Chemical Reactions and Stoichiometry Chemical Stoichiometry The study








![Formulas molecular formula = (empirical formula)n [n = integer] molecular formula = C 6 Formulas molecular formula = (empirical formula)n [n = integer] molecular formula = C 6](https://slidetodoc.com/presentation_image/47a936a708735086b6873005915e4c29/image-16.jpg)









- Slides: 40

Chemical Reactions and Stoichiometry

Chemical Stoichiometry - The study of quantities of materials consumed and produced in chemical reactions.

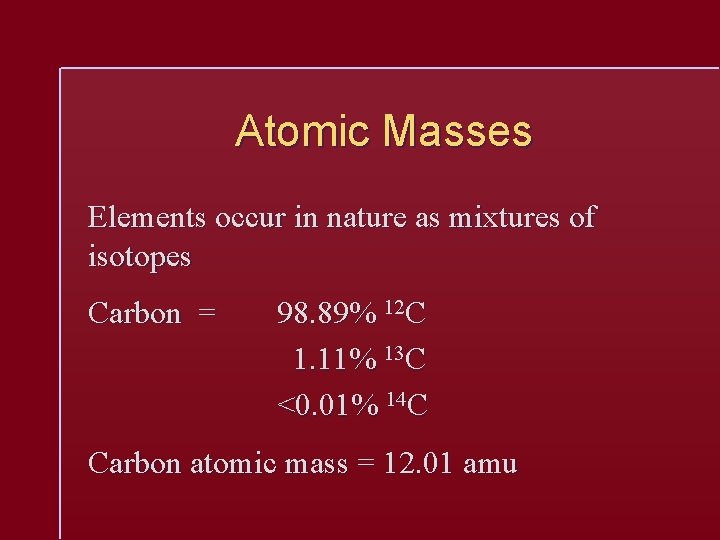
Atomic Masses Elements occur in nature as mixtures of isotopes Carbon = 98. 89% 12 C 1. 11% 13 C <0. 01% 14 C Carbon atomic mass = 12. 01 amu


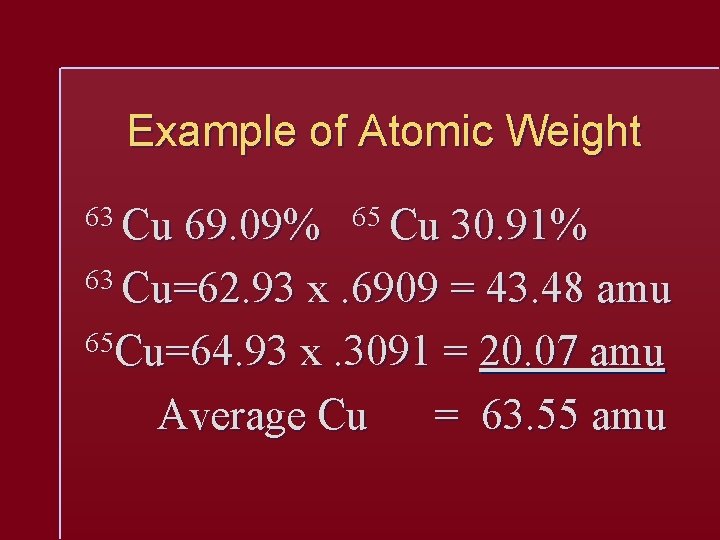
Example of Atomic Weight 63 Cu 69. 09% 65 Cu 30. 91% 63 Cu=62. 93 x. 6909 = 43. 48 amu 65 Cu=64. 93 x. 3091 = 20. 07 amu Average Cu = 63. 55 amu

The Mole The number equal to the number of carbon atoms in exactly 12 grams of pure 12 C. 1 mole of anything = 6. 022 1023 units of that thing

Avogadro’s number equals 23 6. 022 10 units

Molar Mass A substance’s molar mass (molecular weight) is the mass in grams of one mole of the compound. CO 2 = 44. 01 grams per mole

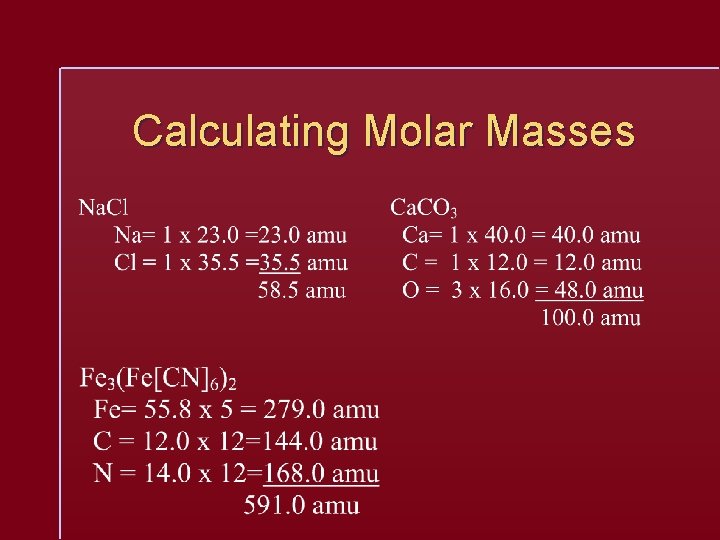
Calculating Molar Masses

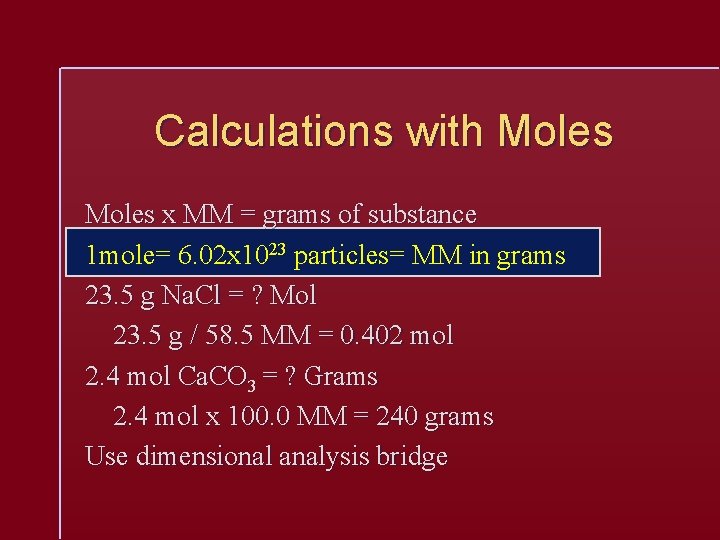
Calculations with Moles x MM = grams of substance 1 mole= 6. 02 x 1023 particles= MM in grams 23. 5 g Na. Cl = ? Mol 23. 5 g / 58. 5 MM = 0. 402 mol 2. 4 mol Ca. CO 3 = ? Grams 2. 4 mol x 100. 0 MM = 240 grams Use dimensional analysis bridge

Mole Calculations

Homework!! p. 117 ff 6, 8, 13, 17 c, 22 a, 23, 26, 29, 32

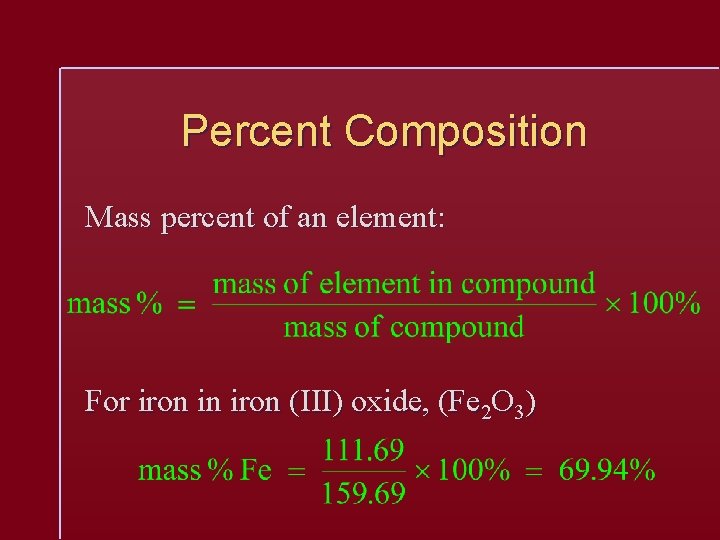
Percent Composition Mass percent of an element: For iron in iron (III) oxide, (Fe 2 O 3)

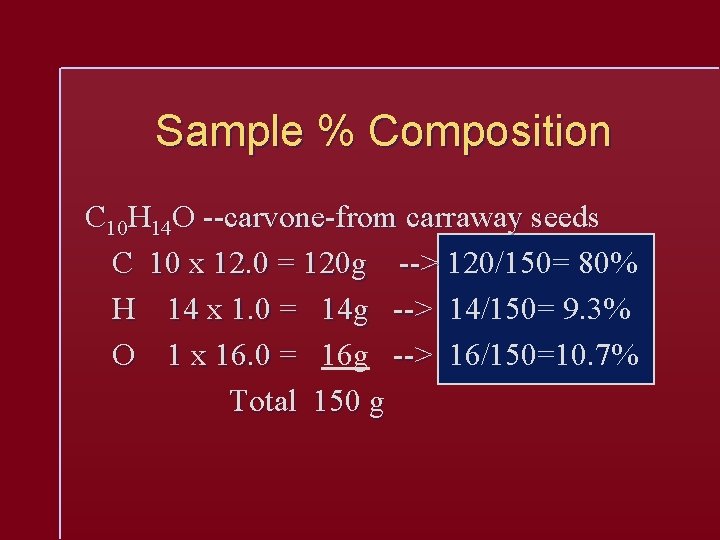
Sample % Composition C 10 H 14 O --carvone-from carraway seeds C 10 x 12. 0 = 120 g --> 120/150= 80% H 14 x 1. 0 = 14 g --> 14/150= 9. 3% O 1 x 16. 0 = 16 g --> 16/150=10. 7% Total 150 g

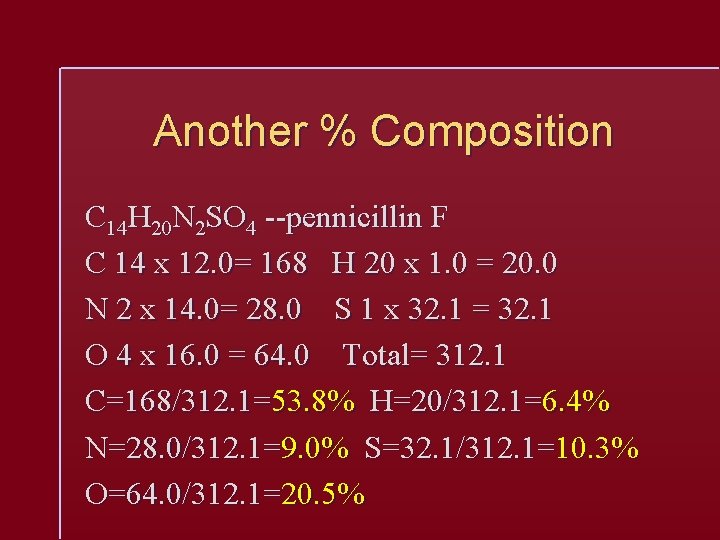
Another % Composition C 14 H 20 N 2 SO 4 --pennicillin F C 14 x 12. 0= 168 H 20 x 1. 0 = 20. 0 N 2 x 14. 0= 28. 0 S 1 x 32. 1 = 32. 1 O 4 x 16. 0 = 64. 0 Total= 312. 1 C=168/312. 1=53. 8% H=20/312. 1=6. 4% N=28. 0/312. 1=9. 0% S=32. 1/312. 1=10. 3% O=64. 0/312. 1=20. 5%
![Formulas molecular formula empirical formulan n integer molecular formula C 6 Formulas molecular formula = (empirical formula)n [n = integer] molecular formula = C 6](https://slidetodoc.com/presentation_image/47a936a708735086b6873005915e4c29/image-16.jpg)
Formulas molecular formula = (empirical formula)n [n = integer] molecular formula = C 6 H 6 = (CH)6 empirical formula = CH

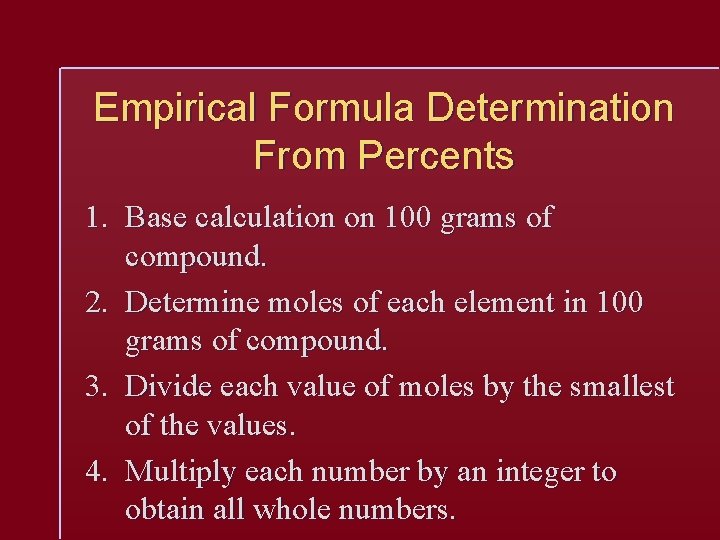
Empirical Formula Determination From Percents 1. Base calculation on 100 grams of compound. 2. Determine moles of each element in 100 grams of compound. 3. Divide each value of moles by the smallest of the values. 4. Multiply each number by an integer to obtain all whole numbers.

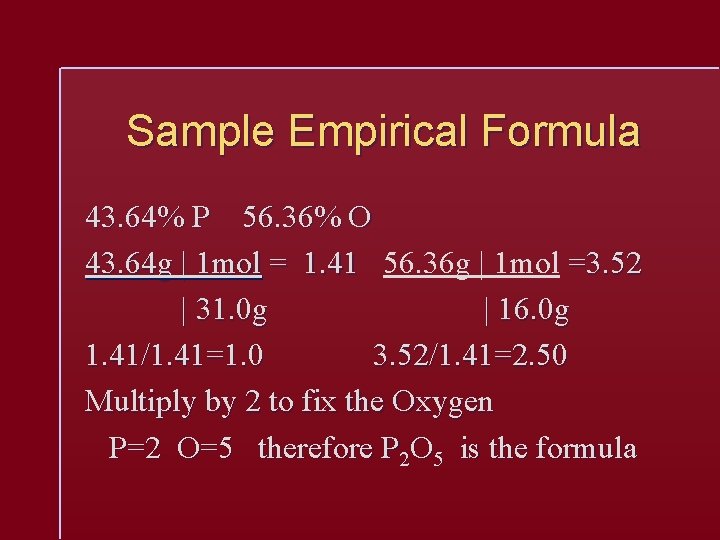
Sample Empirical Formula 43. 64% P 56. 36% O 43. 64 g | 1 mol = 1. 41 56. 36 g | 1 mol =3. 52 | 31. 0 g | 16. 0 g 1. 41/1. 41=1. 0 3. 52/1. 41=2. 50 Multiply by 2 to fix the Oxygen P=2 O=5 therefore P 2 O 5 is the formula

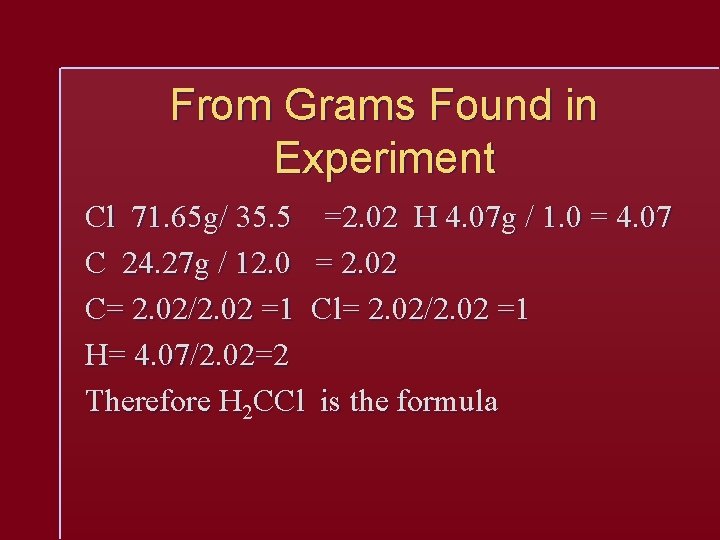
From Grams Found in Experiment Cl 71. 65 g/ 35. 5 =2. 02 H 4. 07 g / 1. 0 = 4. 07 C 24. 27 g / 12. 0 = 2. 02 C= 2. 02/2. 02 =1 Cl= 2. 02/2. 02 =1 H= 4. 07/2. 02=2 Therefore H 2 CCl is the formula

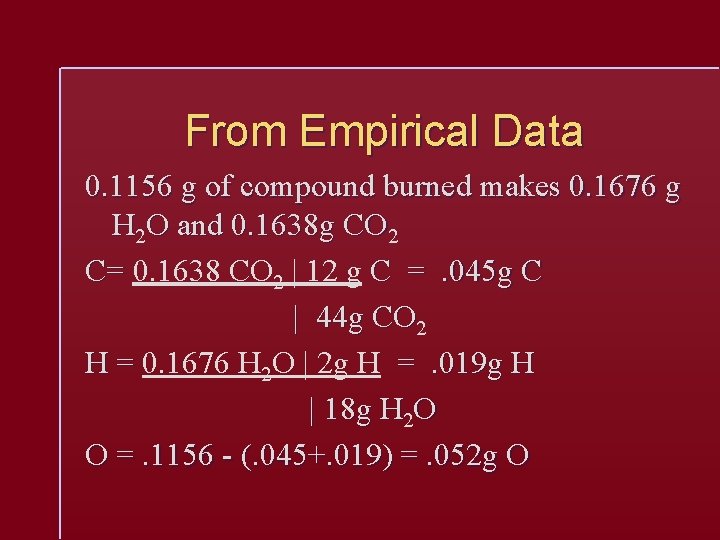
From Empirical Data 0. 1156 g of compound burned makes 0. 1676 g H 2 O and 0. 1638 g CO 2 C= 0. 1638 CO 2 | 12 g C =. 045 g C | 44 g CO 2 H = 0. 1676 H 2 O | 2 g H =. 019 g H | 18 g H 2 O O =. 1156 - (. 045+. 019) =. 052 g O

Continued C=. 045/12. 0 = 0. 00375 H=. 019/1. 0= 0. 019 O=. 052 / 16. 0=0. 00325 C=. 00375/. 00325 = 1. 15 H =. 019 /. 00325 = 5. 84 O=. 00325/. 00325=1. 0 Multiply by 7 (. 15 is about one-seventh) C 8 H 41 O 7

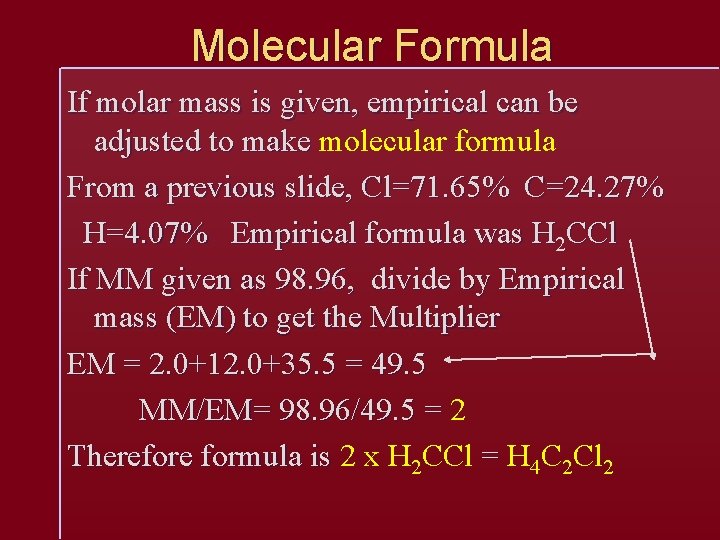
Molecular Formula If molar mass is given, empirical can be adjusted to make molecular formula From a previous slide, Cl=71. 65% C=24. 27% H=4. 07% Empirical formula was H 2 CCl If MM given as 98. 96, divide by Empirical mass (EM) to get the Multiplier EM = 2. 0+12. 0+35. 5 = 49. 5 MM/EM= 98. 96/49. 5 = 2 Therefore formula is 2 x H 2 CCl = H 4 C 2 Cl 2

Homework!! p. 119 ff 35, 40, 43, 48, 54, 57, 58

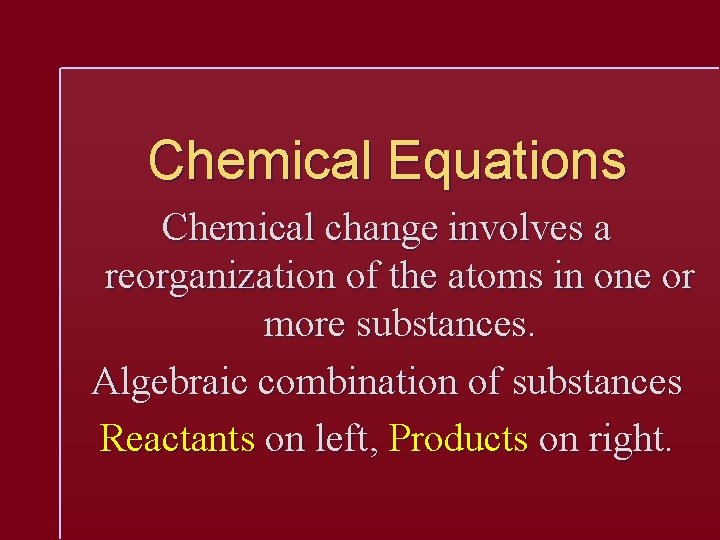
Chemical Equations Chemical change involves a reorganization of the atoms in one or more substances. Algebraic combination of substances Reactants on left, Products on right.

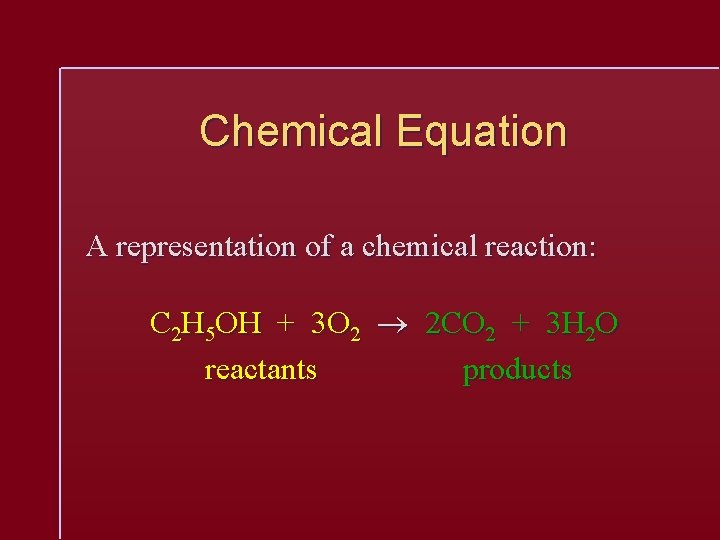
Chemical Equation A representation of a chemical reaction: C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O reactants products

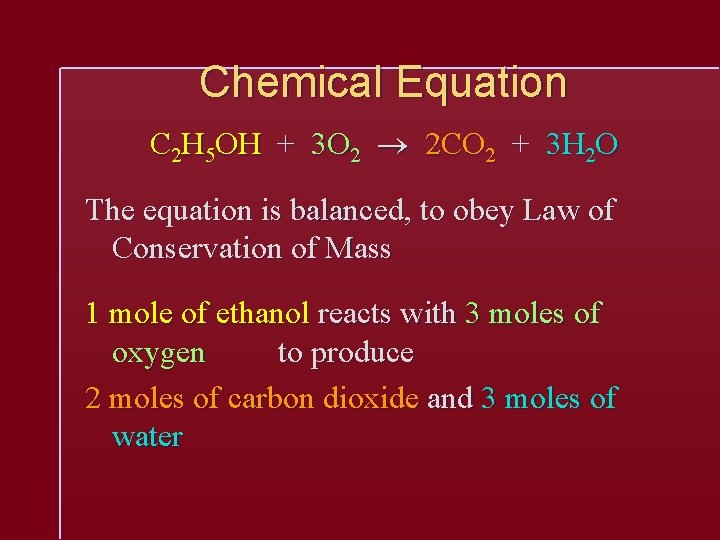
Chemical Equation C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O The equation is balanced, to obey Law of Conservation of Mass 1 mole of ethanol reacts with 3 moles of oxygen to produce 2 moles of carbon dioxide and 3 moles of water

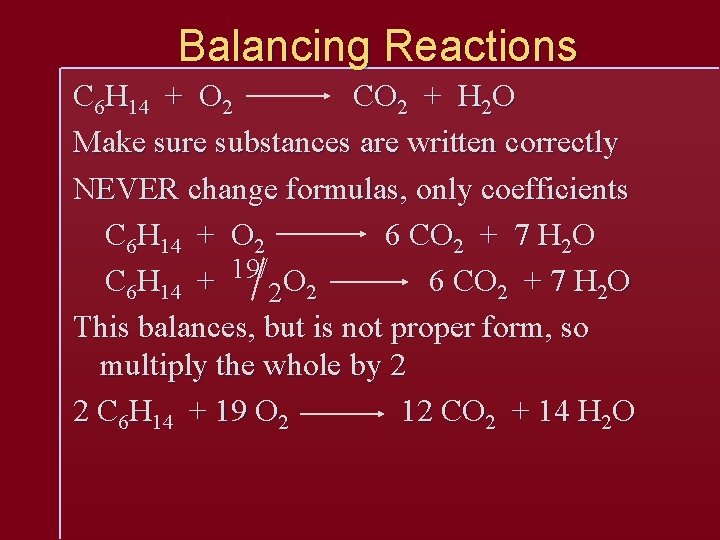
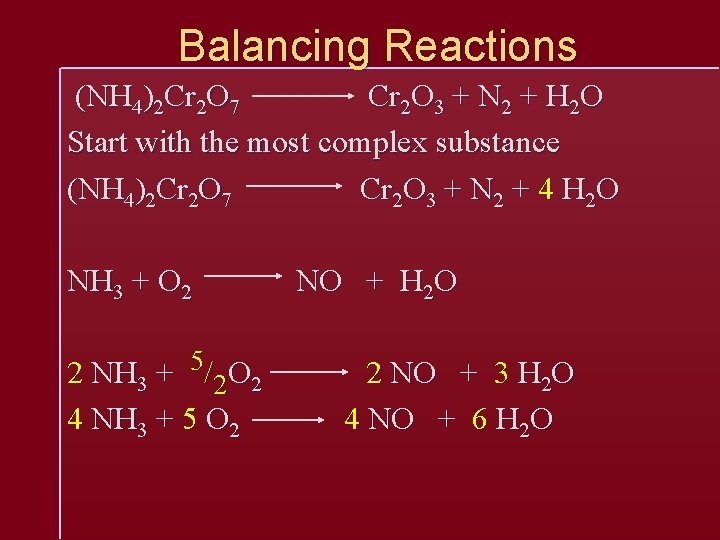
Balancing Reactions C 6 H 14 + O 2 CO 2 + H 2 O Make sure substances are written correctly NEVER change formulas, only coefficients C 6 H 14 + O 2 6 CO 2 + 7 H 2 O C 6 H 14 + 19/2 O 2 6 CO 2 + 7 H 2 O This balances, but is not proper form, so multiply the whole by 2 2 C 6 H 14 + 19 O 2 12 CO 2 + 14 H 2 O

Balancing Reactions (NH 4)2 Cr 2 O 7 Cr 2 O 3 + N 2 + H 2 O Start with the most complex substance (NH 4)2 Cr 2 O 7 Cr 2 O 3 + N 2 + 4 H 2 O NH 3 + O 2 2 NH 3 + 5/2 O 2 4 NH 3 + 5 O 2 NO + H 2 O 2 NO + 3 H 2 O 4 NO + 6 H 2 O

Homework!! p. 121 61, 62, 63, 64, 65, 66

Calculating Masses of Reactants and Products 1. 2. 3. Balance the equation. Set up the mole bridge. Calculate moles of desired substance. 4. Convert moles to grams, if required from the problem.

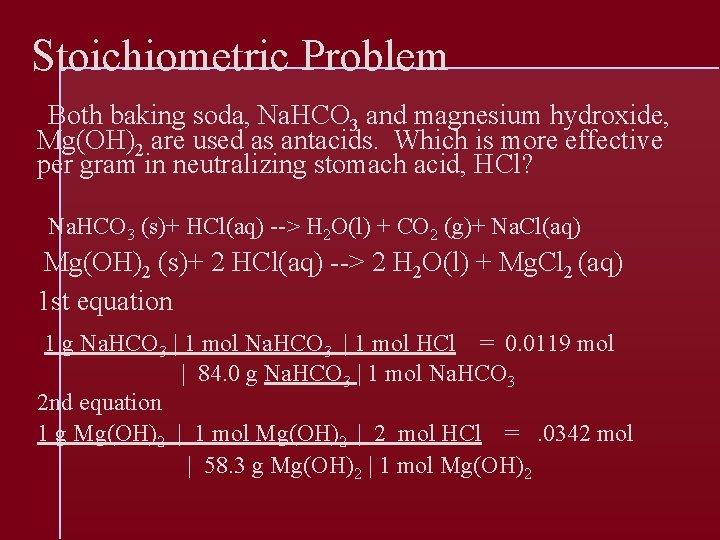
Stoichiometric Problem Both baking soda, Na. HCO 3 and magnesium hydroxide, Mg(OH)2 are used as antacids. Which is more effective per gram in neutralizing stomach acid, HCl? Na. HCO 3 (s)+ HCl(aq) --> H 2 O(l) + CO 2 (g)+ Na. Cl(aq) Mg(OH)2 (s)+ 2 HCl(aq) --> 2 H 2 O(l) + Mg. Cl 2 (aq) 1 st equation 1 g Na. HCO 3 | 1 mol HCl = 0. 0119 mol | 84. 0 g Na. HCO 3 | 1 mol Na. HCO 3 2 nd equation 1 g Mg(OH)2 | 1 mol Mg(OH)2 | 2 mol HCl =. 0342 mol | 58. 3 g Mg(OH)2 | 1 mol Mg(OH)2

Another Normal Problem Lithium hydroxide is used in spacecraft to eliminate CO 2. How much CO 2 is absorbed by 1. 00 kg of Li. OH? Li. OH(s) + CO 2 (g) --> Li 2 CO 3 (s) + H 2 O (l) 2 Li. OH(s) + CO 2 (g) --> Li 2 CO 3 (s) + H 2 O (l) 1000 g Li. OH | 1 mol Li. OH | 1 mol CO 2 | 44 g CO 2 | 23. 9 g Li. OH | 2 mol Li. OH | 1 mol CO 2 = 920. g CO 2

Limiting Reactant The limiting reactant is the reactant that is consumed first, limiting the amounts of products formed.

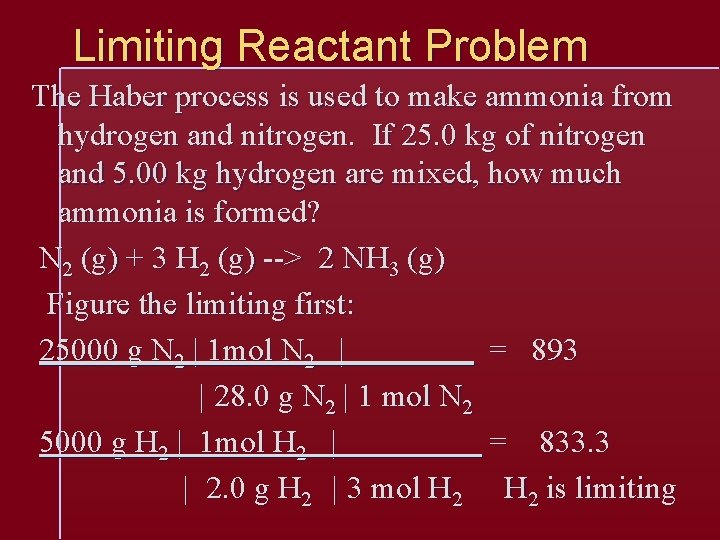
Limiting Reactant Problem The Haber process is used to make ammonia from hydrogen and nitrogen. If 25. 0 kg of nitrogen and 5. 00 kg hydrogen are mixed, how much ammonia is formed? N 2 (g) + 3 H 2 (g) --> 2 NH 3 (g) Figure the limiting first: 25000 g N 2 | 1 mol N 2 | = 893 | 28. 0 g N 2 | 1 mol N 2 5000 g H 2 | 1 mol H 2 | = 833. 3 | 2. 0 g H 2 | 3 mol H 2 is limiting

Continue the problem with the limiting reactant: 5000 g H 2 | 1 mol H 2 | 2 mol NH 3| 17. 0 g NH 3 | 2. 0 g H 2 | 3 mol H 2 | 1 mol NH 3 = 28000 g NH 3

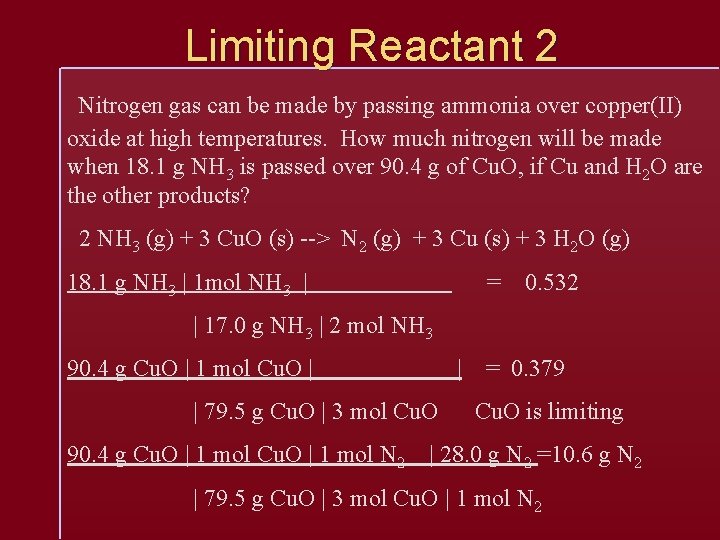
Limiting Reactant 2 Nitrogen gas can be made by passing ammonia over copper(II) oxide at high temperatures. How much nitrogen will be made when 18. 1 g NH 3 is passed over 90. 4 g of Cu. O, if Cu and H 2 O are the other products? 2 NH 3 (g) + 3 Cu. O (s) --> N 2 (g) + 3 Cu (s) + 3 H 2 O (g) 18. 1 g NH 3 | 1 mol NH 3 | = 0. 532 | 17. 0 g NH 3 | 2 mol NH 3 90. 4 g Cu. O | 1 mol Cu. O | | | 79. 5 g Cu. O | 3 mol Cu. O 90. 4 g Cu. O | 1 mol N 2 = 0. 379 Cu. O is limiting | 28. 0 g N 2 =10. 6 g N 2 | 79. 5 g Cu. O | 3 mol Cu. O | 1 mol N 2

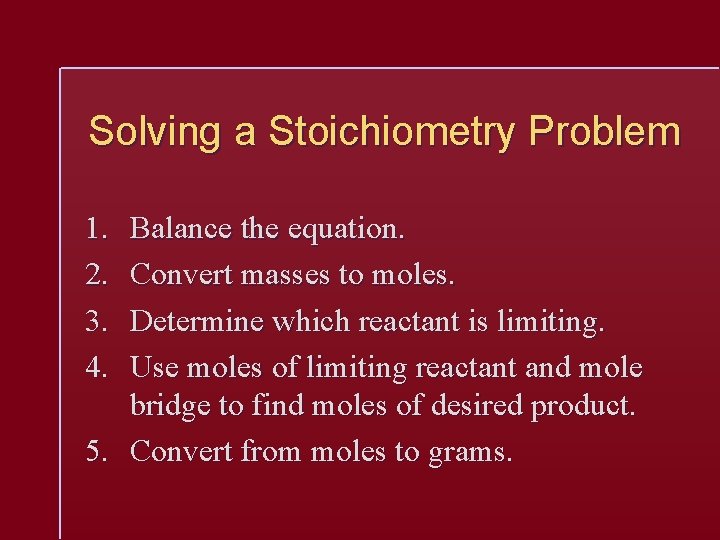
Solving a Stoichiometry Problem 1. 2. 3. 4. Balance the equation. Convert masses to moles. Determine which reactant is limiting. Use moles of limiting reactant and mole bridge to find moles of desired product. 5. Convert from moles to grams.

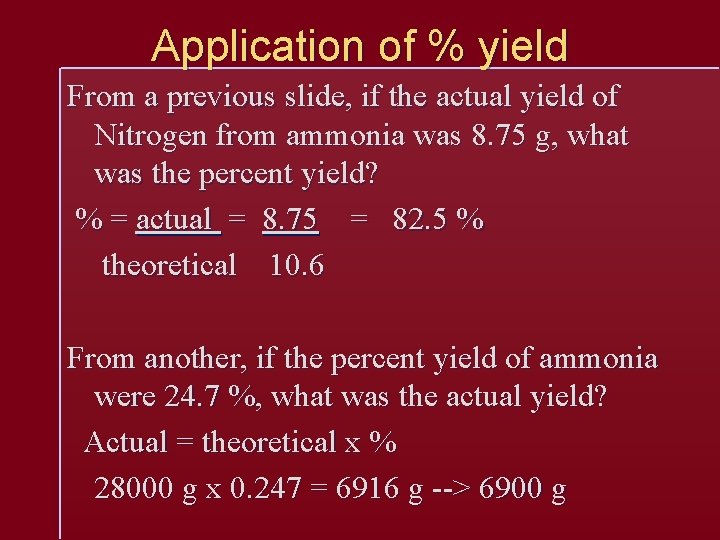
Percent Yield You never get the full or Theoretical amount from a chemical reaction, due to errors and other circumstances. Percent yield is the percent of theoretical you did receive % yield = actual yield theoretical yield

Application of % yield From a previous slide, if the actual yield of Nitrogen from ammonia was 8. 75 g, what was the percent yield? % = actual = 8. 75 = 82. 5 % theoretical 10. 6 From another, if the percent yield of ammonia were 24. 7 %, what was the actual yield? Actual = theoretical x % 28000 g x 0. 247 = 6916 g --> 6900 g

Homework!! p. 121 ff 69, 70, 76, 81, 82, 84, 90, 95, 108 SUPER XCR!!! 112
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Chemical reactions section 3 reactions in aqueous solutions
Chemical reactions section 3 reactions in aqueous solutions Stoichiometry map for chemical reactions
Stoichiometry map for chemical reactions Solubility rules
Solubility rules Chapter 9 chemical reactions answers
Chapter 9 chemical reactions answers Chapter 9 study guide chemical reactions
Chapter 9 study guide chemical reactions Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Aqueous reactions and solution stoichiometry
Aqueous reactions and solution stoichiometry 5 examples of redox reaction
5 examples of redox reaction Unit 5 chemical reactions answers
Unit 5 chemical reactions answers How to determine if a single replacement reaction occurs
How to determine if a single replacement reaction occurs Chemical reactions reactants and products
Chemical reactions reactants and products Chemical equations and reactions chapter 8 review
Chemical equations and reactions chapter 8 review Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Reactions of copper and percent yield
Reactions of copper and percent yield What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Building vocabulary: chemical bonds and reactions
Building vocabulary: chemical bonds and reactions Chapter 19 review oxidation-reduction reactions
Chapter 19 review oxidation-reduction reactions Stoichiometry mole island diagram
Stoichiometry mole island diagram Different types of redox reactions
Different types of redox reactions Types of reactions
Types of reactions Types of reactions chemistry
Types of reactions chemistry Types of reaction
Types of reaction Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chemical reactions answer key
Chapter 10 chemical reactions answer key The calculation of quantities in chemical reactions
The calculation of quantities in chemical reactions Principles of immunochemical reaction
Principles of immunochemical reaction Predicting products of chemical reactions
Predicting products of chemical reactions Predicting synthesis reactions
Predicting synthesis reactions Combination reaction equation
Combination reaction equation
































































