Stoichiometry Chemical Analyses and Formulas Stoichiometry Chemical analyses












- Slides: 24

Stoichiometry Chemical Analyses and Formulas

Stoichiometry • Chemical analyses of oxygen bearing minerals are given as weight percents of oxides. • We need to be able to recalculate oxide analyses to cations per given number of oxygens to derive a chemical formula.

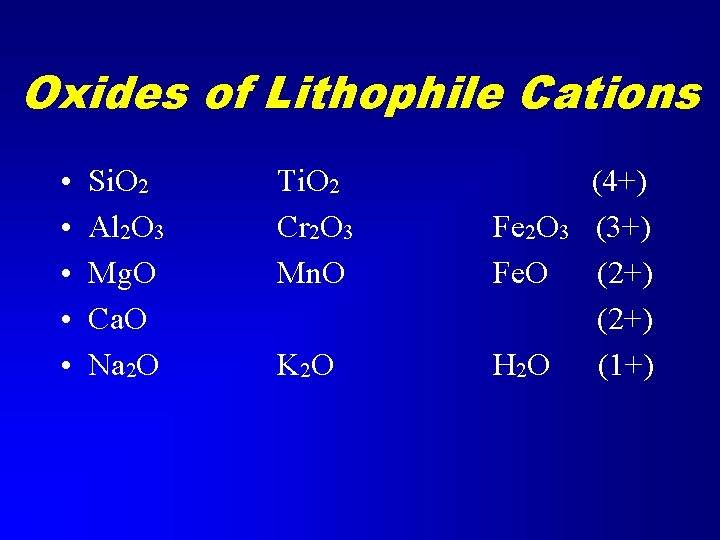
Oxides of Lithophile Cations • • • Si. O 2 Al 2 O 3 Mg. O Ca. O Na 2 O Ti. O 2 Cr 2 O 3 Mn. O Fe 2 O 3 Fe. O K 2 O H 2 O (4+) (3+) (2+) (1+)


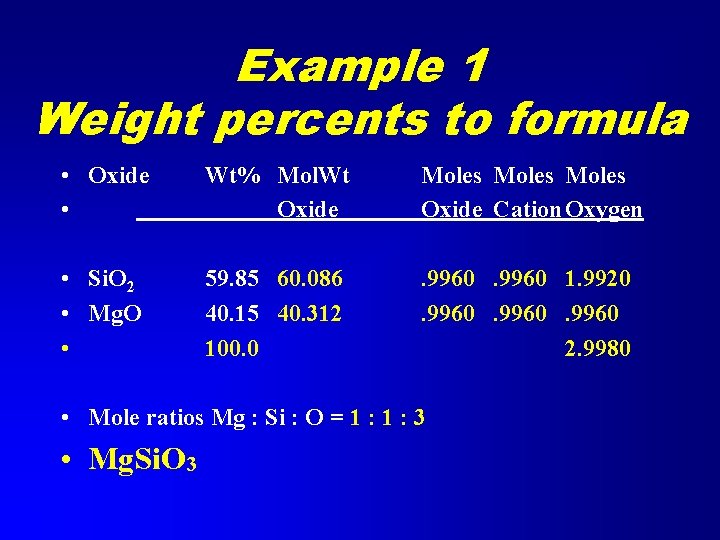
Example 1 Weight percents to formula • Oxide • Wt% Mol. Wt Oxide Moles Oxide Cation Oxygen • Si. O 2 • Mg. O • 59. 85 60. 086 40. 15 40. 312 100. 0 . 9960 1. 9920. 9960 2. 9980 • Mole ratios Mg : Si : O = 1 : 3 • Mg. Si. O 3

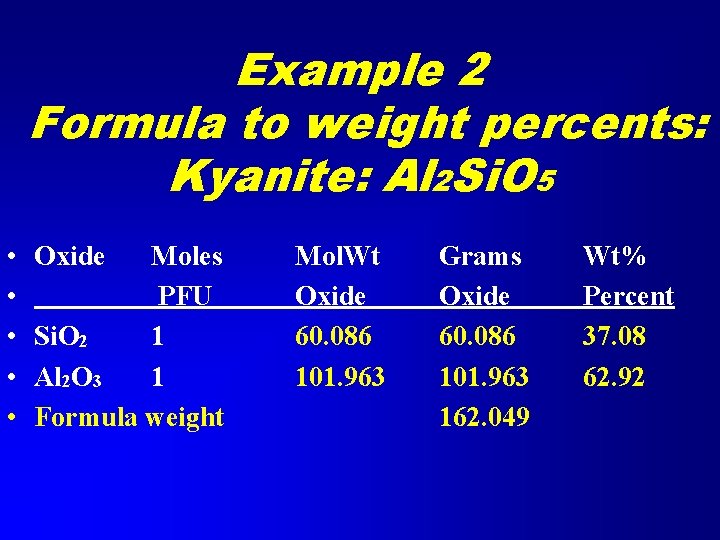
Example 2 Formula to weight percents • • Kyanite is Al 2 Si. O 5 Calculate the weight percents of the oxides Si. O 2 Al 2 O 3

Example 2 Formula to weight percents: Kyanite: Al 2 Si. O 5 • • • Oxide Moles PFU Si. O 2 1 Al 2 O 3 1 Formula weight Mol. Wt Oxide 60. 086 101. 963 Grams Oxide 60. 086 101. 963 162. 049 Wt% Percent 37. 08 62. 92

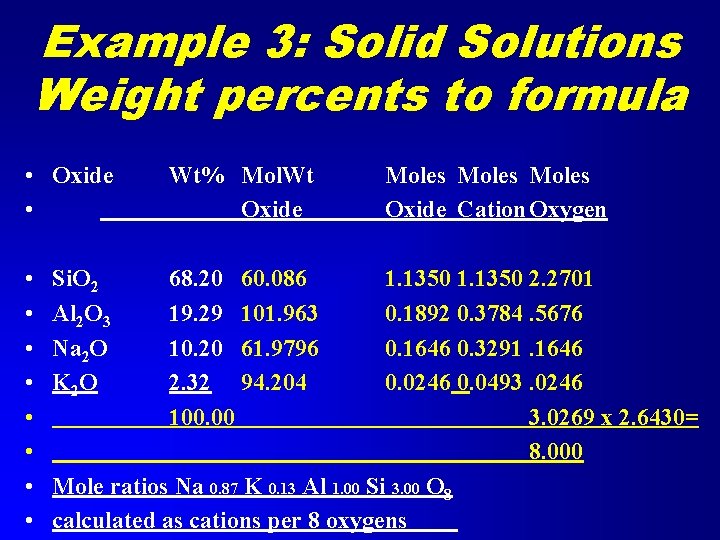
Example 3: Solid Solutions Weight percents to formula • Alkali Feldspars may exist with any composition between Na. Al. Si 3 O 8 and KAl. Si 3 O 8. • Formula has 8 oxygens: (Na, K)Al. Si 3 O 8 • The alkalis may substitute in any ratio, but total alkalis to Al is 1 to 1.

Example 3: Solid Solutions Weight percents to formula • Oxide • Wt% Mol. Wt Oxide • • 68. 20 60. 086 19. 29 101. 963 10. 20 61. 9796 2. 32 94. 204 100. 00 Si. O 2 Al 2 O 3 Na 2 O K 2 O Moles Oxide Cation Oxygen 1. 1350 2. 2701 0. 1892 0. 3784. 5676 0. 1646 0. 3291. 1646 0. 0246 0. 0493. 0246 3. 0269 x 2. 6430= 8. 000 Mole ratios Na 0. 87 K 0. 13 Al 1. 00 Si 3. 00 O 8 calculated as cations per 8 oxygens

Simple Solid Solutions • Na. Al. Si 3 O 8 - KAl. Si 3 O 8 Alkali Feldspars • • Mg. Si. O 3 - Fe. Si. O 3 Enstatite-Ferrosilite (pyroxene) Mg. Ca. Si 2 O 6 -Fe. Ca. Si 2 O 6 Diopside-Hedenbergite Mg 2 Si. O 4 - Fe 2 Si. O 4 Forsterite-Fayalite Mg 3 Al 2 Si 3 O 12 - Fe 3 Al 2 Si 3 O 12 Pyrope - Almandine

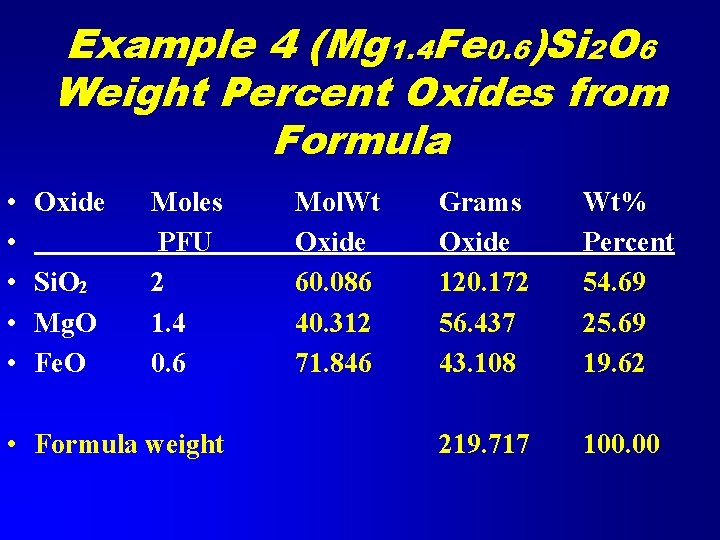
Example 4 Weight Percent Oxides from Formula • Given the formula En 70 Fs 30 for an orthopyroxene, calculate the weight percent oxides. • En = enstatite = Mg 2 Si 2 O 6 • Fs = ferrosilite = Fe 2 Si 2 O 6 • Formula is (Mg 0. 7 Fe 0. 3)2 Si 2 O 6 = (Mg 1. 4 Fe 0. 6)Si 2 O 6

Example 4 (Mg 1. 4 Fe 0. 6)Si 2 O 6 Weight Percent Oxides from Formula • • • Oxide Si. O 2 Mg. O Fe. O Moles PFU 2 1. 4 0. 6 • Formula weight Mol. Wt Oxide 60. 086 40. 312 71. 846 Grams Oxide 120. 172 56. 437 43. 108 Wt% Percent 54. 69 25. 69 19. 62 219. 717 100. 00

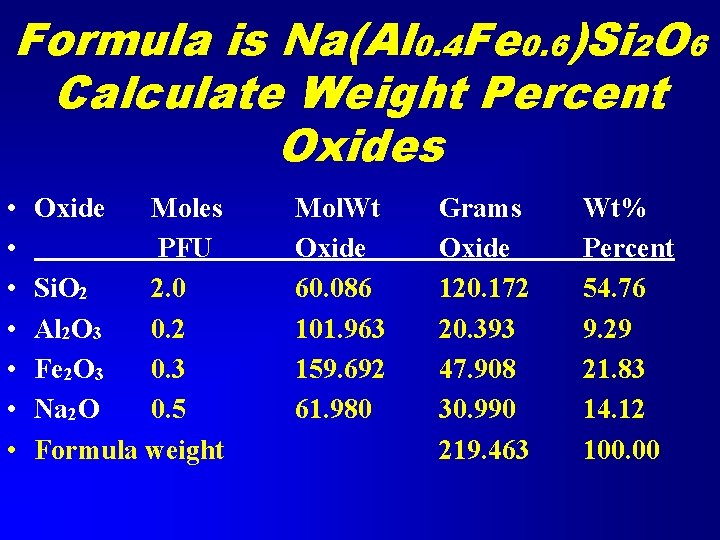
Example 5 Weight Percent Oxides from Formula • A pyroxene is a solid solution of 40% jadeite (Na. Al. Si 2 O 6) and 60% acmite (Na. Fe. Si 2 O 6). • Calculate the weight percent oxides • Formula is Na(Al 0. 4 Fe 0. 6)Si 2 O 6

Formula is Na(Al 0. 4 Fe 0. 6)Si 2 O 6 Calculate Weight Percent Oxides • • Oxide Moles PFU Si. O 2 2. 0 Al 2 O 3 0. 2 Fe 2 O 3 0. 3 Na 2 O 0. 5 Formula weight Mol. Wt Oxide 60. 086 101. 963 159. 692 61. 980 Grams Oxide 120. 172 20. 393 47. 908 30. 990 219. 463 Wt% Percent 54. 76 9. 29 21. 83 14. 12 100. 00

Coupled Substitutions • Plagioclase feldspar Na. Al. Si 3 O 8 - Ca. Al 2 Si 2 O 8 • Jadeite-diopside Na. Al. Si 2 O 6 - Ca. Mg. Si 2 O 6

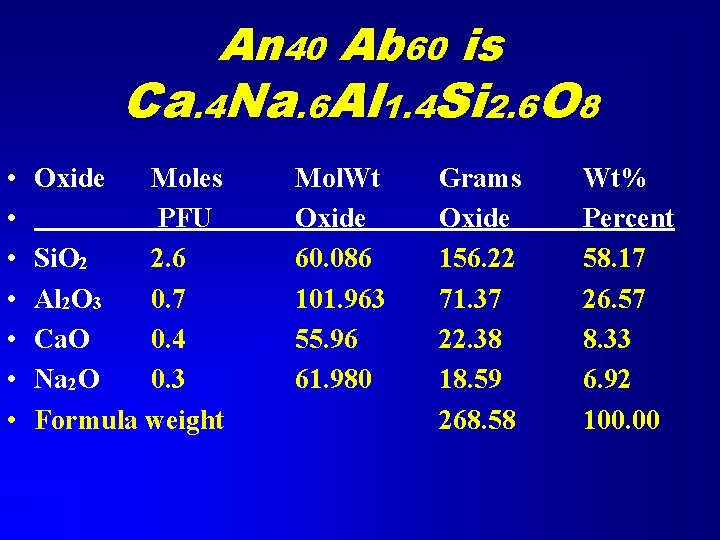
Coupled Substitution 40% Anorthite; 60% Albite Calculate Weight percent Oxides • First write the formula • Anorthite is Ca. Al 2 Si 2 O 8 • Albite is Na. Al. Si 3 O 8 • An 40 Ab 60 is Ca. 4 Na. 6 Al 1. 4 Si 2. 6 O 8

An 40 Ab 60 is Ca. 4 Na. 6 Al 1. 4 Si 2. 6 O 8 • • Oxide Moles PFU Si. O 2 2. 6 Al 2 O 3 0. 7 Ca. O 0. 4 Na 2 O 0. 3 Formula weight Mol. Wt Oxide 60. 086 101. 963 55. 96 61. 980 Grams Oxide 156. 22 71. 37 22. 38 18. 59 268. 58 Wt% Percent 58. 17 26. 57 8. 33 6. 92 100. 00

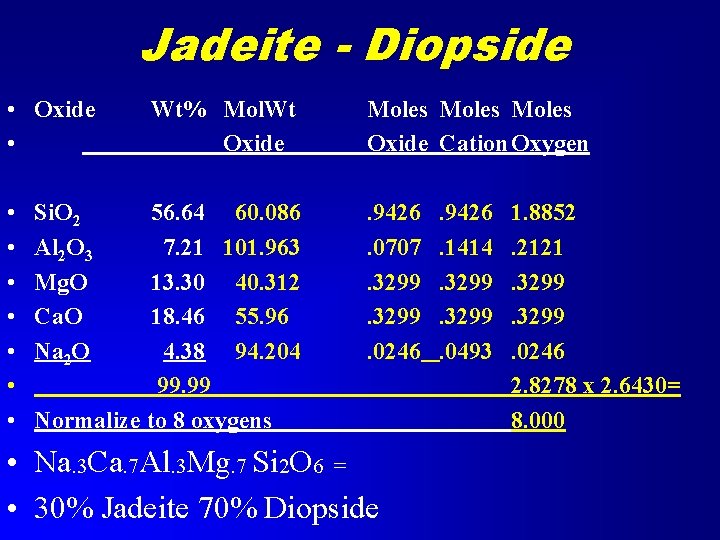
Example Given Analysis Compute Mole percents of Jadeite and Diopside • Jadeite is Na. Al. Si 2 O 6 • Diopside is Ca. Mg. Si 2 O 6

Jadeite - Diopside • Oxide • • Si. O 2 Al 2 O 3 Mg. O Ca. O Na 2 O Wt% Mol. Wt Oxide 56. 64 60. 086 7. 21 101. 963 13. 30 40. 312 18. 46 55. 96 4. 38 94. 204 99. 99 Normalize to 8 oxygens Moles Oxide Cation Oxygen. 9426. 0707. 3299. 0246 • Na. 3 Ca. 7 Al. 3 Mg. 7 Si 2 O 6 = • 30% Jadeite 70% Diopside . 9426. 1414. 3299. 0493 1. 8852. 2121. 3299. 0246 2. 8278 x 2. 6430= 8. 000

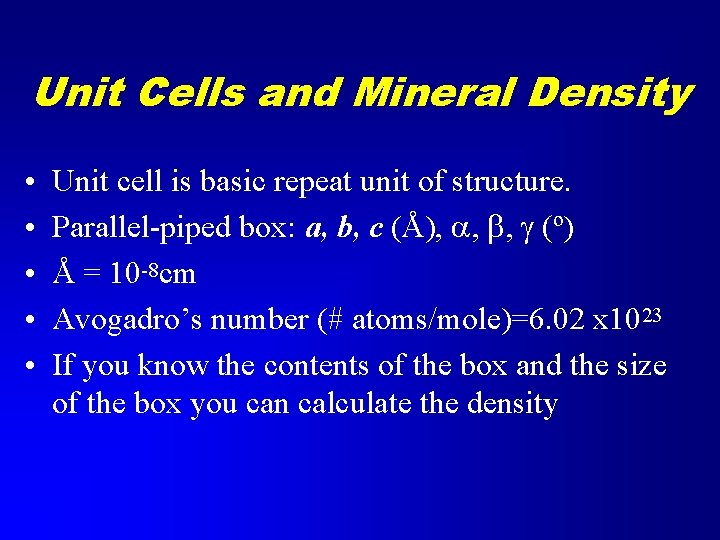
Unit Cells and Mineral Density • • • Unit cell is basic repeat unit of structure. Parallel-piped box: a, b, c (Å), a, b, g (º) Å = 10 -8 cm Avogadro’s number (# atoms/mole)=6. 02 x 1023 If you know the contents of the box and the size of the box you can calculate the density

Cell Volume (1Å3=10 -24 cm 3) • • • Cubic Tetragonal Hex/Trigonal Orthorhombic Monoclinic Triclinic V = a 3 V = a 2 c sin 120º V = abc sin b • V = abc (1 + 2 cos a cos b cos g - cos 2 a - cos 2 b - cos 2 g)1/2

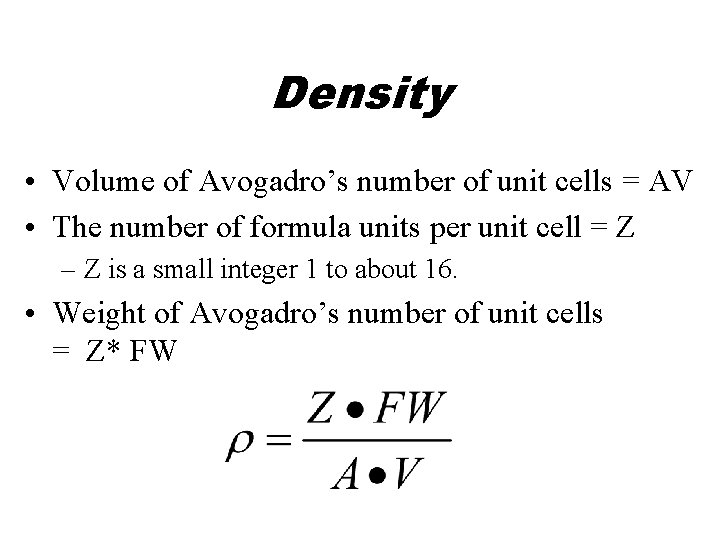
Density • Volume of Avogadro’s number of unit cells = AV • The number of formula units per unit cell = Z – Z is a small integer 1 to about 16. • Weight of Avogadro’s number of unit cells = Z* FW

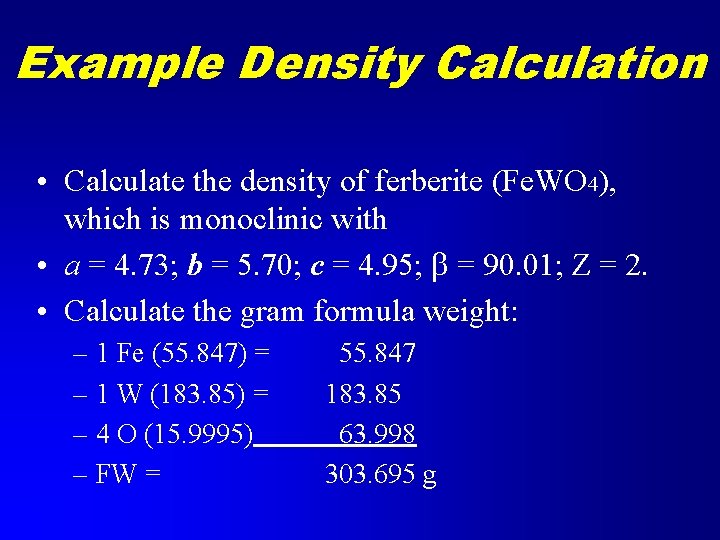
Example Density Calculation • Calculate the density of ferberite (Fe. WO 4), which is monoclinic with • a = 4. 73; b = 5. 70; c = 4. 95; b = 90. 01; Z = 2. • Calculate the gram formula weight: – 1 Fe (55. 847) = – 1 W (183. 85) = – 4 O (15. 9995) – FW = 55. 847 183. 85 63. 998 303. 695 g

Example Density Calculation: Ferberite Fe. WO 4 • • • V = abc sin b = (4. 73)(5. 70)(4. 95)(sin 90. 01º) V = 133. 46 Å3 V = 1. 335 x 10 -22 cm 3 r = ZFw/AV = 2 (303. 70) / 6. 02 1023 *1. 335 10 -22 r = 7. 56 g/cm 3
 Chapter 7 chemical formulas and chemical compounds test
Chapter 7 chemical formulas and chemical compounds test Love formula in chemistry
Love formula in chemistry Critical thinking example in nursing
Critical thinking example in nursing Stoichiometry packet
Stoichiometry packet Stoichiometry equation
Stoichiometry equation How to find mols from grams
How to find mols from grams Stoichiometric factor
Stoichiometric factor Stoichiometry formulas
Stoichiometry formulas Concln
Concln Rhetorical analyses
Rhetorical analyses Courtage analyses services
Courtage analyses services Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Writing and naming chemical formulas
Writing and naming chemical formulas Chapter 9 chemical names and formulas answer key
Chapter 9 chemical names and formulas answer key Chemistry chapter 9 chemical names and formulas
Chemistry chapter 9 chemical names and formulas Dichlorine octoxide formula
Dichlorine octoxide formula Counting atoms worksheet
Counting atoms worksheet Formula moles
Formula moles Covalent bond formula
Covalent bond formula Rules for naming ionic compounds
Rules for naming ionic compounds A chemical formula shows the types and
A chemical formula shows the types and Chemical rxns/balancing equ./stoichiometry
Chemical rxns/balancing equ./stoichiometry In a chemical reaction stoichiometry refers to
In a chemical reaction stoichiometry refers to Chemical accounting stoichiometry
Chemical accounting stoichiometry