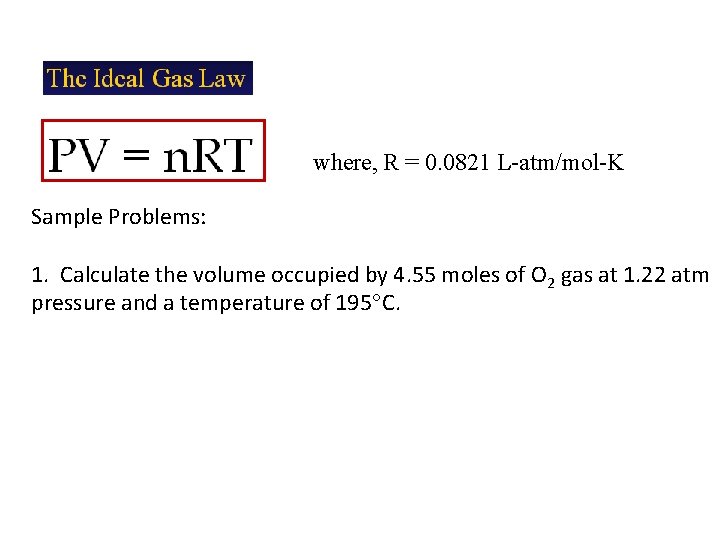
where, R = 0. 0821 L-atm/mol-K Sample Problems: 1. Calculate the volume occupied by 4. 55 moles of O 2 gas at 1. 22 atm pressure and a temperature of 195 C.

2. How many moles of N 2 gas occupy 6. 2 Liters at 2. 05 atm and 37 C? 3. If 0. 58 mole of Argon occupies 8. 5 Liters at 25 C, what is the pressure?

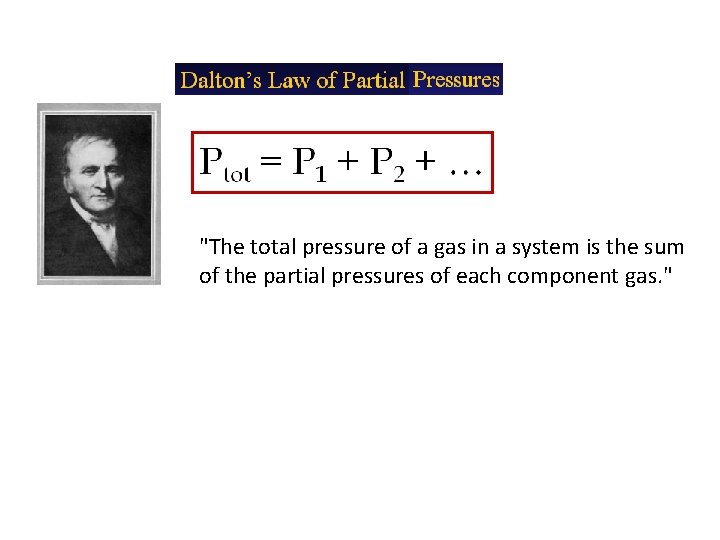
"The total pressure of a gas in a system is the sum of the partial pressures of each component gas. "

Sample Problems: 1. A mixture of gases contains N 2, He and O 2. Their partial pressures are 0. 40 atm, 0. 55 atm & 0. 15 atm. What is the total pressure? 2. A mixture of CO 2 and N 2 gases is at an atmospheric pressure of 758 torr. If the pressure of the nitrogen is 633 torr, what is the pressure of the carbon dioxide?

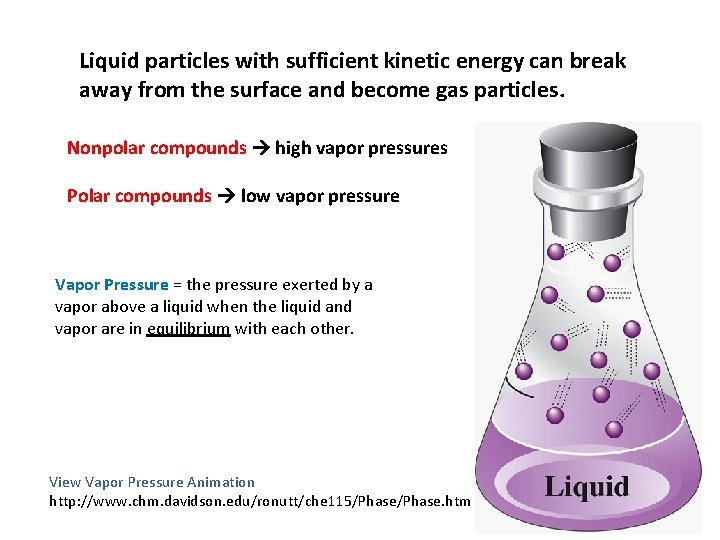
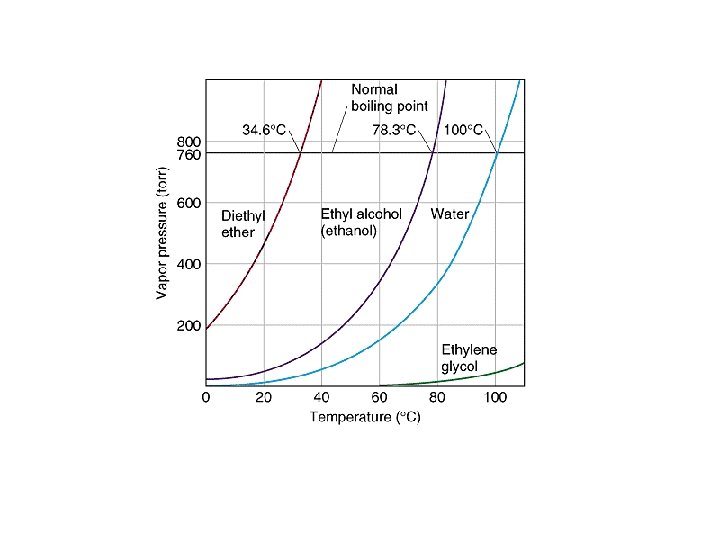
Liquid particles with sufficient kinetic energy can break away from the surface and become gas particles. Nonpolar compounds high vapor pressures Polar compounds low vapor pressure Vapor Pressure = the pressure exerted by a vapor above a liquid when the liquid and vapor are in equilibrium with each other. View Vapor Pressure Animation http: //www. chm. davidson. edu/ronutt/che 115/Phase. htm

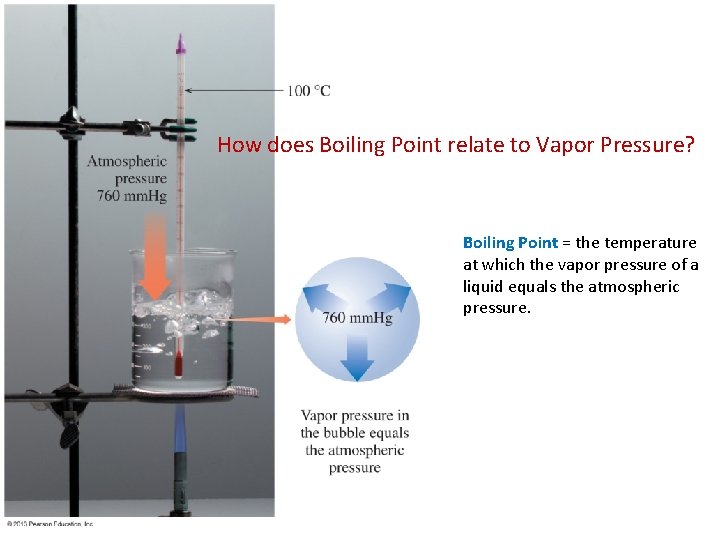
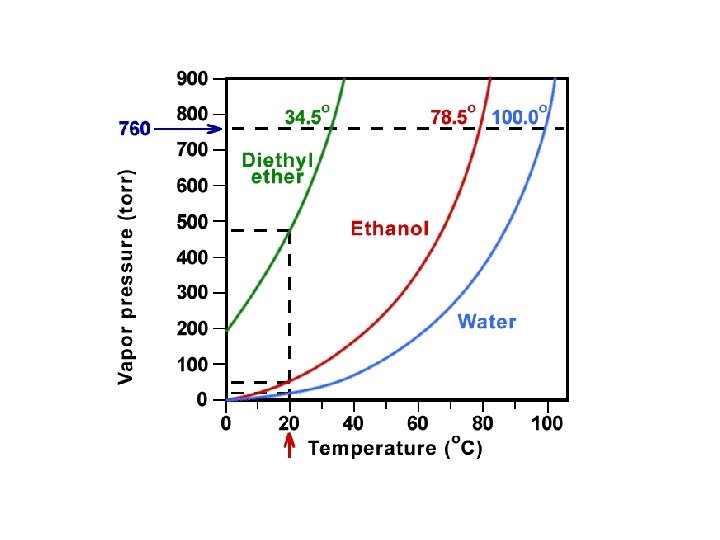
How does Boiling Point relate to Vapor Pressure? Boiling Point = the temperature at which the vapor pressure of a liquid equals the atmospheric pressure.


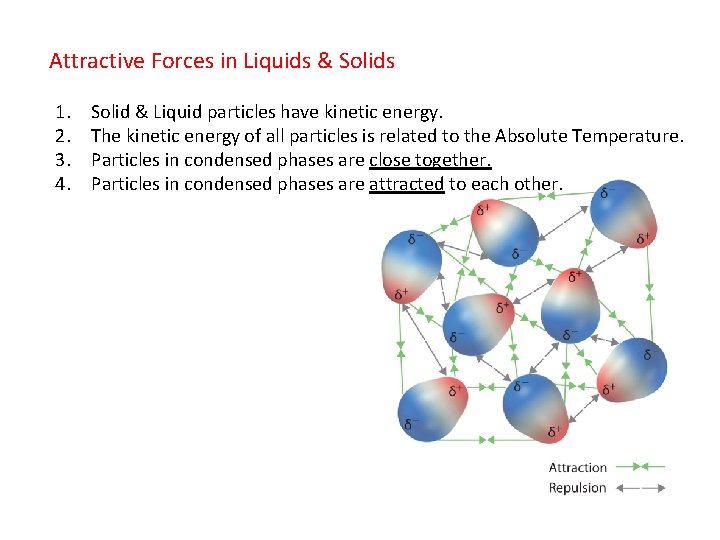
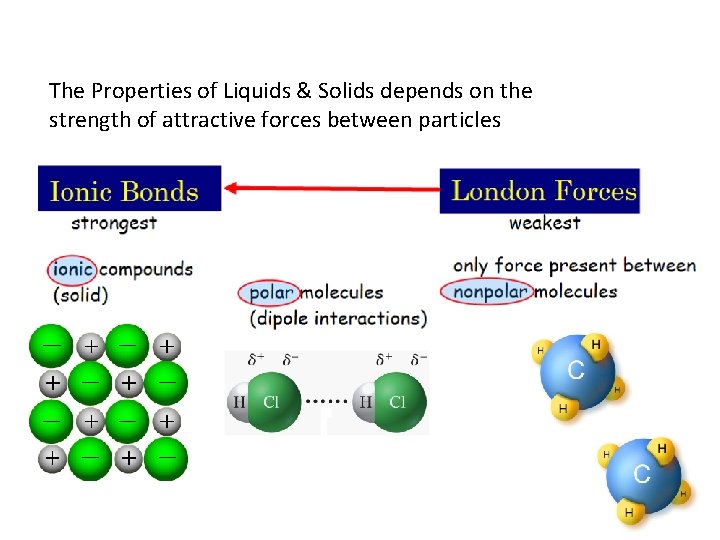
Attractive Forces in Liquids & Solids 1. 2. 3. 4. Solid & Liquid particles have kinetic energy. The kinetic energy of all particles is related to the Absolute Temperature. Particles in condensed phases are close together. Particles in condensed phases are attracted to each other.

The Properties of Liquids & Solids depends on the strength of attractive forces between particles

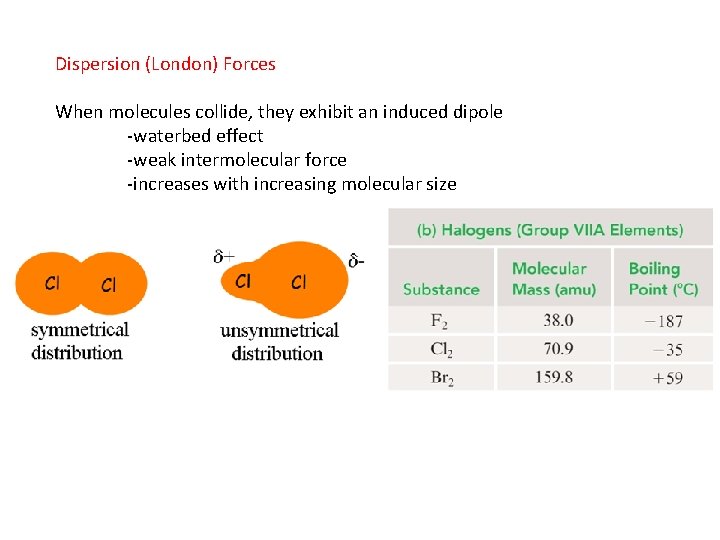
Dispersion (London) Forces When molecules collide, they exhibit an induced dipole -waterbed effect -weak intermolecular force -increases with increasing molecular size

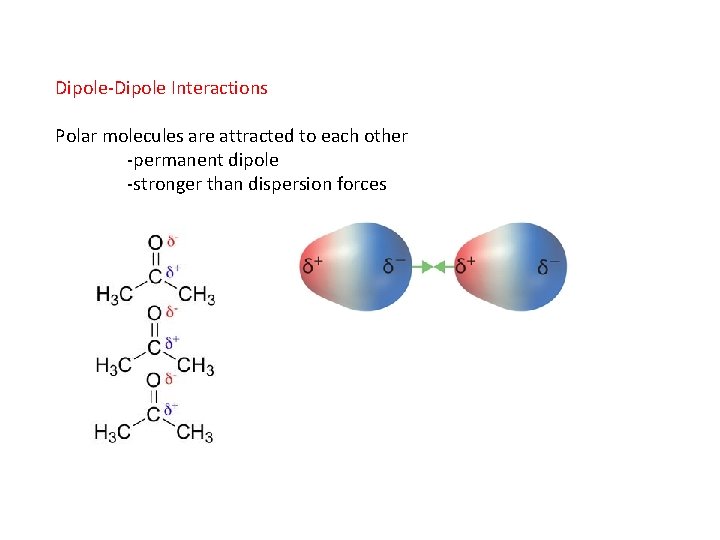
Dipole-Dipole Interactions Polar molecules are attracted to each other -permanent dipole -stronger than dispersion forces

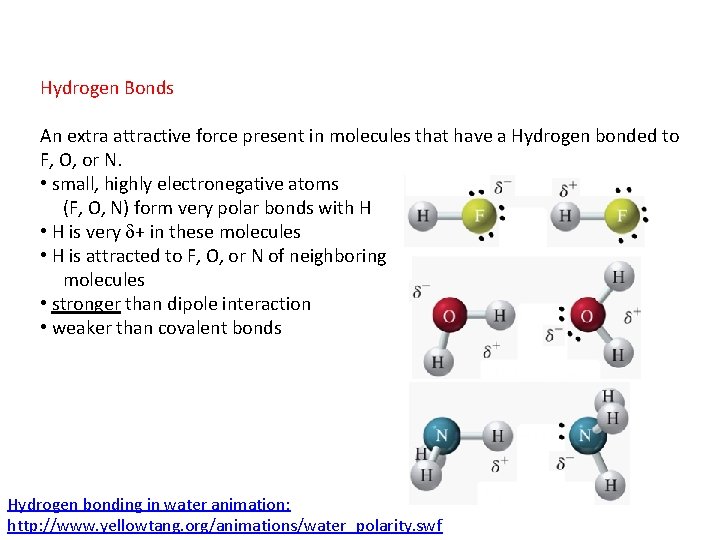
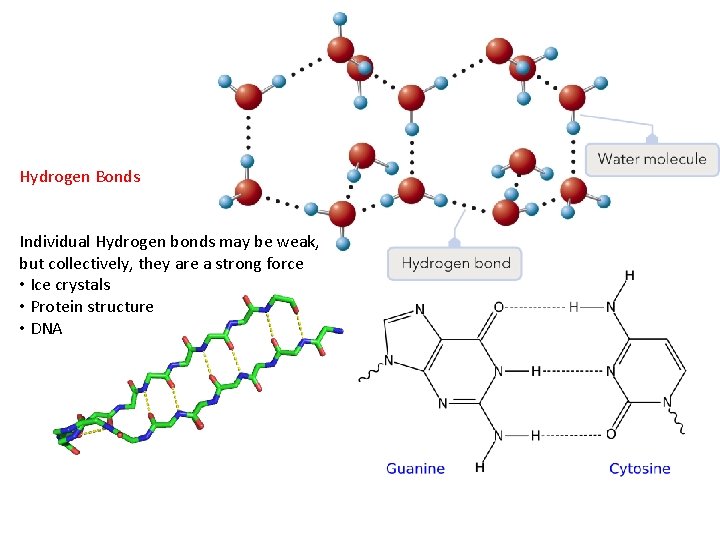
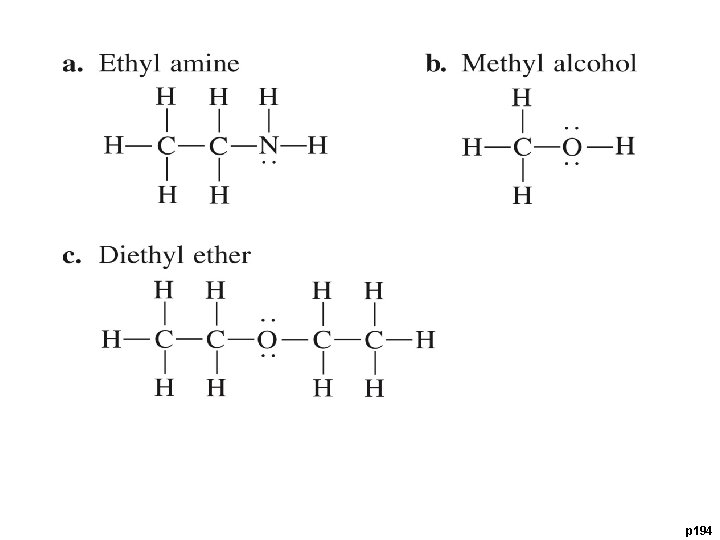
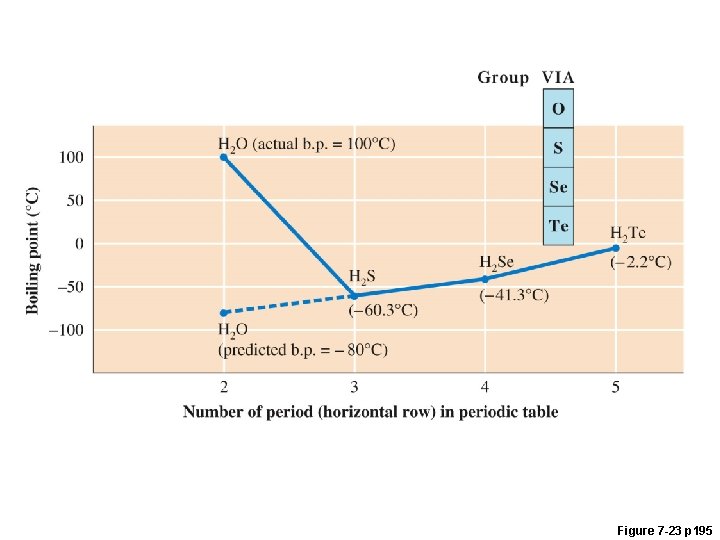
Hydrogen Bonds An extra attractive force present in molecules that have a Hydrogen bonded to F, O, or N. • small, highly electronegative atoms (F, O, N) form very polar bonds with H • H is very + in these molecules • H is attracted to F, O, or N of neighboring molecules • stronger than dipole interaction • weaker than covalent bonds Hydrogen bonding in water animation: http: //www. yellowtang. org/animations/water_polarity. swf

Hydrogen Bonds Individual Hydrogen bonds may be weak, but collectively, they are a strong force • Ice crystals • Protein structure • DNA

p 194


Figure 7 -23 p 195

Mixed Gas Law Problems: 1. A mixture of hydrogen gas and water vapor has a pressure of 659. 0 mm Hg at 20 C. The vapor pressure of water is 17. 5 mm Hg at 20 C. What is the pressure of the hydrogen? 2. A certain amount of gas occupies 256 ml at 98 C and 756. 9 mm Hg. What is its volume at 25 C and 733. 1 mm Hg?

3. How many moles of a gas occupy 212 ml at 25 C and 733. 1 torr? 4. If a gas has a volume of 1. 6 Liters at a pressure of 4. 5 atm, what will it’s volume be at 0. 85 atm?

5. In an experiment, oxygen gas is collected over water at 25 C. The total pressure is 756. 9 mm Hg. If the vapor pressure of water is 23. 8 mm Hg, what is the pressure of the oxygen? 6. In an experiment, 0. 25 moles of hydrogen gas were generated. What volume would this amount of gas occupy at STP? Additional Practice Questions are posted on Canvas in the Chapter 7 folder.