Chemical Equations Vocabulary Coefficient is a small whole

Chemical Equations
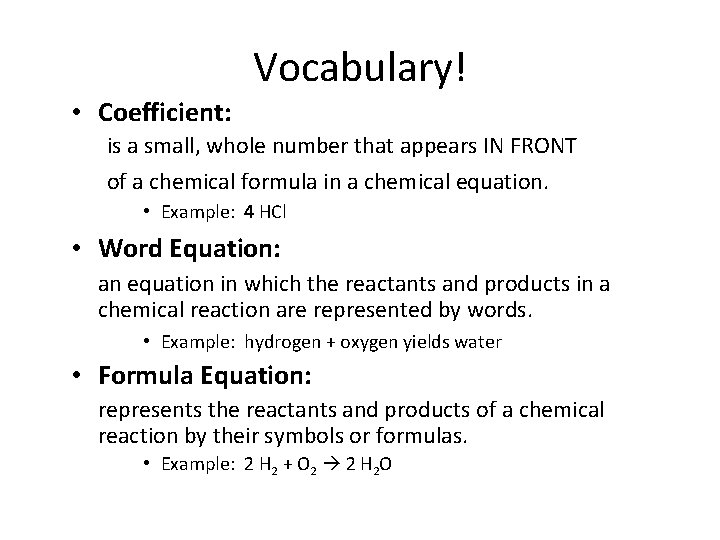
Vocabulary! • Coefficient: is a small, whole number that appears IN FRONT of a chemical formula in a chemical equation. • Example: 4 HCl • Word Equation: an equation in which the reactants and products in a chemical reaction are represented by words. • Example: hydrogen + oxygen yields water • Formula Equation: represents the reactants and products of a chemical reaction by their symbols or formulas. • Example: 2 H 2 + O 2 2 H 2 O
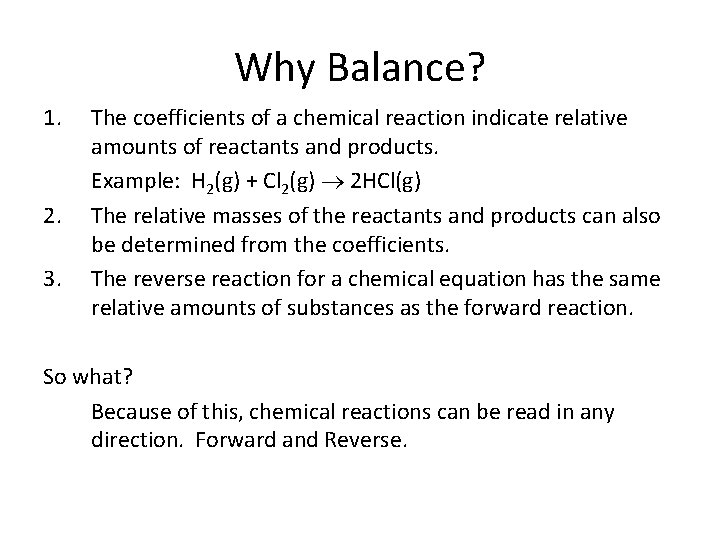
Why Balance? 1. 2. 3. The coefficients of a chemical reaction indicate relative amounts of reactants and products. Example: H 2(g) + Cl 2(g) 2 HCl(g) The relative masses of the reactants and products can also be determined from the coefficients. The reverse reaction for a chemical equation has the same relative amounts of substances as the forward reaction. So what? Because of this, chemical reactions can be read in any direction. Forward and Reverse.
Law of Conservation of Mass The law of conservation of mass states that matter is neither lost nor gained in chemical reactions. It simply changes form. h c t a W ! e M
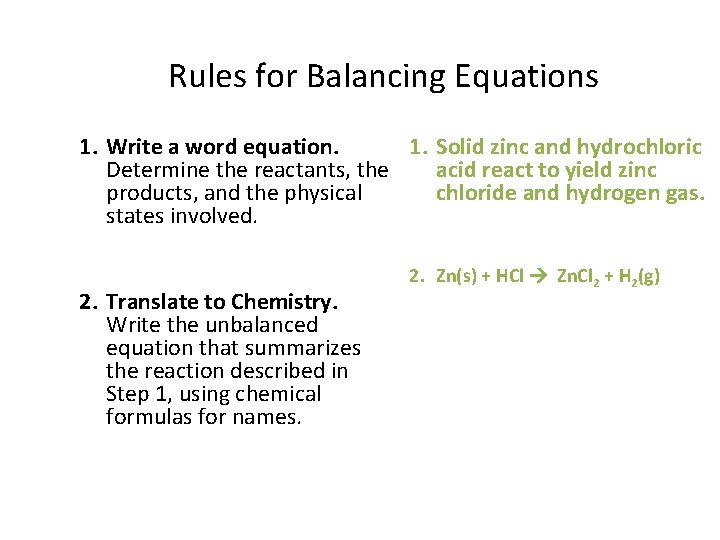
Rules for Balancing Equations 1. Write a word equation. 1. Solid zinc and hydrochloric Determine the reactants, the acid react to yield zinc products, and the physical chloride and hydrogen gas. states involved. 2. Translate to Chemistry. Write the unbalanced equation that summarizes the reaction described in Step 1, using chemical formulas for names. 2. Zn(s) + HCl Zn. Cl 2 + H 2(g)
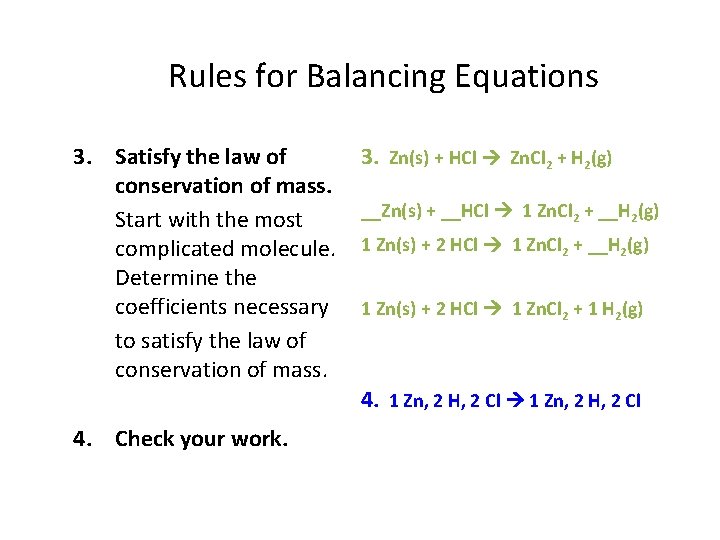
Rules for Balancing Equations 3. Satisfy the law of conservation of mass. Start with the most complicated molecule. Determine the coefficients necessary to satisfy the law of conservation of mass. 3. Zn(s) + HCl Zn. Cl 2 + H 2(g) __Zn(s) + __HCl 1 Zn. Cl 2 + __H 2(g) 1 Zn(s) + 2 HCl 1 Zn. Cl 2 + 1 H 2(g) 4. 1 Zn, 2 H, 2 Cl 4. Check your work.
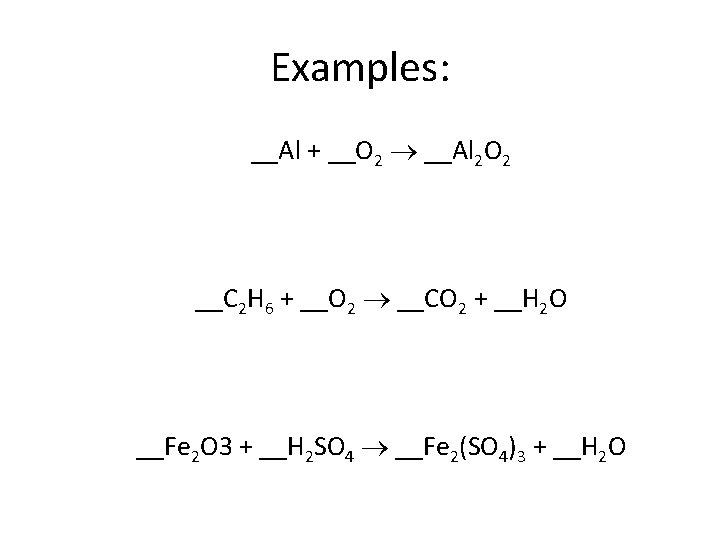
Examples: __Al + __O 2 __Al 2 O 2 __C 2 H 6 + __O 2 __CO 2 + __H 2 O __Fe 2 O 3 + __H 2 SO 4 __Fe 2(SO 4)3 + __H 2 O
- Slides: 7