Section 9 1 Using Chemical Equations Stoichiometry Section








- Slides: 60

Section 9. 1 Using Chemical Equations (Stoichiometry)

Section 9. 1 Using Chemical Equations (Stoichiometry)

Section 9. 1 Using Chemical Equations (Stoichiometry) Objectives 1. To understand the information given in a balanced equation 2. To use a balanced equation to determine relationships between moles of reactant and products (mole ratio) (Stoichiometry)

Section 9. 1 Using Chemical Equations (Stoichiometry) Sandwich Shoppe! 1. Let’s create a sandwich recipe…PHe. T 1. Slice, dozen, 100, 6. 02 X 1023, one mole (pg 281)

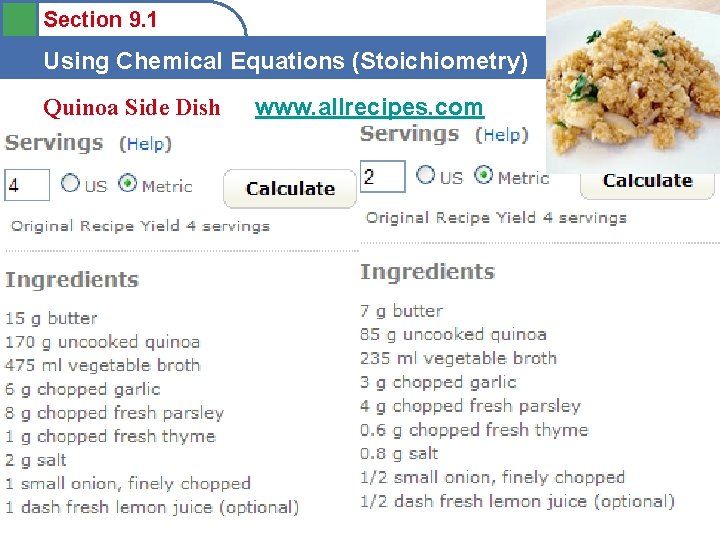
Section 9. 1 Using Chemical Equations (Stoichiometry) Quinoa Side Dish www. allrecipes. com

Section 9. 1 Using Chemical Equations (Stoichiometry) 1. Information Given by Chemical Equations • The coefficients of a balanced equation give the relative numbers of molecules. • A balanced chemical equation gives relative numbers (or moles) of reactant and product molecules that participate in a chemical reaction.

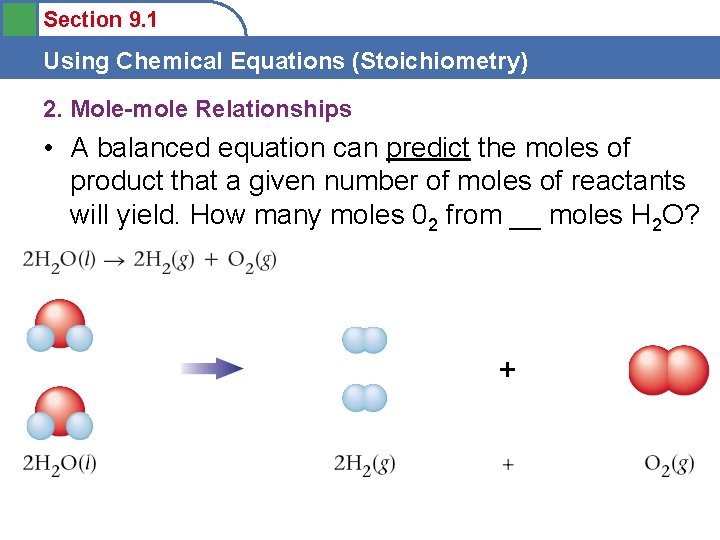
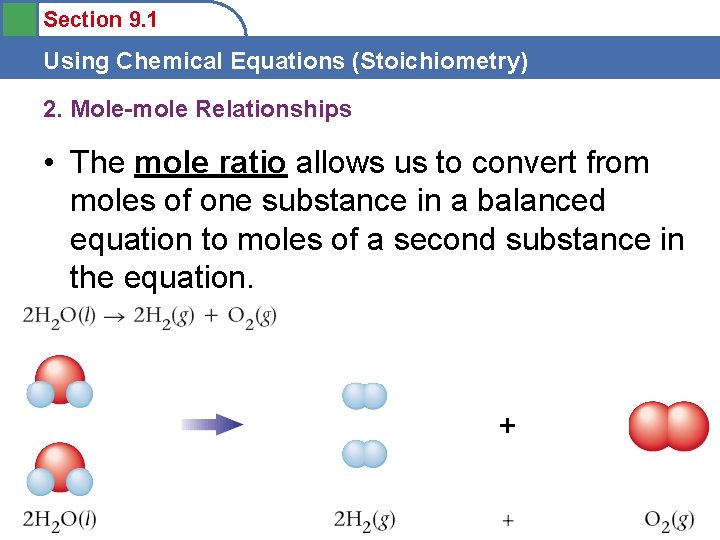
Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • A balanced equation can predict the moles of product that a given number of moles of reactants will yield. How many moles 02 from __ moles H 2 O?

Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • The mole ratio allows us to convert from moles of one substance in a balanced equation to moles of a second substance in the equation.

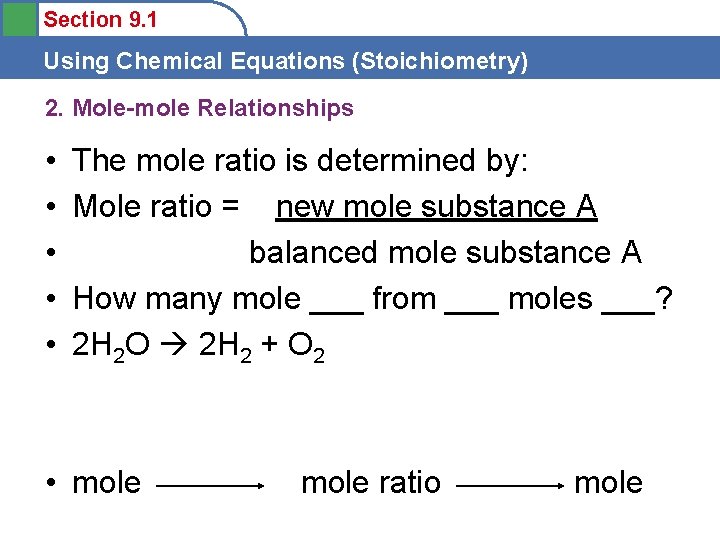
Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • • • The mole ratio is determined by: Mole ratio = new mole substance A balanced mole substance A How many mole ___ from ___ moles ___? 2 H 2 O 2 H 2 + O 2 • mole ratio mole

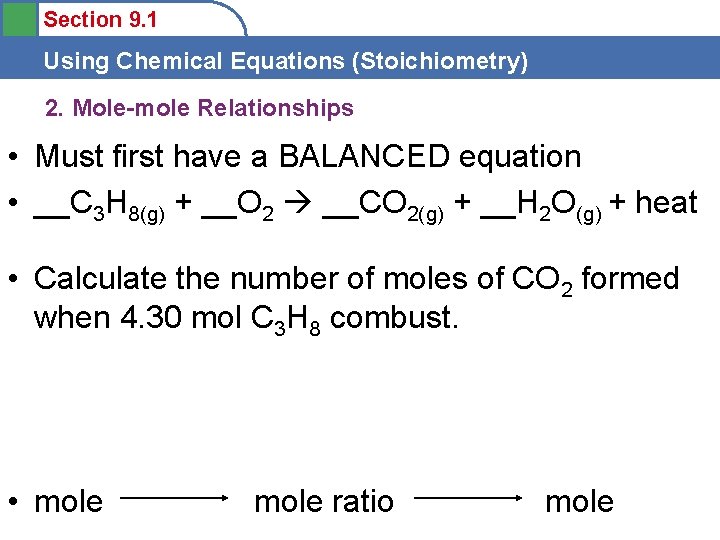
Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • Must first have a BALANCED equation • __C 3 H 8(g) + __O 2 __CO 2(g) + __H 2 O(g) + heat • Calculate the number of moles of CO 2 formed when 4. 30 mol C 3 H 8 combust. • mole ratio mole

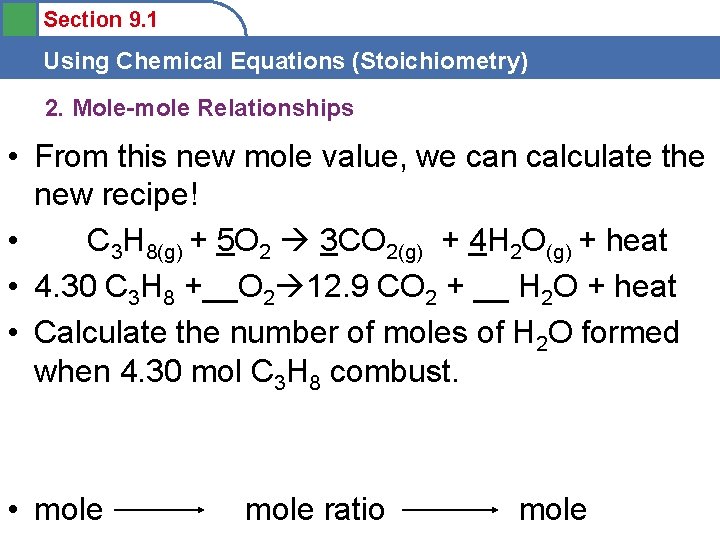
Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • From this new mole value, we can calculate the new recipe! • C 3 H 8(g) + 5 O 2 3 CO 2(g) + 4 H 2 O(g) + heat • 4. 30 C 3 H 8 +__O 2 12. 9 CO 2 + __ H 2 O + heat • Calculate the number of moles of H 2 O formed when 4. 30 mol C 3 H 8 combust. • mole ratio mole

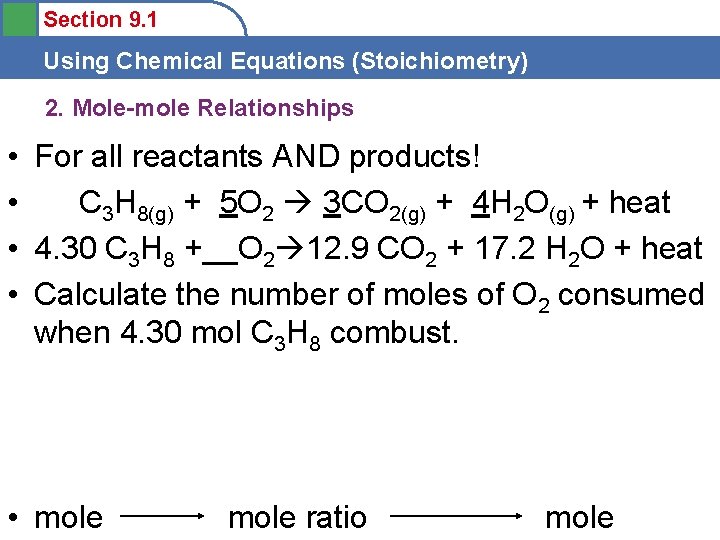
Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • For all reactants AND products! • C 3 H 8(g) + 5 O 2 3 CO 2(g) + 4 H 2 O(g) + heat • 4. 30 C 3 H 8 +__O 2 12. 9 CO 2 + 17. 2 H 2 O + heat • Calculate the number of moles of O 2 consumed when 4. 30 mol C 3 H 8 combust. • mole ratio mole

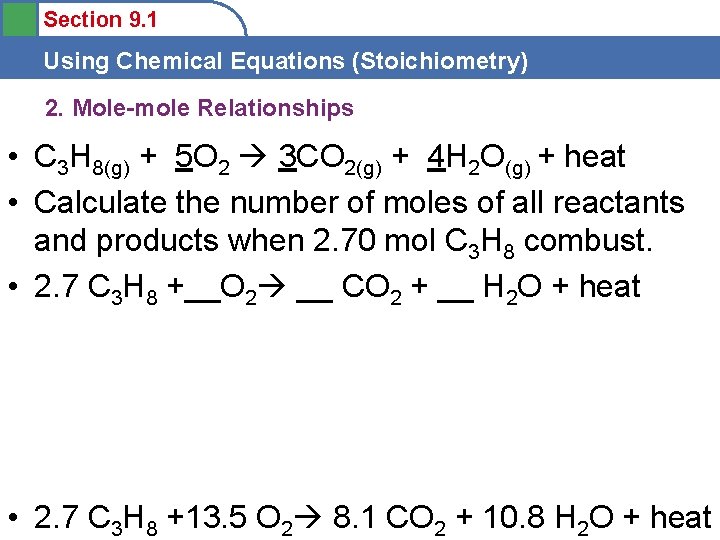
Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • C 3 H 8(g) + 5 O 2 3 CO 2(g) + 4 H 2 O(g) + heat • Calculate the number of moles of all reactants and products when 2. 70 mol C 3 H 8 combust. • 2. 7 C 3 H 8 +__O 2 __ CO 2 + __ H 2 O + heat • 2. 7 C 3 H 8 +13. 5 O 2 8. 1 CO 2 + 10. 8 H 2 O + heat

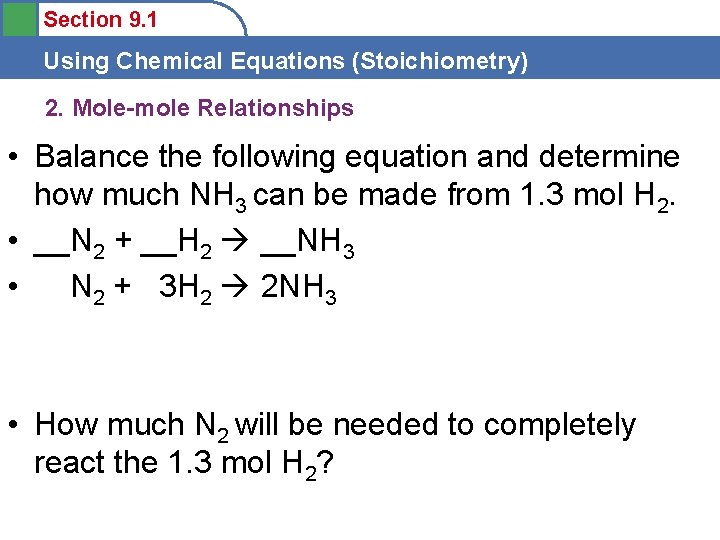
Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • Balance the following equation and determine how much NH 3 can be made from 1. 3 mol H 2. • __N 2 + __H 2 __NH 3 • N 2 + 3 H 2 2 NH 3 • How much N 2 will be needed to completely react the 1. 3 mol H 2?

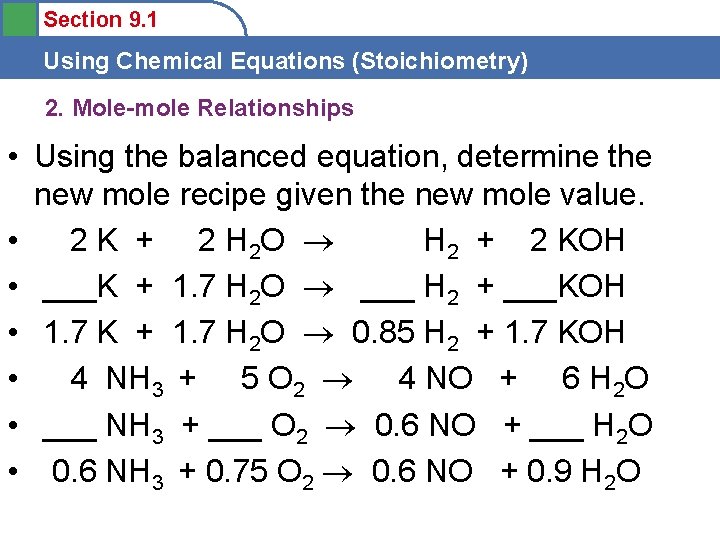
Section 9. 1 Using Chemical Equations (Stoichiometry) 2. Mole-mole Relationships • Using the balanced equation, determine the new mole recipe given the new mole value. • 2 K + 2 H 2 O H 2 + 2 KOH • ___K + 1. 7 H 2 O ___ H 2 + ___KOH • 1. 7 K + 1. 7 H 2 O 0. 85 H 2 + 1. 7 KOH • 4 NH 3 + 5 O 2 4 NO + 6 H 2 O • ___ NH 3 + ___ O 2 0. 6 NO + ___ H 2 O • 0. 6 NH 3 + 0. 75 O 2 0. 6 NO + 0. 9 H 2 O

Section 9. 1 Using Chemical Equations (Stoichiometry) Objectives Review 1. To understand the information given in a balanced equation 2. To use a balanced equation to determine relationships between moles of reactant and products (mole ratio) (Stoichiometry) 3. Work Session: Page 287 #2 -5 4. QUIZ!!

Section 9. 2 Using Chemical Equations to Calculate Mass Objectives 1. To learn to use Stoichiometry to relate masses of reactants and products in a chemical reaction 2. To perform mass calculations that involve scientific notation 3. Calculate theoretical predications for a reaction that will be performed in the lab

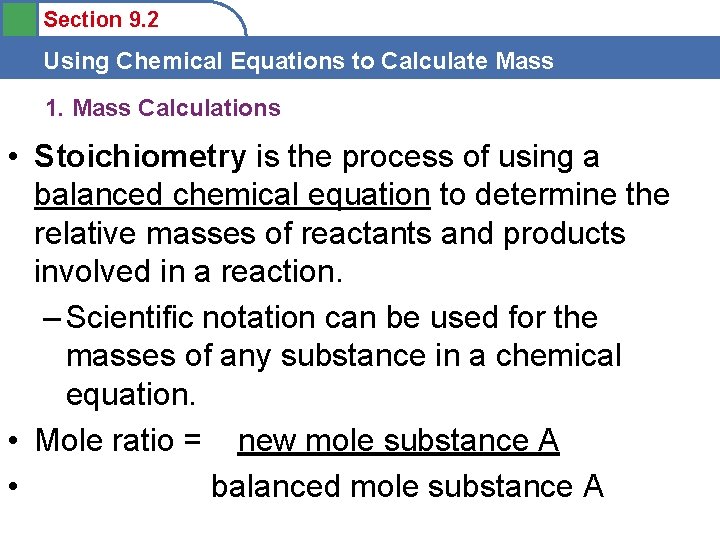
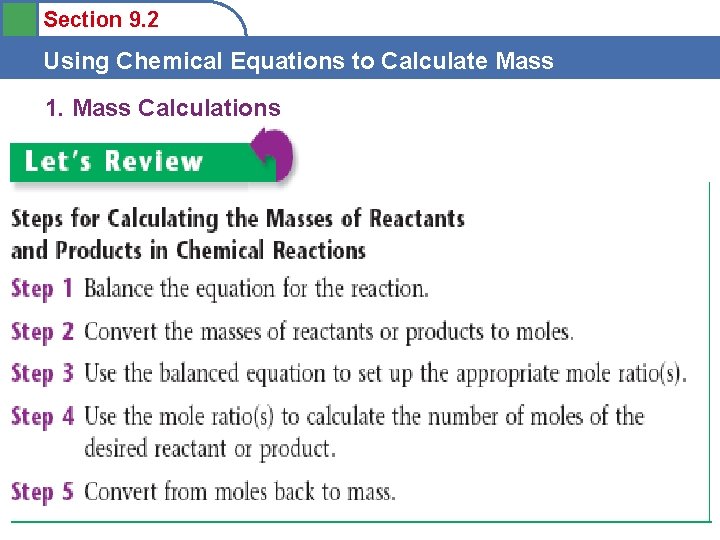
Section 9. 2 Using Chemical Equations to Calculate Mass 1. Mass Calculations • Stoichiometry is the process of using a balanced chemical equation to determine the relative masses of reactants and products involved in a reaction. – Scientific notation can be used for the masses of any substance in a chemical equation. • Mole ratio = new mole substance A • balanced mole substance A

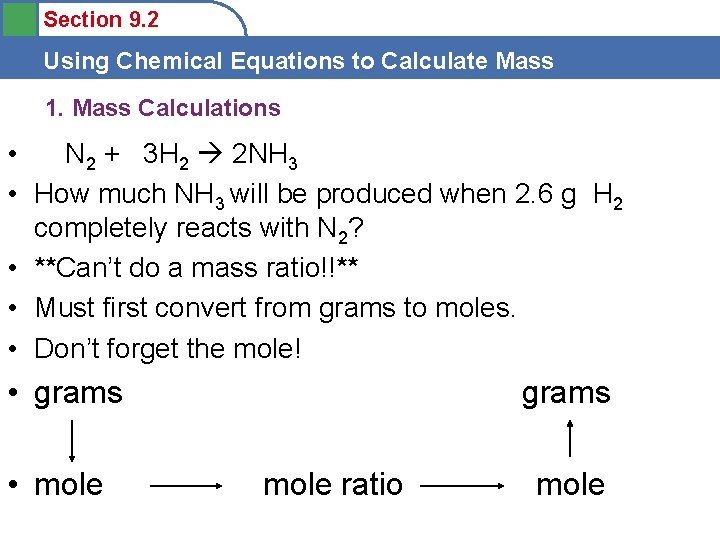
Section 9. 2 Using Chemical Equations to Calculate Mass 1. Mass Calculations • N 2 + 3 H 2 2 NH 3 • How much NH 3 will be produced when 2. 6 g H 2 completely reacts with N 2? • **Can’t do a mass ratio!!** • Must first convert from grams to moles. • Don’t forget the mole! • grams • mole grams mole ratio mole

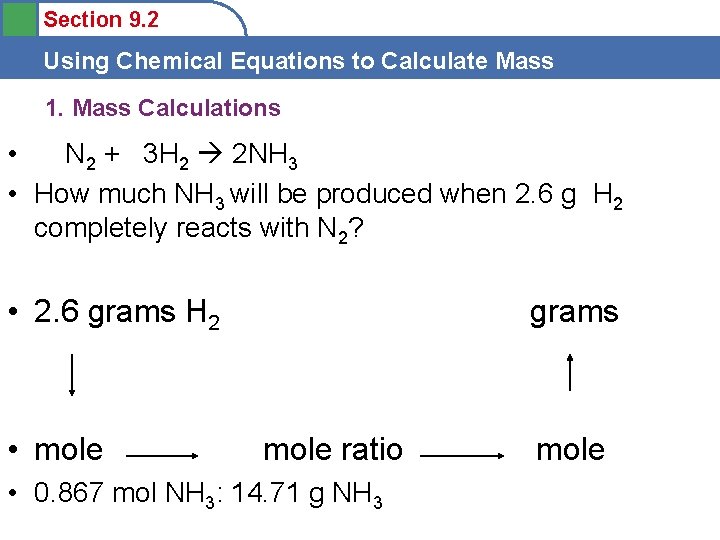
Section 9. 2 Using Chemical Equations to Calculate Mass 1. Mass Calculations • N 2 + 3 H 2 2 NH 3 • How much NH 3 will be produced when 2. 6 g H 2 completely reacts with N 2? • 2. 6 grams H 2 • mole grams mole ratio • 0. 867 mol NH 3: 14. 71 g NH 3 mole

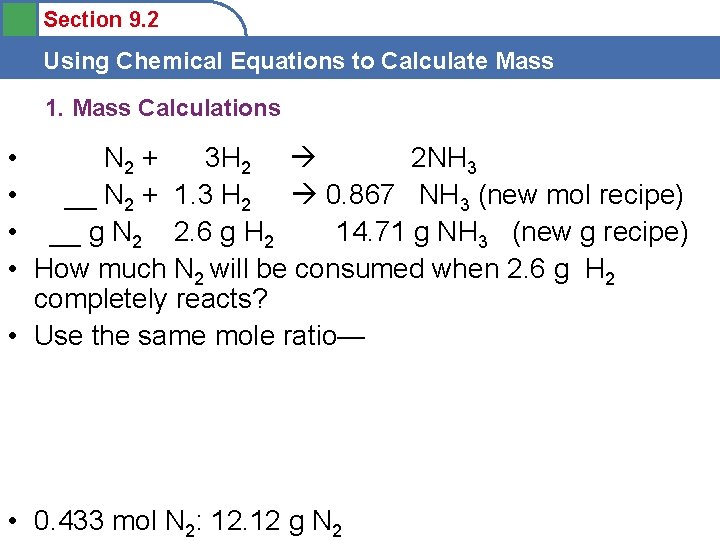
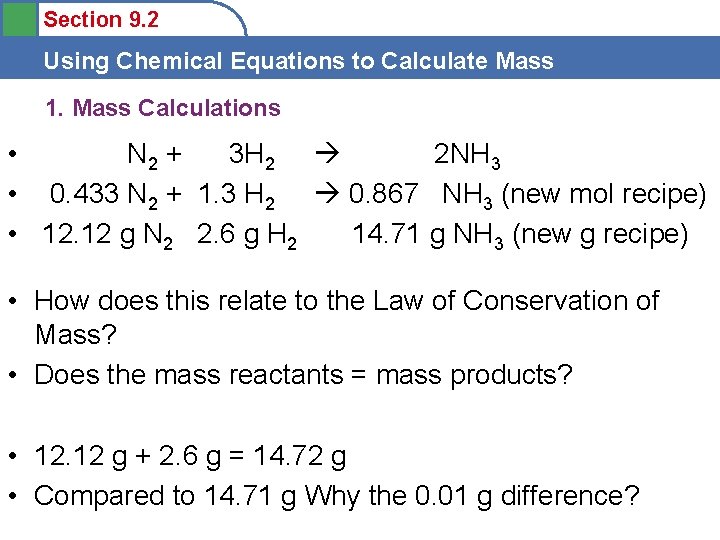
Section 9. 2 Using Chemical Equations to Calculate Mass 1. Mass Calculations • N 2 + 3 H 2 2 NH 3 • __ N 2 + 1. 3 H 2 0. 867 NH 3 (new mol recipe) • __ g N 2 2. 6 g H 2 14. 71 g NH 3 (new g recipe) • How much N 2 will be consumed when 2. 6 g H 2 completely reacts? • Use the same mole ratio— • 0. 433 mol N 2: 12. 12 g N 2

Section 9. 2 Using Chemical Equations to Calculate Mass 1. Mass Calculations • N 2 + 3 H 2 2 NH 3 • 0. 433 N 2 + 1. 3 H 2 0. 867 NH 3 (new mol recipe) • 12. 12 g N 2 2. 6 g H 2 14. 71 g NH 3 (new g recipe) • How does this relate to the Law of Conservation of Mass? • Does the mass reactants = mass products? • 12. 12 g + 2. 6 g = 14. 72 g • Compared to 14. 71 g Why the 0. 01 g difference?

Section 9. 2 Using Chemical Equations to Calculate Mass 1. Mass Calculations

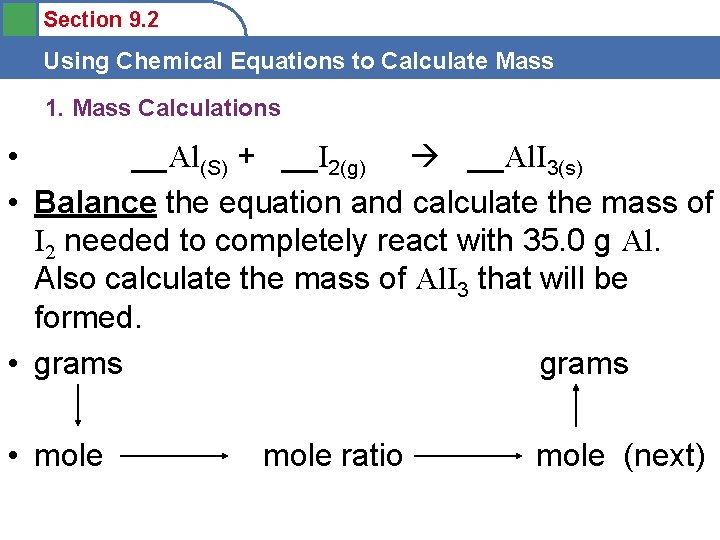
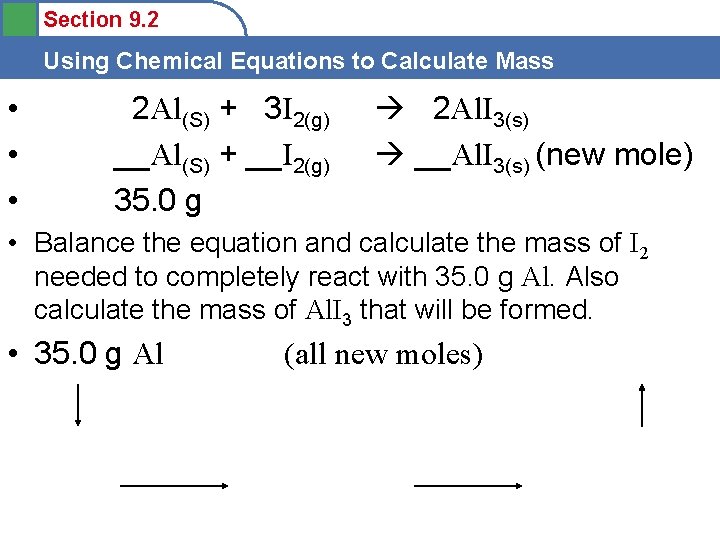
Section 9. 2 Using Chemical Equations to Calculate Mass 1. Mass Calculations • __Al(S) + __I 2(g) __Al. I 3(s) • Balance the equation and calculate the mass of I 2 needed to completely react with 35. 0 g Al. Also calculate the mass of Al. I 3 that will be formed. • grams • mole ratio mole (next)

Section 9. 2 Using Chemical Equations to Calculate Mass • • • 2 Al(S) + 3 I 2(g) __Al(S) + __I 2(g) 35. 0 g 2 Al. I 3(s) __Al. I 3(s) (new mole) • Balance the equation and calculate the mass of I 2 needed to completely react with 35. 0 g Al. Also calculate the mass of Al. I 3 that will be formed. • 35. 0 g Al (all new moles)

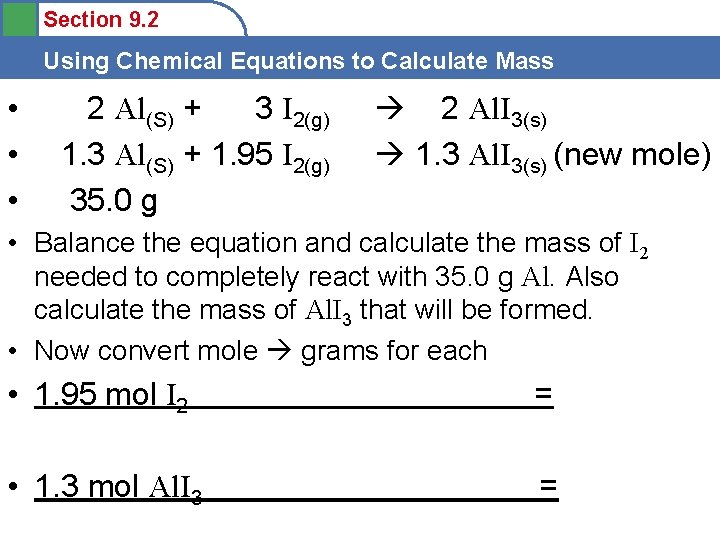
Section 9. 2 Using Chemical Equations to Calculate Mass • • • 2 Al(S) + 3 I 2(g) 1. 3 Al(S) + 1. 95 I 2(g) 35. 0 g 2 Al. I 3(s) 1. 3 Al. I 3(s) (new mole) • Balance the equation and calculate the mass of I 2 needed to completely react with 35. 0 g Al. Also calculate the mass of Al. I 3 that will be formed. • Now convert mole grams for each • 1. 95 mol I 2 = • 1. 3 mol Al. I 3 =

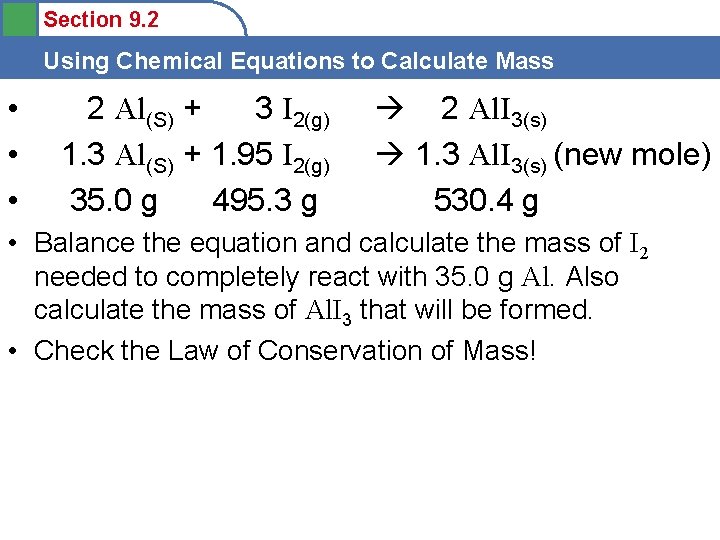
Section 9. 2 Using Chemical Equations to Calculate Mass • • • 2 Al(S) + 3 I 2(g) 1. 3 Al(S) + 1. 95 I 2(g) 35. 0 g 495. 3 g 2 Al. I 3(s) 1. 3 Al. I 3(s) (new mole) 530. 4 g • Balance the equation and calculate the mass of I 2 needed to completely react with 35. 0 g Al. Also calculate the mass of Al. I 3 that will be formed. • Check the Law of Conservation of Mass!

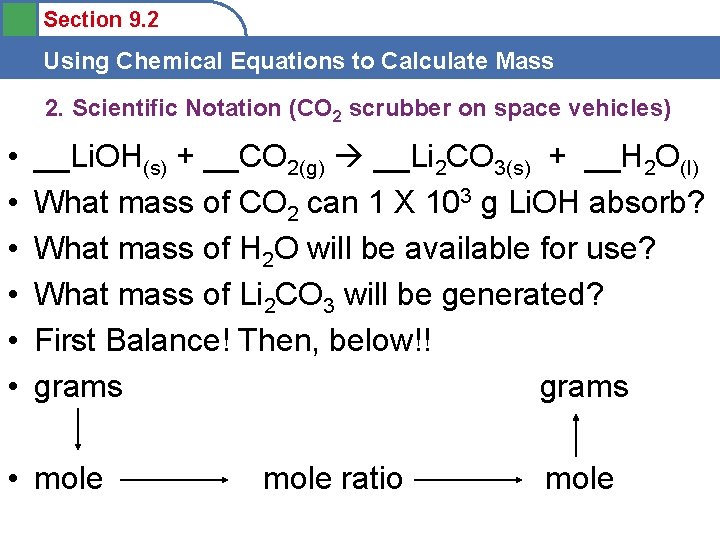
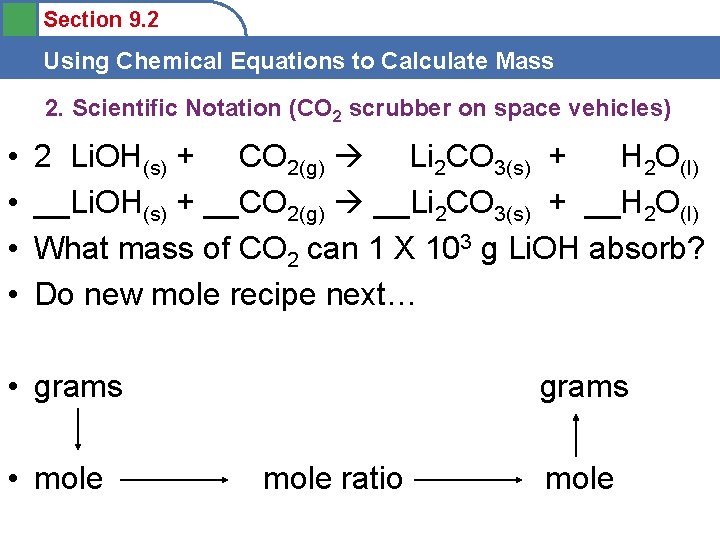
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Scientific Notation (CO 2 scrubber on space vehicles) • • • __Li. OH(s) + __CO 2(g) __Li 2 CO 3(s) + __H 2 O(l) What mass of CO 2 can 1 X 103 g Li. OH absorb? What mass of H 2 O will be available for use? What mass of Li 2 CO 3 will be generated? First Balance! Then, below!! grams • mole ratio mole

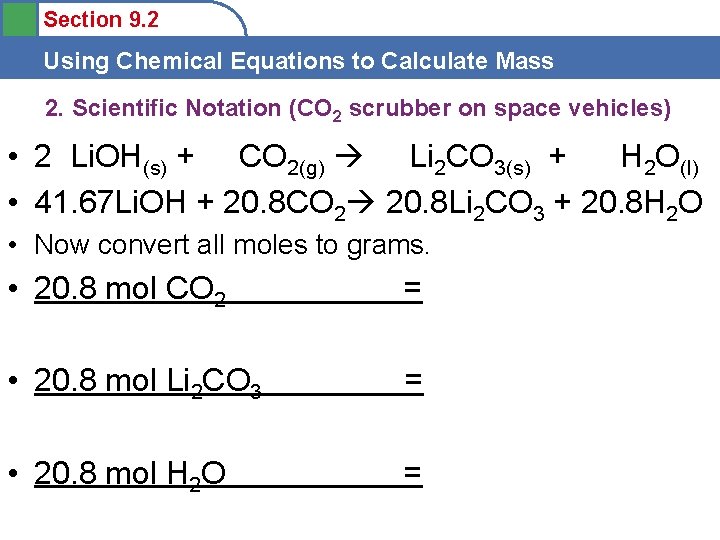
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Scientific Notation (CO 2 scrubber on space vehicles) • • 2 Li. OH(s) + CO 2(g) Li 2 CO 3(s) + H 2 O(l) __Li. OH(s) + __CO 2(g) __Li 2 CO 3(s) + __H 2 O(l) What mass of CO 2 can 1 X 103 g Li. OH absorb? Do new mole recipe next… • grams • mole grams mole ratio mole

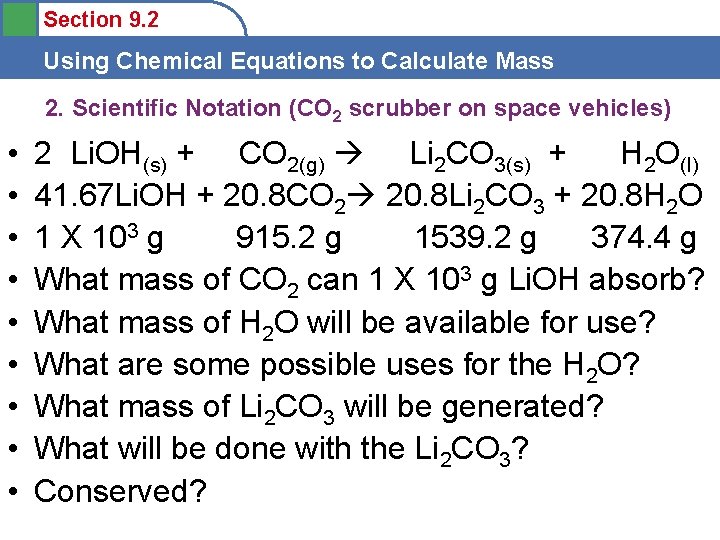
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Scientific Notation (CO 2 scrubber on space vehicles) • 2 Li. OH(s) + CO 2(g) Li 2 CO 3(s) + H 2 O(l) • 41. 67 Li. OH + 20. 8 CO 2 20. 8 Li 2 CO 3 + 20. 8 H 2 O • Now convert all moles to grams. • 20. 8 mol CO 2 = • 20. 8 mol Li 2 CO 3 = • 20. 8 mol H 2 O =

Section 9. 2 Using Chemical Equations to Calculate Mass 2. Scientific Notation (CO 2 scrubber on space vehicles) • • • 2 Li. OH(s) + CO 2(g) Li 2 CO 3(s) + H 2 O(l) 41. 67 Li. OH + 20. 8 CO 2 20. 8 Li 2 CO 3 + 20. 8 H 2 O 1 X 103 g 915. 2 g 1539. 2 g 374. 4 g What mass of CO 2 can 1 X 103 g Li. OH absorb? What mass of H 2 O will be available for use? What are some possible uses for the H 2 O? What mass of Li 2 CO 3 will be generated? What will be done with the Li 2 CO 3? Conserved?

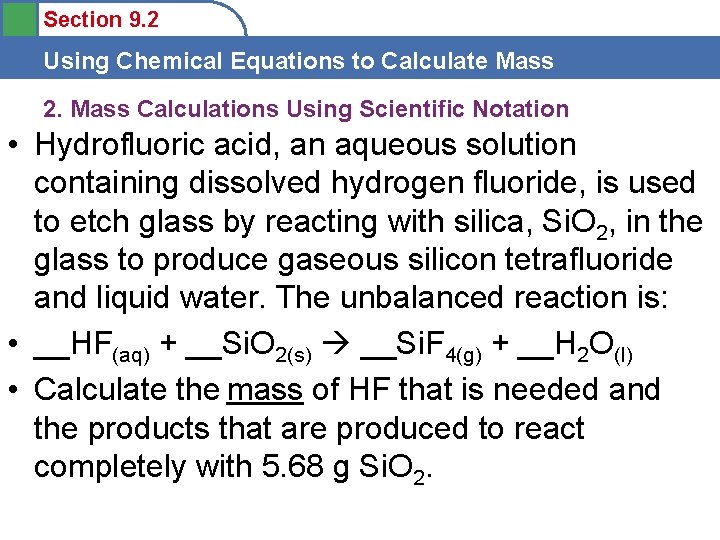
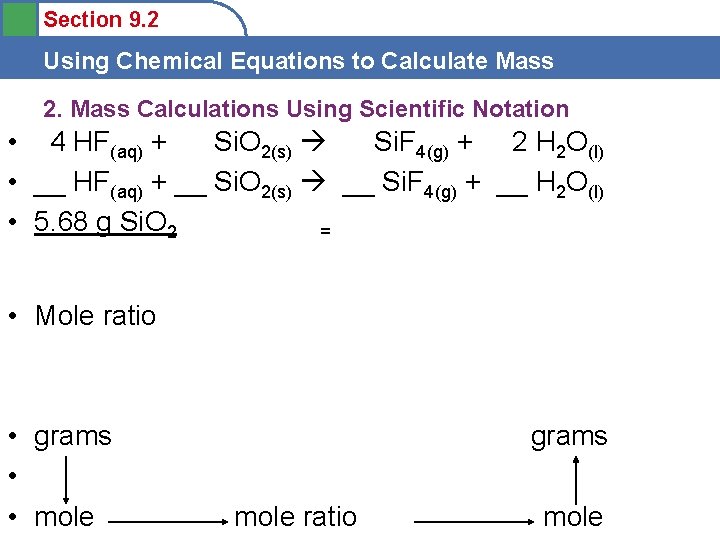
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations Using Scientific Notation • Hydrofluoric acid, an aqueous solution containing dissolved hydrogen fluoride, is used to etch glass by reacting with silica, Si. O 2, in the glass to produce gaseous silicon tetrafluoride and liquid water. The unbalanced reaction is: • __HF(aq) + __Si. O 2(s) __Si. F 4(g) + __H 2 O(l) • Calculate the mass of HF that is needed and the products that are produced to react completely with 5. 68 g Si. O 2.

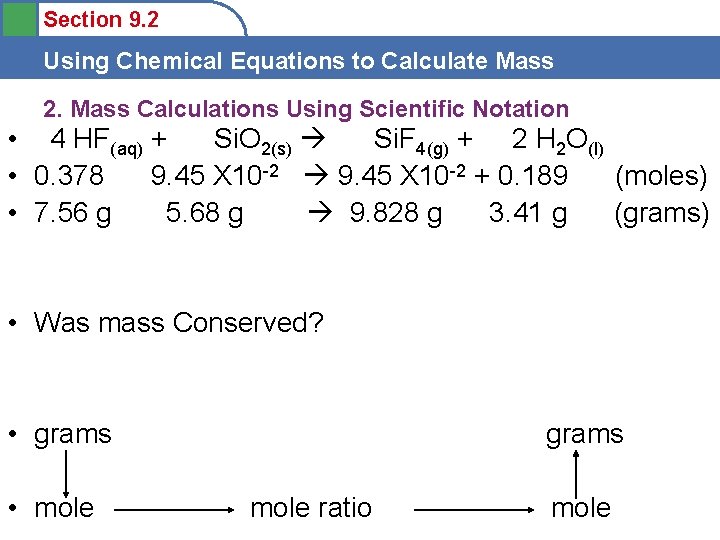
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations Using Scientific Notation • 4 HF(aq) + Si. O 2(s) Si. F 4(g) + 2 H 2 O(l) • __ HF(aq) + __ Si. O 2(s) __ Si. F 4(g) + __ H 2 O(l) • 5. 68 g Si. O 2 = • Mole ratio • grams • • mole grams mole ratio mole

Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations Using Scientific Notation • 4 HF(aq) + Si. O 2(s) Si. F 4(g) + 2 H 2 O(l) • 0. 378 9. 45 X 10 -2 + 0. 189 (moles) • 7. 56 g 5. 68 g 9. 828 g 3. 41 g (grams) • Was mass Conserved? • grams • mole grams mole ratio mole

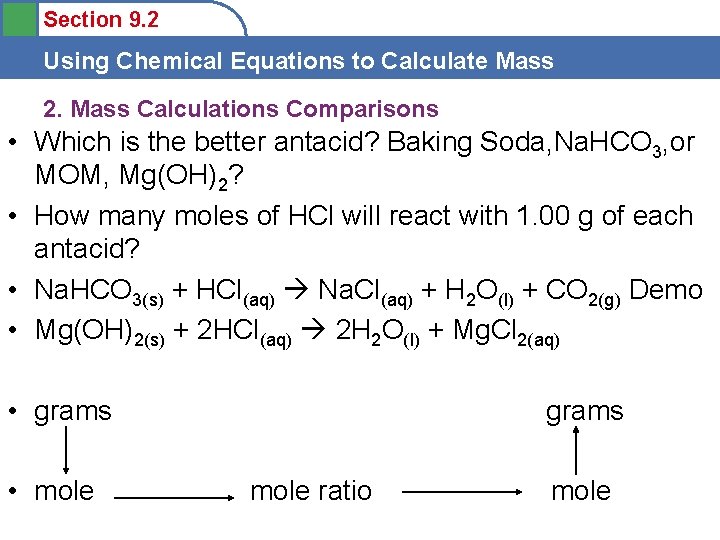
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations Comparisons • Which is the better antacid? Baking Soda, Na. HCO 3, or MOM, Mg(OH)2? • How many moles of HCl will react with 1. 00 g of each antacid? • Na. HCO 3(s) + HCl(aq) Na. Cl(aq) + H 2 O(l) + CO 2(g) Demo • Mg(OH)2(s) + 2 HCl(aq) 2 H 2 O(l) + Mg. Cl 2(aq) • grams • mole grams mole ratio mole

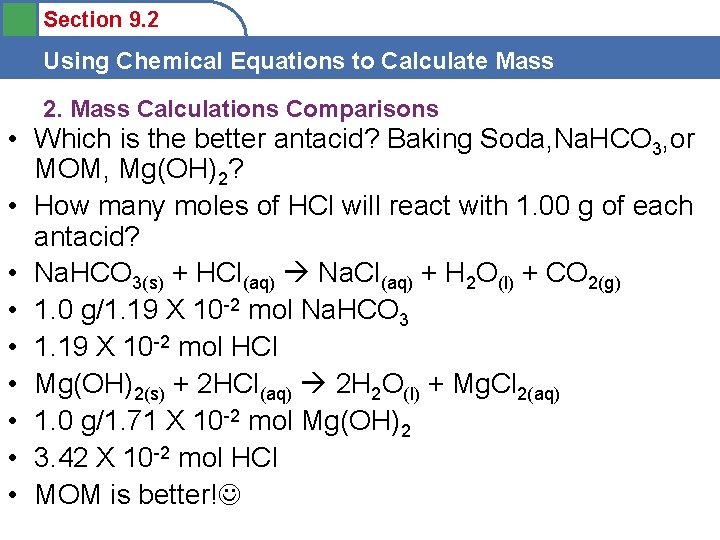
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations Comparisons • Which is the better antacid? Baking Soda, Na. HCO 3, or MOM, Mg(OH)2? • How many moles of HCl will react with 1. 00 g of each antacid? • Na. HCO 3(s) + HCl(aq) Na. Cl(aq) + H 2 O(l) + CO 2(g) • 1. 0 g/1. 19 X 10 -2 mol Na. HCO 3 • 1. 19 X 10 -2 mol HCl • Mg(OH)2(s) + 2 HCl(aq) 2 H 2 O(l) + Mg. Cl 2(aq) • 1. 0 g/1. 71 X 10 -2 mol Mg(OH)2 • 3. 42 X 10 -2 mol HCl • MOM is better!

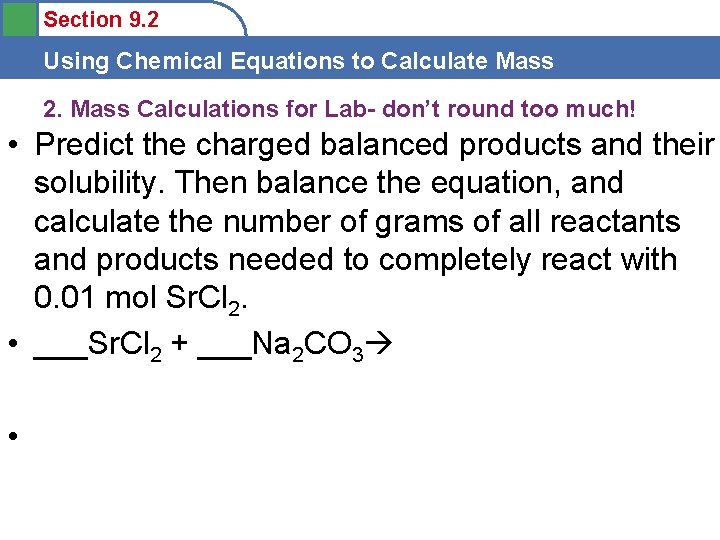
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations for Lab- don’t round too much! • Predict the charged balanced products and their solubility. Then balance the equation, and calculate the number of grams of all reactants and products needed to completely react with 0. 01 mol Sr. Cl 2. • ___Sr. Cl 2 + ___Na 2 CO 3 •

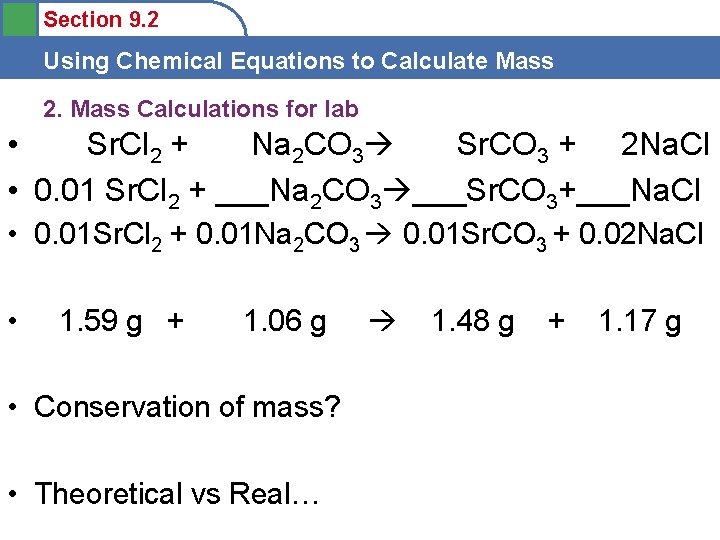
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations for lab • Sr. Cl 2 + Na 2 CO 3 Sr. CO 3 + 2 Na. Cl • 0. 01 Sr. Cl 2 + ___Na 2 CO 3 ___Sr. CO 3+___Na. Cl • 0. 01 Sr. Cl 2 + 0. 01 Na 2 CO 3 0. 01 Sr. CO 3 + 0. 02 Na. Cl • 1. 59 g + 1. 06 g • Conservation of mass? • Theoretical vs Real… 1. 48 g + 1. 17 g

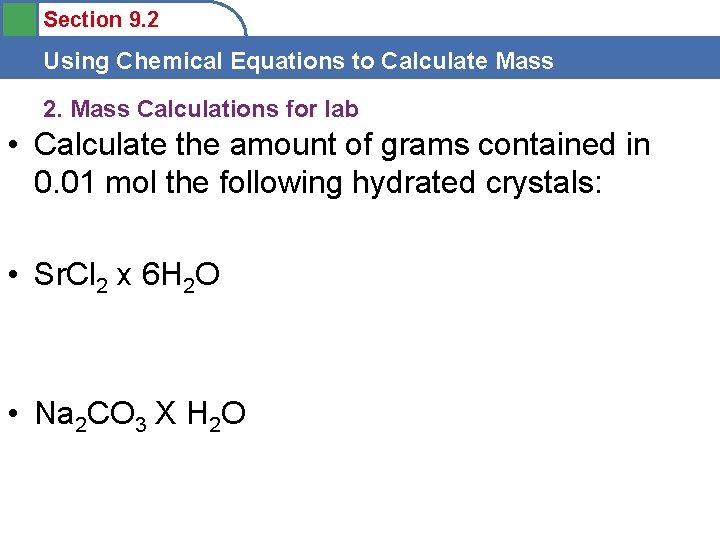
Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations for lab • Calculate the amount of grams contained in 0. 01 mol the following hydrated crystals: • Sr. Cl 2 x 6 H 2 O • Na 2 CO 3 X H 2 O

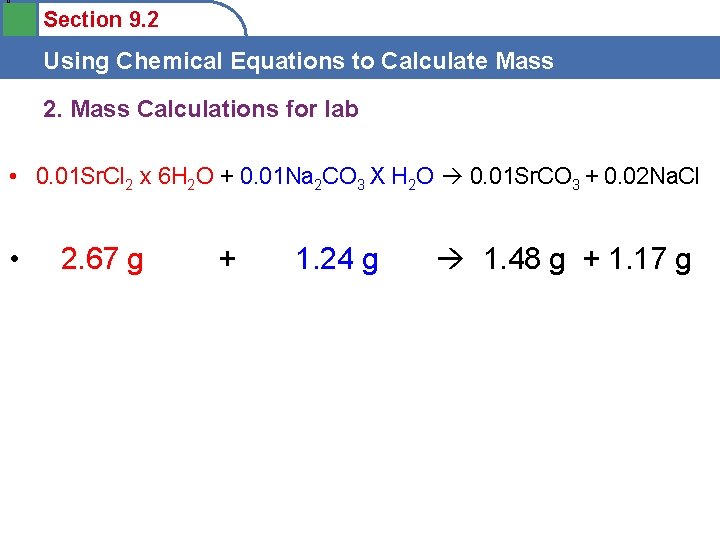
0 Section 9. 2 Using Chemical Equations to Calculate Mass 2. Mass Calculations for lab • 0. 01 Sr. Cl 2 x 6 H 2 O + 0. 01 Na 2 CO 3 X H 2 O 0. 01 Sr. CO 3 + 0. 02 Na. Cl • 2. 67 g + 1. 24 g 1. 48 g + 1. 17 g

Section 9. 2 Using Chemical Equations to Calculate Mass Objectives Review 1. To understand the information given in a balanced equation 2. To use a balanced equation to determine relationships between moles of reactant and products (mole ratio) (Stoichiometry) 3. Calculate theoretical predications for a reaction that will be performed in the lab 4. Work Session: Page 295 1, 2, 4, 5, 6

Section 9. 3 Limiting Reactants and Percent Yield Objectives 1. To understand the concept of limiting reactants 2. To learn to recognize the limiting reactant in a reaction 3. To learn to use the limiting reactant to do stoichiometric calculations 4. To learn to calculate percent yield

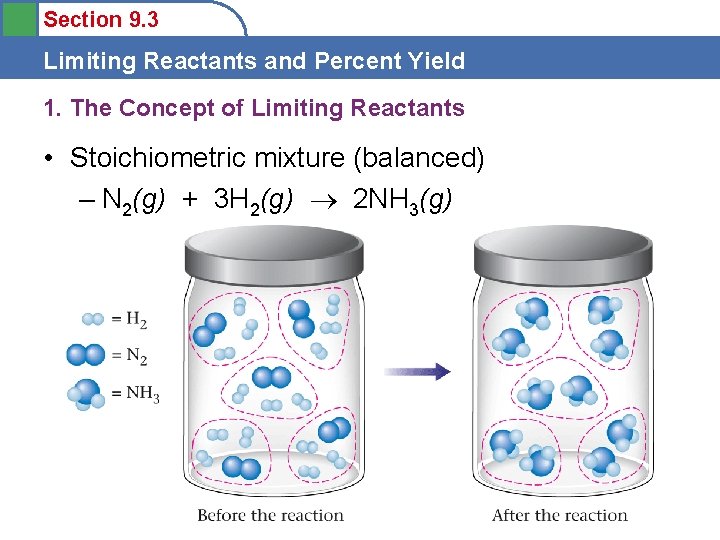
Section 9. 3 Limiting Reactants and Percent Yield 1. The Concept of Limiting Reactants • Stoichiometric mixture (balanced) – N 2(g) + 3 H 2(g) 2 NH 3(g)

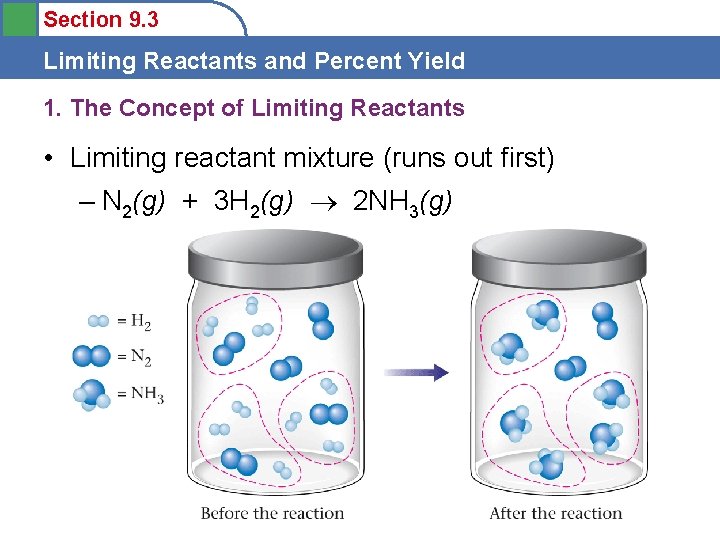
Section 9. 3 Limiting Reactants and Percent Yield 1. The Concept of Limiting Reactants • Limiting reactant mixture (runs out first) – N 2(g) + 3 H 2(g) 2 NH 3(g)

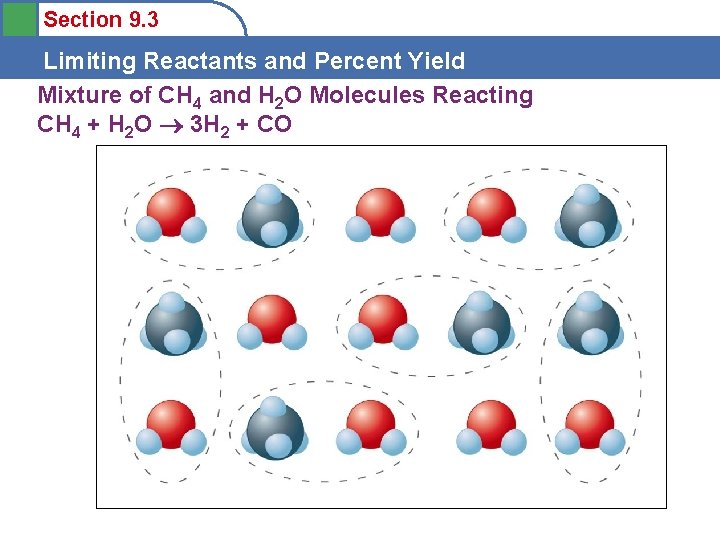
Section 9. 3 Limiting Reactants and Percent Yield Mixture of CH 4 and H 2 O Molecules Reacting CH 4 + H 2 O 3 H 2 + CO

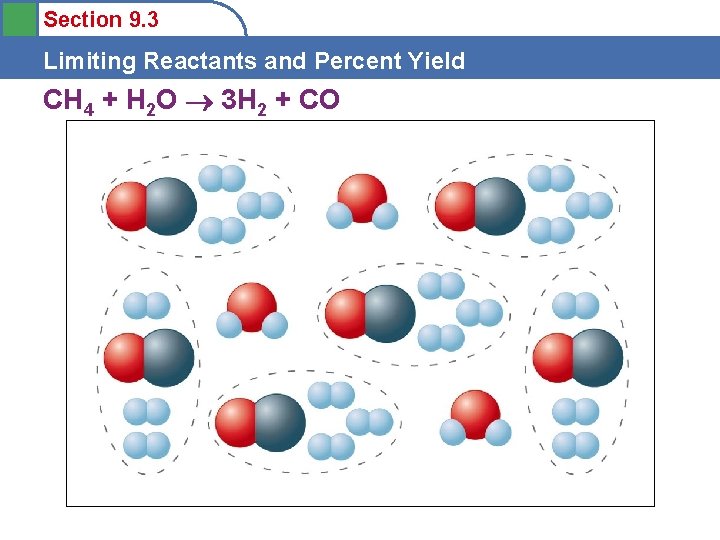
Section 9. 3 Limiting Reactants and Percent Yield CH 4 + H 2 O 3 H 2 + CO

Section 9. 3 Limiting Reactants and Percent Yield Limiting Reactants • • The amount of products that can form is limited by the methane. Methane is the limiting reactant. Water is in excess. Limiting and Excess Reactants depend on the actual amounts of reactants present and must be calculated for each different reaction. (Water won’t always be in excess!)

Section 9. 3 Limiting Reactants and Percent Yield Limiting Reactants

Section 9. 3 Limiting Reactants and Percent Yield 2. Calculations Involving a Limiting Reactant • What mass of water is needed to react with 249 g methane (CH 4)? • CH 4 + H 2 O 3 H 2 + CO • 249 g CH 4 • How many g of H 2 and CO are produced? • 279 g H 2 O, 93 g H 2, 434 g CO

Section 9. 3 Limiting Reactants and Percent Yield 2. Calculations Involving a Limiting Reactant • How many g of H 2 and CO are produced when 249 g methane (CH 4) reacts with 300 g H 2 O? • CH 4 + H 2 O 3 H 2 + CO • 249 g + 279 g 93 g + 434 g (last slide) • 249 g + 300 g 93 g + 434 g • The 249 g CH 4 will react with only 279 g H 2 O, thereby leaving 21 g H 2 O in excess. CH 4 is the reactant that will run out first and is the LIMITING REACTANT.

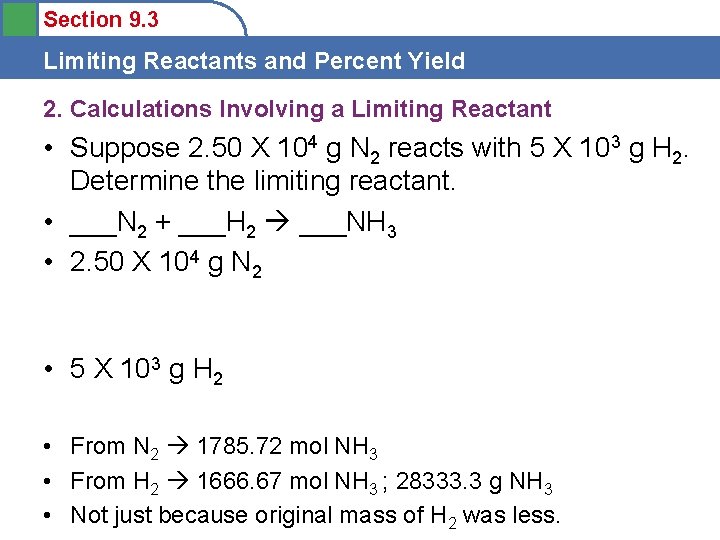
Section 9. 3 Limiting Reactants and Percent Yield 2. Calculations Involving a Limiting Reactant • So, how do we figure this out using Stoich? • 1) Balance Reaction • 2) Do Stoich to relate EACH reactant to one product • 3) Whichever reactant produces the LEAST amount of product is the LIMITING REACTANT

Section 9. 3 Limiting Reactants and Percent Yield 2. Calculations Involving a Limiting Reactant

Section 9. 3 Limiting Reactants and Percent Yield 2. Calculations Involving a Limiting Reactant • Suppose 2. 50 X 104 g N 2 reacts with 5 X 103 g H 2. Determine the limiting reactant. • ___N 2 + ___H 2 ___NH 3 • 2. 50 X 104 g N 2 • 5 X 103 g H 2 • From N 2 1785. 72 mol NH 3 • From H 2 1666. 67 mol NH 3 ; 28333. 3 g NH 3 • Not just because original mass of H 2 was less.

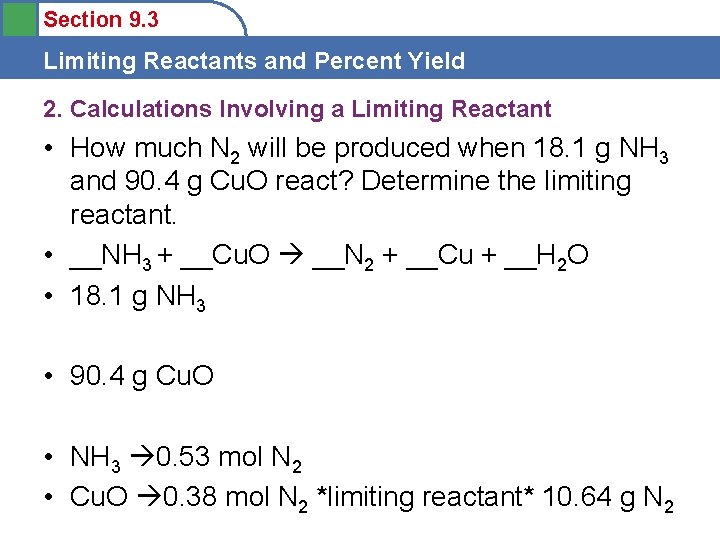
Section 9. 3 Limiting Reactants and Percent Yield 2. Calculations Involving a Limiting Reactant • How much N 2 will be produced when 18. 1 g NH 3 and 90. 4 g Cu. O react? Determine the limiting reactant. • __NH 3 + __Cu. O __N 2 + __Cu + __H 2 O • 18. 1 g NH 3 • 90. 4 g Cu. O • NH 3 0. 53 mol N 2 • Cu. O 0. 38 mol N 2 *limiting reactant* 10. 64 g N 2

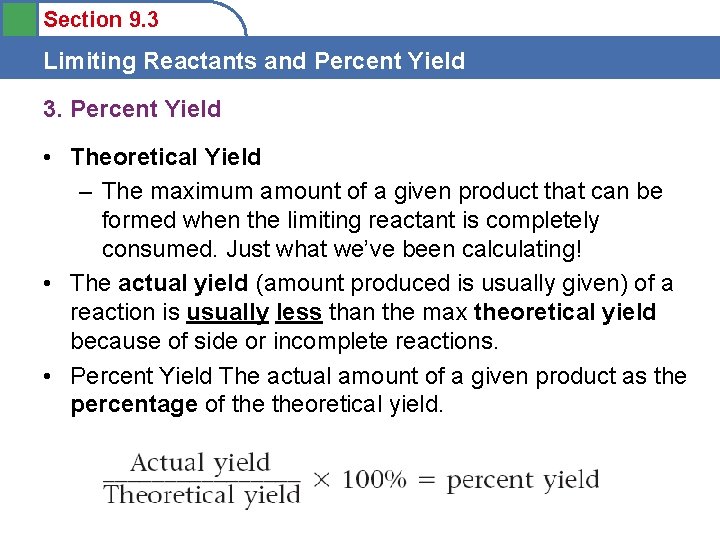
Section 9. 3 Limiting Reactants and Percent Yield 3. Percent Yield • Theoretical Yield – The maximum amount of a given product that can be formed when the limiting reactant is completely consumed. Just what we’ve been calculating! • The actual yield (amount produced is usually given) of a reaction is usually less than the max theoretical yield because of side or incomplete reactions. • Percent Yield The actual amount of a given product as the percentage of theoretical yield.

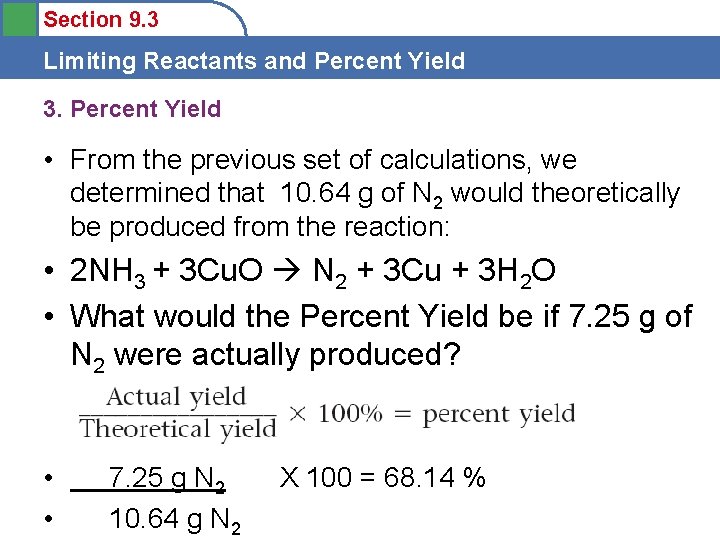
Section 9. 3 Limiting Reactants and Percent Yield 3. Percent Yield • From the previous set of calculations, we determined that 10. 64 g of N 2 would theoretically be produced from the reaction: • 2 NH 3 + 3 Cu. O N 2 + 3 Cu + 3 H 2 O • What would the Percent Yield be if 7. 25 g of N 2 were actually produced? • • 7. 25 g N 2 10. 64 g N 2 X 100 = 68. 14 %

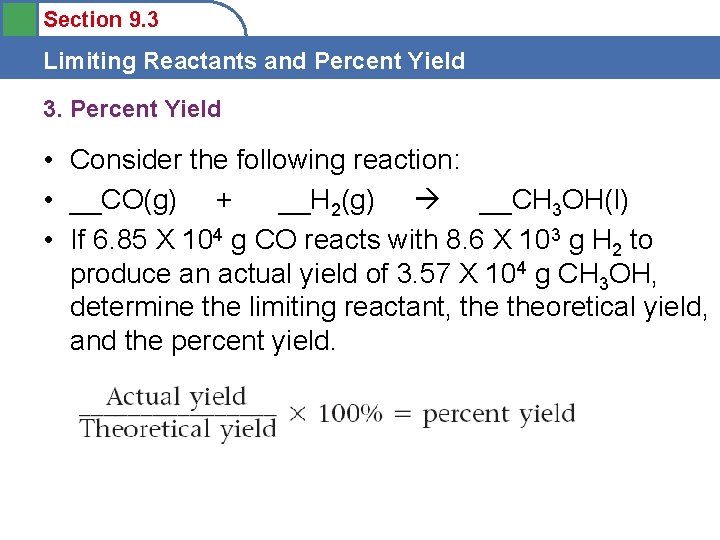
Section 9. 3 Limiting Reactants and Percent Yield 3. Percent Yield • Consider the following reaction: • __CO(g) + __H 2(g) __CH 3 OH(l) • If 6. 85 X 104 g CO reacts with 8. 6 X 103 g H 2 to produce an actual yield of 3. 57 X 104 g CH 3 OH, determine the limiting reactant, theoretical yield, and the percent yield.

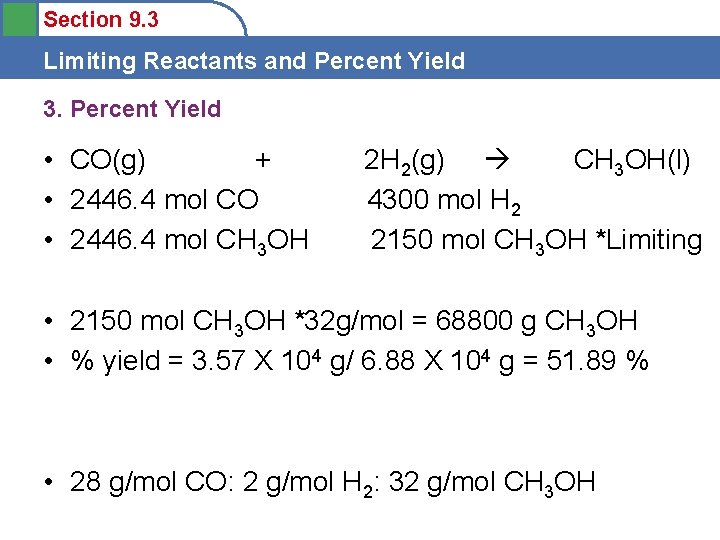
Section 9. 3 Limiting Reactants and Percent Yield 3. Percent Yield • CO(g) + • 2446. 4 mol CO • 2446. 4 mol CH 3 OH 2 H 2(g) CH 3 OH(l) 4300 mol H 2 2150 mol CH 3 OH *Limiting • 2150 mol CH 3 OH *32 g/mol = 68800 g CH 3 OH • % yield = 3. 57 X 104 g/ 6. 88 X 104 g = 51. 89 % • 28 g/mol CO: 2 g/mol H 2: 32 g/mol CH 3 OH

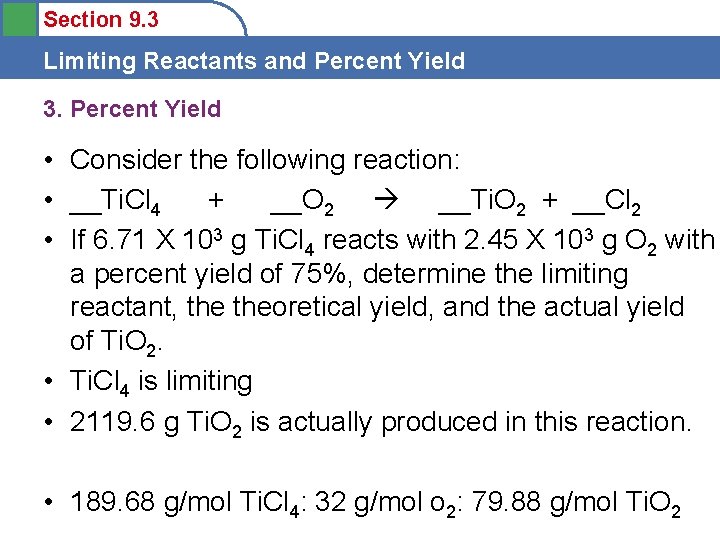
Section 9. 3 Limiting Reactants and Percent Yield 3. Percent Yield • Consider the following reaction: • __Ti. Cl 4 + __O 2 __Ti. O 2 + __Cl 2 • If 6. 71 X 103 g Ti. Cl 4 reacts with 2. 45 X 103 g O 2 with a percent yield of 75%, determine the limiting reactant, theoretical yield, and the actual yield of Ti. O 2. • Ti. Cl 4 is limiting • 2119. 6 g Ti. O 2 is actually produced in this reaction. • 189. 68 g/mol Ti. Cl 4: 32 g/mol o 2: 79. 88 g/mol Ti. O 2

Section 9. 3 Limiting Reactants and Percent Yield Objectives Review 1. To understand the concept of limiting reactants 2. To learn to recognize the limiting reactant in a reaction 3. To learn to use the limiting reactant to do stoichiometric calculations 4. To learn to calculate percent yield 5. Work Session: Page 308 2, 3, 4, 5