The Mole Stoichiometry Formulas Dr Ron Rusay Spring




















- Slides: 35

The Mole, Stoichiometry, & Formulas Dr. Ron Rusay Spring 2008 © Copyright 2008 R. J. Rusay

Chemical Stoichiometry ð Stoichiometry is the study of chemicals and their quantities that are consumed and produced in chemical reactions. ð It quantitatively relates the behavior of atoms and molecules to observable chemical change and measurable mass effects.

QUESTION The fuel in small portable lighters is butane (C 4 H 10). Suppose after using such a lighter for a few minutes (perhaps to encourage your favorite concert performer to play one more encore) you had used 1. 0 gram of fuel. How many moles of butane would this be? 1. 2. 3. 4. 58 moles 0. 077 moles 1. 7 10– 24 moles 0. 017 moles

ANSWER 4) 0. 017 mol is the answer for a proper grams to mole conversion. You need to know the molar mass of butane: 4 12 g/mol = 48 for carbon + 10 1 g/mol = 10. 0 for hydrogen. Total = 48. 0 + 10. 0 = 58. 0 g/mol. Next; 1. 0 gram of butane 1 mol/58. 0 g = 0. 017 mol Section 3. 3: Molar Mass

Mass Calculations: Products Reactants © Copyright 1995 -2002 R. J. Rusay

Mass Calculations: Products Reactants © Copyright 1995 -2008 R. J. Rusay

QUESTION The fuel in small portable lighters is butane (C 4 H 10). Suppose after using such a lighter for a few minutes (perhaps to encourage your favorite concert performer to play one more encore) you had used 1. 0 gram of fuel. . How many moles of butane would this be? 0. 017 moles How many grams of carbon dioxide would this produce? 1. ) 750 mg 2. ) 6. 0 g 3) 1. 5 g 4. ) 3. 0 g

ANSWER Choice 4. ) 3. 0 g is the answer for the correct grams to moles to grams conversion. 1. 0 g C 4 H 10 mol C 4 H 10 1. 0 g 8 mol CO 2 2 mol C 4 H 10 58 g C 4 H 10 ? g CO 2 44 g CO 2 mol CO 2 3. 0 g CO 2 ? g CO 2

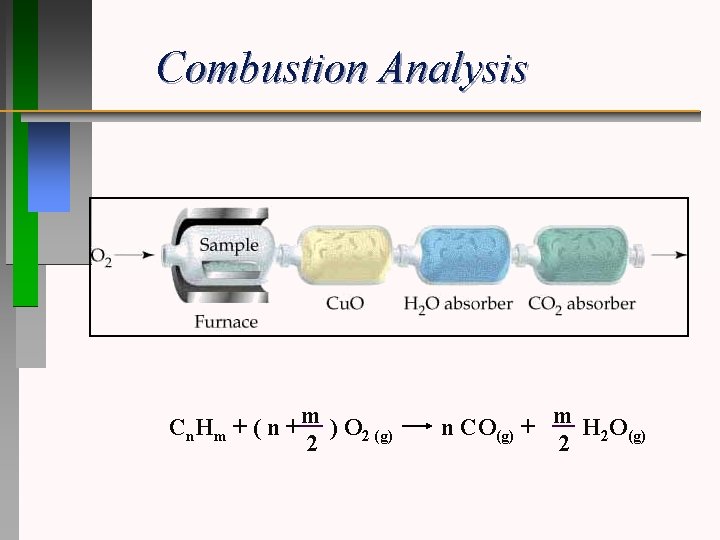
Combustion Analysis Cn. Hm + ( n + m ) O 2 (g) 2 n CO(g) + m H 2 O(g) 2

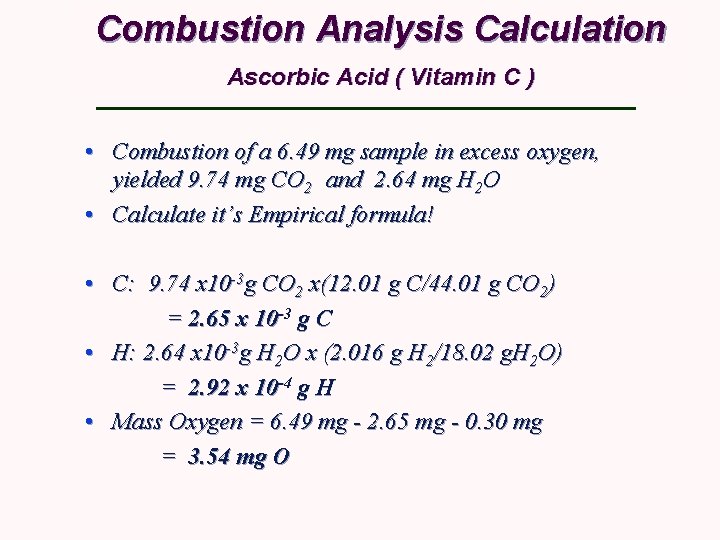
Combustion Analysis Calculation Ascorbic Acid ( Vitamin C ) • Combustion of a 6. 49 mg sample in excess oxygen, yielded 9. 74 mg CO 2 and 2. 64 mg H 2 O • Calculate it’s Empirical formula! • C: 9. 74 x 10 -3 g CO 2 x(12. 01 g C/44. 01 g CO 2) = 2. 65 x 10 -3 g C • H: 2. 64 x 10 -3 g H 2 O x (2. 016 g H 2/18. 02 g. H 2 O) = 2. 92 x 10 -4 g H • Mass Oxygen = 6. 49 mg - 2. 65 mg - 0. 30 mg = 3. 54 mg O

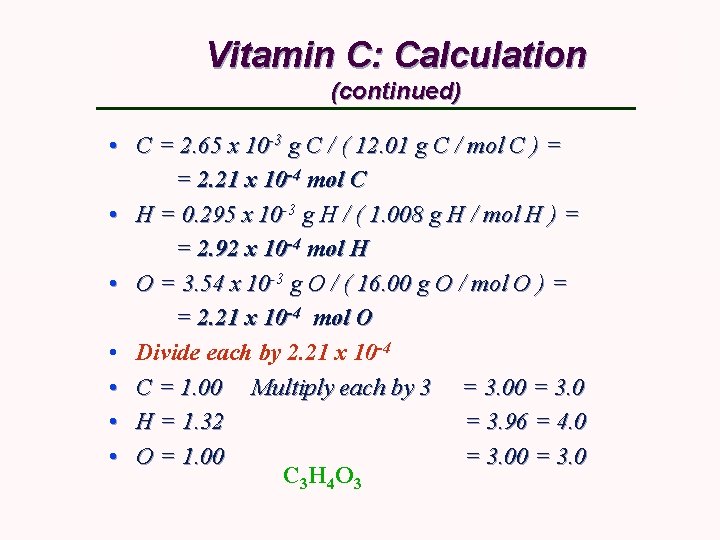
Vitamin C: Calculation (continued) • C = 2. 65 x 10 -3 g C / ( 12. 01 g C / mol C ) = = 2. 21 x 10 -4 mol C • H = 0. 295 x 10 -3 g H / ( 1. 008 g H / mol H ) = = 2. 92 x 10 -4 mol H • O = 3. 54 x 10 -3 g O / ( 16. 00 g O / mol O ) = = 2. 21 x 10 -4 mol O • Divide each by 2. 21 x 10 -4 • C = 1. 00 Multiply each by 3 = 3. 00 = 3. 0 • H = 1. 32 = 3. 96 = 4. 0 • O = 1. 00 = 3. 0 C 3 H 4 O 3

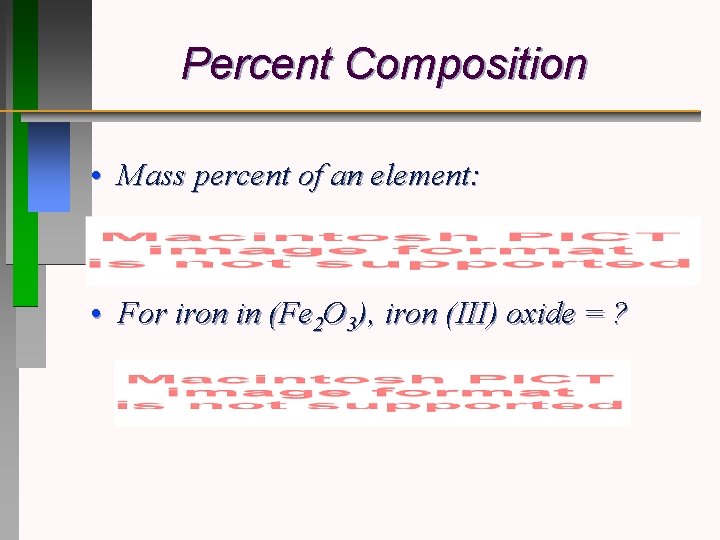
Percent Composition • Mass percent of an element: • For iron in (Fe 2 O 3), iron (III) oxide = ?

QUESTION

ANSWER 5) All of these Section 3. 5 Percent Composition of Compounds (p. 89) The ratio of C to H in C 8 H 8 is 1: 1. This is the same ratio found for each of the compounds, so all have the same percent composition by mass.

QUESTION Morphine, derived from opium plants, has the potential for use and abuse. It’s formula is C 17 H 19 NO 3. What percent, by mass, is the carbon in this compound? 1. 2. 3. 4. 42. 5% 27. 9% 71. 6% This cannot be solved until the mass of the sample is given.

ANSWER Choice 3 is correct. First determine the molar mass of the compound, then divide that into the total mass of carbon present, and finally, multiply that by 10. (17 12) / ((17 12) + (19 1) + (1 14) + 3 16)) = 0. 716 100 = 71. 6 % Section 3. 4: Percent Composition of Compounds

QUESTION

ANSWER 3) 4. 04 g Section 3. 5 Percent Composition of Compounds (p. 89) The molar mass of K 2 Cr. O 7 is 2 ´ 39. 10 + 52. 00 + 7 ´ 16. 00 = 242. 2. The mass fraction of potassium is (2 ´ 39. 10)/242. 2 = 0. 3229 ´ 12. 5 g = 4. 04 g.

Formulas: Dalton’s Law • Dalton’s law of multiple proportions: When two elements form different compounds, the mass ratio of the elements in one compound is related to the mass ratio in the other by a small whole number.

Formulas: Multiple Proportions

Formulas & Multiple Proportions Components of acid rain, SO 2(g) and SO 3(g) • Compound A contains: 1. 000 g Sulfur & 1. 500 g Oxygen • Compound B contains: 1. 000 g Sulfur & 1. 000 g Oxygen • Mass ratio A: 2 to 3; Mass ratio B: 1 to 1 • Adjusting for atomic mass differences: AW sulfur is 2 x the AW oxygen; the atom ratios therefore are S 1 O 3 and S 1 O 2 respectively

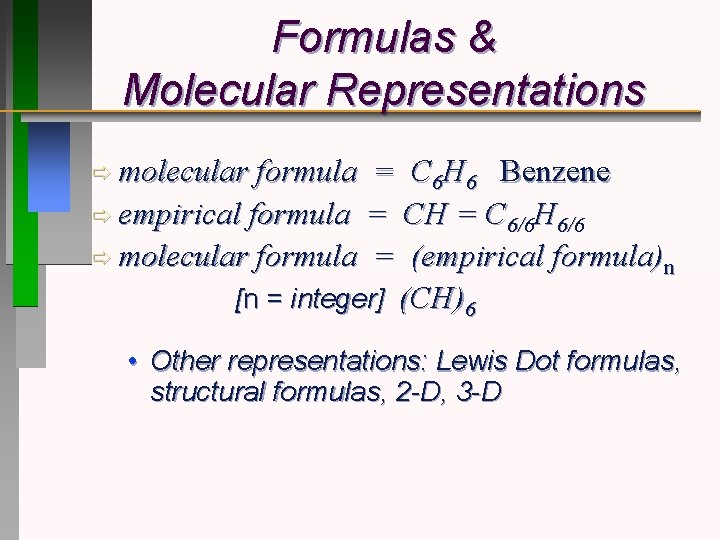
Formulas & Molecular Representations ð molecular formula = C 6 H 6 Benzene ð empirical formula = CH = C 6/6 H 6/6 ð molecular formula = (empirical formula)n [n = integer] (CH)6 • Other representations: Lewis Dot formulas, structural formulas, 2 -D, 3 -D

Formulas & Molecular Representations

Empirical Formulas from Analyses

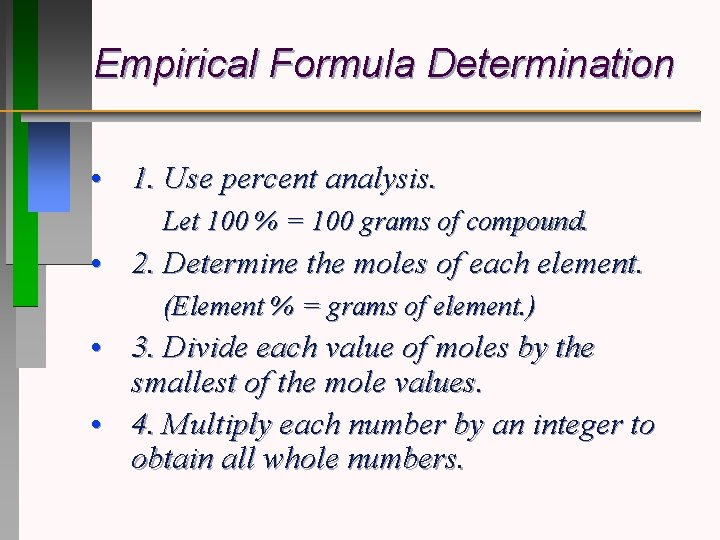
Empirical Formula Determination • 1. Use percent analysis. Let 100 % = 100 grams of compound. • 2. Determine the moles of each element. (Element % = grams of element. ) • 3. Divide each value of moles by the smallest of the mole values. • 4. Multiply each number by an integer to obtain all whole numbers.

QUESTION The dye indigo is a compound with tremendous economic importance (blue jeans wouldn’t be blue without it. ) Indigo’s percent composition is: 73. 27% C; 3. 84% H; 10. 68%N and 12. 21% O. What is the empirical formula of indigo? 1. 2. 3. 4. C 6 H 4 NO C 8 H 3 NO C 8 H 5 NO I know this should be whole numbers for each atom, but I do not know how to accomplish that.

ANSWER Choice 3 is the smallest whole number ratio of the atoms that make up a molecule of indigo. The percentage must be converted to a mass, then the mass is converted to moles of the atoms and finally, the smallest is divided into the others to obtain the proper ratio. Section 3. 5: Determining the Formula of a Compound

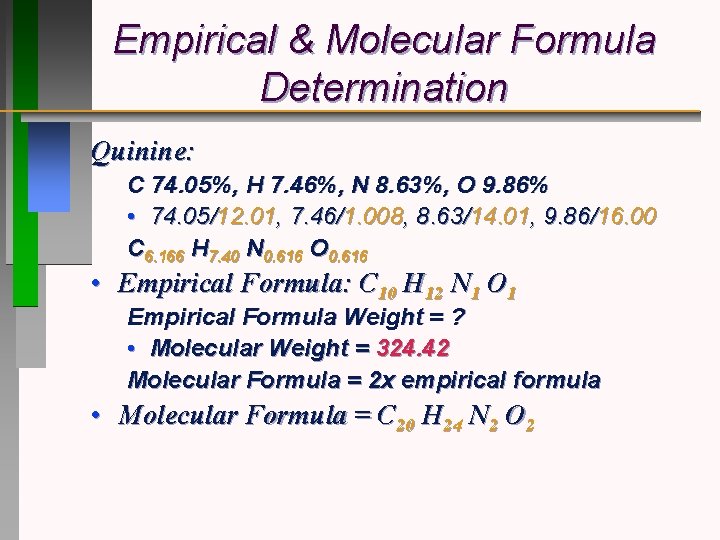
Empirical & Molecular Formula Determination Quinine: C 74. 05%, H 7. 46%, N 8. 63%, O 9. 86% • 74. 05/12. 01, 7. 46/1. 008, 8. 63/14. 01, 9. 86/16. 00 C 6. 166 H 7. 40 N 0. 616 O 0. 616 • Empirical Formula: C 10 H 12 N 1 O 1 Empirical Formula Weight = ? • Molecular Weight = 324. 42 Molecular Formula = 2 x empirical formula • Molecular Formula = C 20 H 24 N 2 O 2

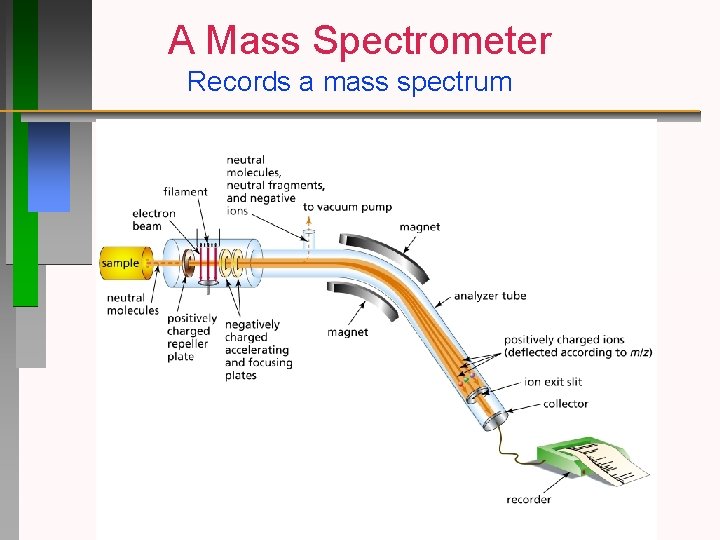
A Mass Spectrometer Records a mass spectrum


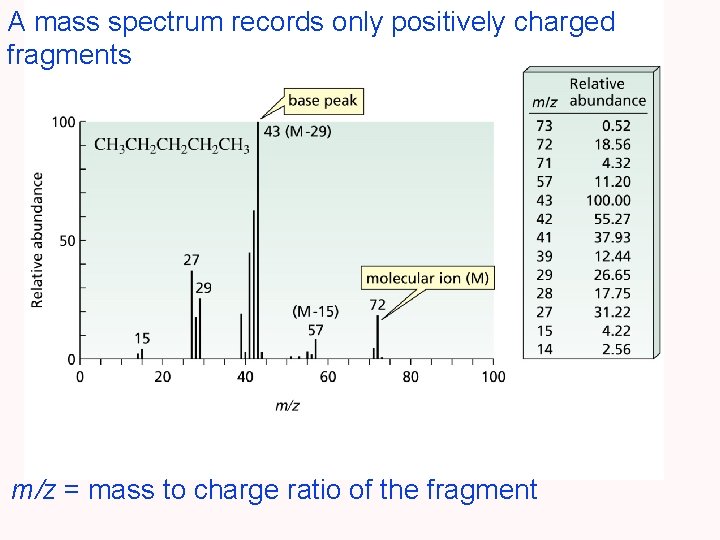
A mass spectrum records only positively charged fragments m/z = mass to charge ratio of the fragment

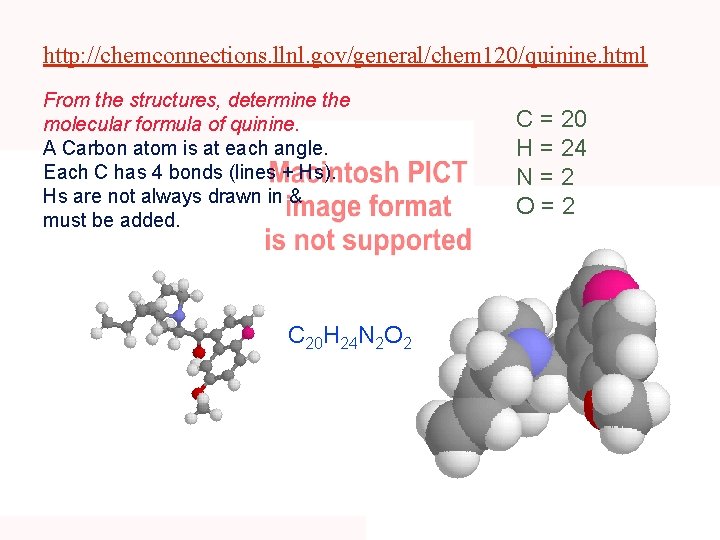
http: //chemconnections. llnl. gov/general/chem 120/quinine. html From the structures, determine the molecular formula of quinine. A Carbon atom is at each angle. Each C has 4 bonds (lines + Hs). Hs are not always drawn in & must be added. C 20 H 24 N 2 O 2 C = 20 H = 24 N=2 O=2

QUESTION

ANSWER 2) C 8 H 8 Section 3. 6 Determining the Formula of a Compound (p. 91) The mass of CH is 13. 018 divides into 104. 1 about 8 times. Therefore there are 8 CH groups in this compound. C 8 H 8 is the molecular formula.

More Chemical Equations / Stoichiometric Calculations