Chapter 7 Quantitative Relationships in Chemical Reactions Malone













- Slides: 38

Chapter 7 Quantitative Relationships in Chemical Reactions Malone and Dolter - Basic Concepts of Chemistry 9 e

Setting the Stage – Limiting Reactants and Energy n n n One possible result of the increase in CO 2 as a result of global warming is an increase in vegetation. However, what is found is that some vegetation is increasing, (converting CO 2 to carbohydrates) but not all. Photosynthesis often requires more than CO 2 and sunlight – increasing one reactant does not always result in the increase in product, unless the other reactants are increased). Malone and Dolter - Basic Concepts of Chemistry 9 e 2

Setting a Goal - Part A Mass Relationships in Chemical Reactions n You will gain command over the aspects of stoichiometric calculation involving masses, moles, and molecules under both ideal and realistic conditions. Malone and Dolter - Basic Concepts of Chemistry 9 e 3

Objective for Section 7 -1 n Perform stoichiometry calculations using mole ratios from balanced equations. Malone and Dolter - Basic Concepts of Chemistry 9 e 4

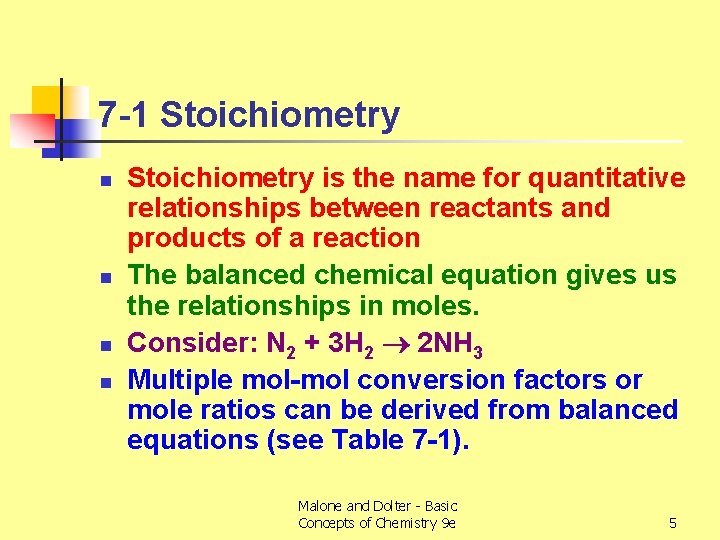
7 -1 Stoichiometry n n Stoichiometry is the name for quantitative relationships between reactants and products of a reaction The balanced chemical equation gives us the relationships in moles. Consider: N 2 + 3 H 2 2 NH 3 Multiple mol-mol conversion factors or mole ratios can be derived from balanced equations (see Table 7 -1). Malone and Dolter - Basic Concepts of Chemistry 9 e 5

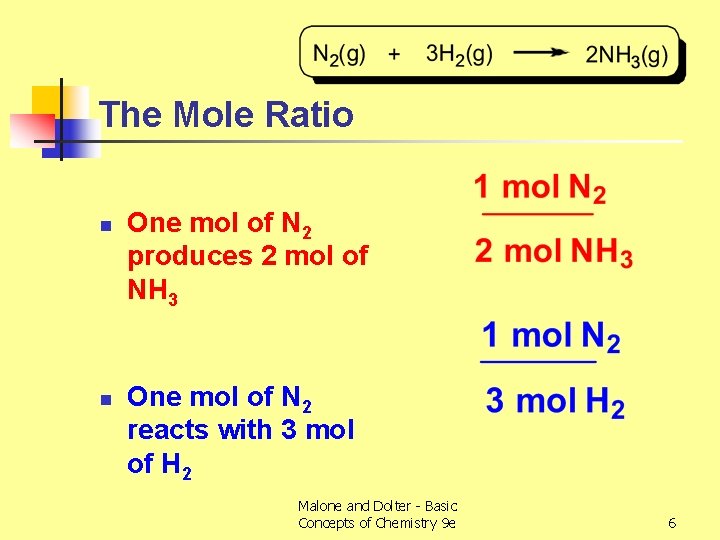
The Mole Ratio n n One mol of N 2 produces 2 mol of NH 3 One mol of N 2 reacts with 3 mol of H 2 Malone and Dolter - Basic Concepts of Chemistry 9 e 6

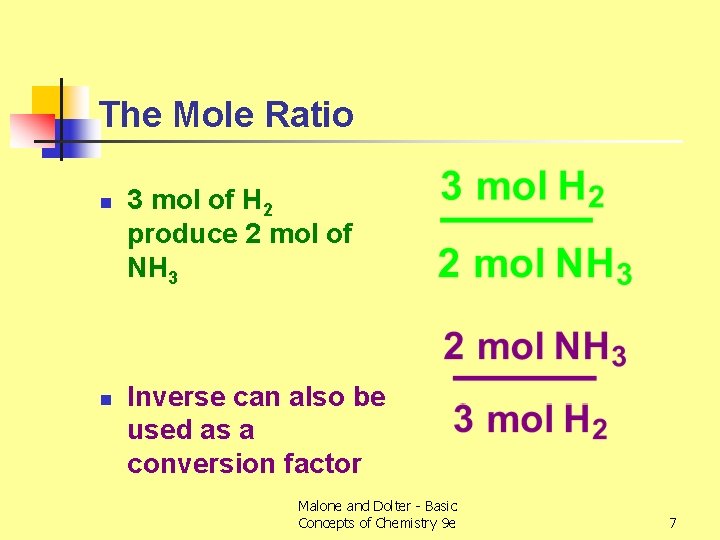
The Mole Ratio n n 3 mol of H 2 produce 2 mol of NH 3 Inverse can also be used as a conversion factor Malone and Dolter - Basic Concepts of Chemistry 9 e 7

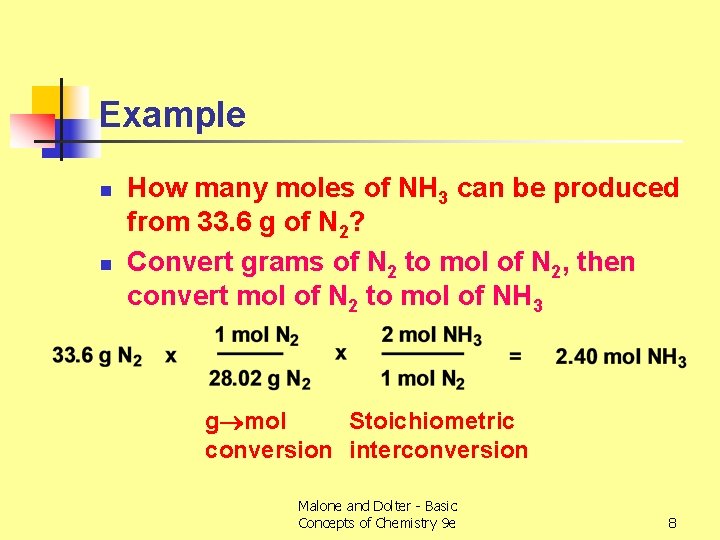
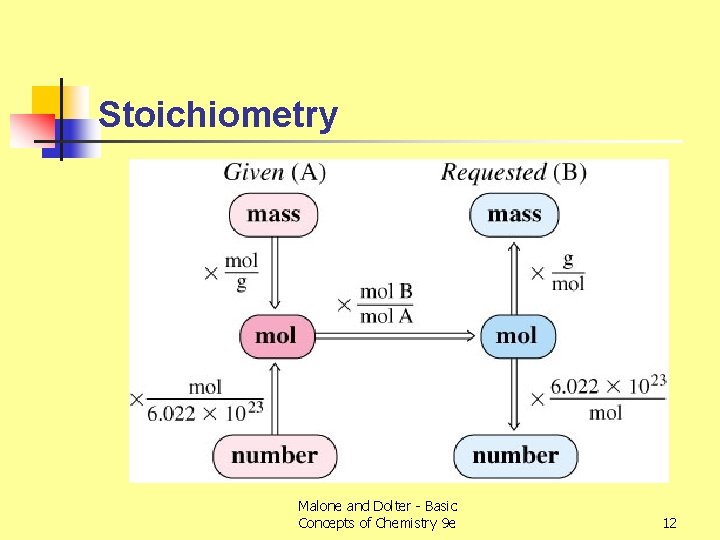
Example n n How many moles of NH 3 can be produced from 33. 6 g of N 2? Convert grams of N 2 to mol of N 2, then convert mol of N 2 to mol of NH 3 g mol Stoichiometric conversion interconversion Malone and Dolter - Basic Concepts of Chemistry 9 e 8

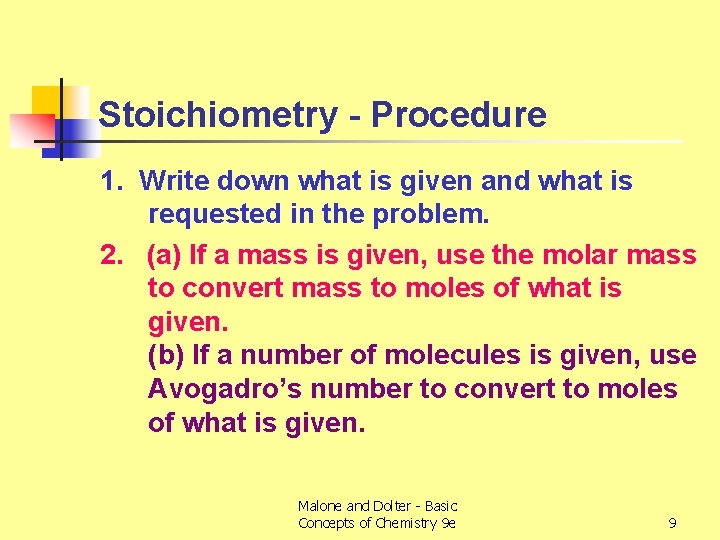
Stoichiometry - Procedure 1. Write down what is given and what is requested in the problem. 2. (a) If a mass is given, use the molar mass to convert mass to moles of what is given. (b) If a number of molecules is given, use Avogadro’s number to convert to moles of what is given. Malone and Dolter - Basic Concepts of Chemistry 9 e 9

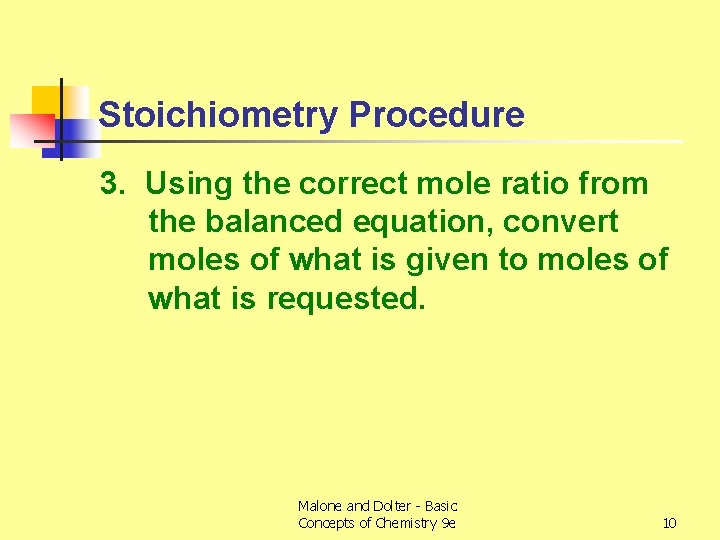
Stoichiometry Procedure 3. Using the correct mole ratio from the balanced equation, convert moles of what is given to moles of what is requested. Malone and Dolter - Basic Concepts of Chemistry 9 e 10

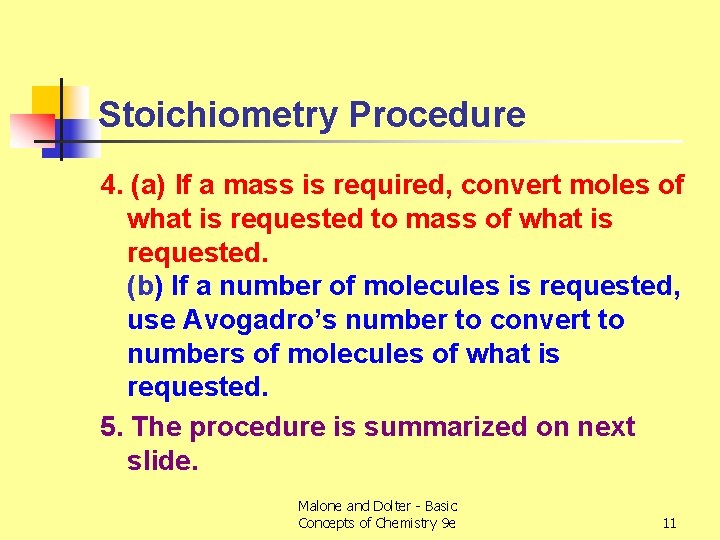
Stoichiometry Procedure 4. (a) If a mass is required, convert moles of what is requested to mass of what is requested. (b) If a number of molecules is requested, use Avogadro’s number to convert to numbers of molecules of what is requested. 5. The procedure is summarized on next slide. Malone and Dolter - Basic Concepts of Chemistry 9 e 11

Stoichiometry Malone and Dolter - Basic Concepts of Chemistry 9 e 12

Objective for Section 7 -2 n Given the masses of two different reactants, determine the limiting reactant and calculate the yield of product. Malone and Dolter - Basic Concepts of Chemistry 9 e 13

7 -2 Limiting Reactant n n If specific amounts of each reactant are mixed, the reactant that produces the least amount of product is called the limiting reactant. Think of hot dogs and buns: n n n hot dogs are sold in packs of ten hot dog buns are sold in packs of twelve how many hot dog-hot dog bun combinations can you make with one pack of hot dogs and one pack of hot dog buns? Malone and Dolter - Basic Concepts of Chemistry 9 e 14

Think of Hot Dogs and Hot Dog Buns Malone and Dolter - Basic Concepts of Chemistry 9 e 15

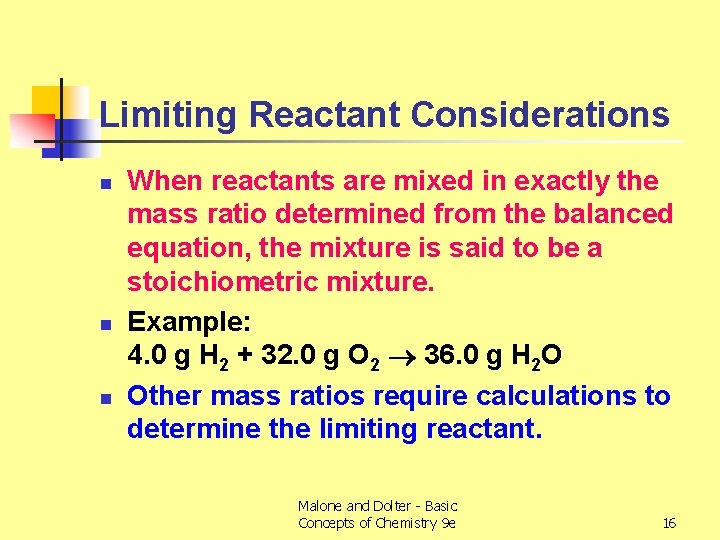
Limiting Reactant Considerations n n n When reactants are mixed in exactly the mass ratio determined from the balanced equation, the mixture is said to be a stoichiometric mixture. Example: 4. 0 g H 2 + 32. 0 g O 2 36. 0 g H 2 O Other mass ratios require calculations to determine the limiting reactant. Malone and Dolter - Basic Concepts of Chemistry 9 e 16

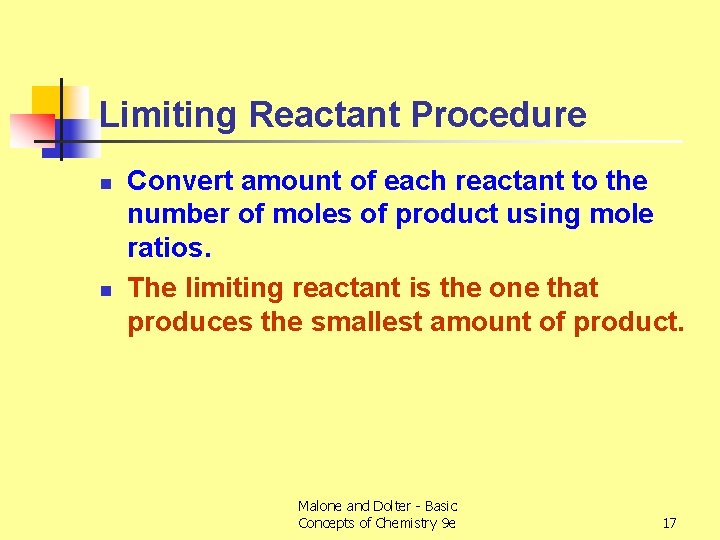
Limiting Reactant Procedure n n Convert amount of each reactant to the number of moles of product using mole ratios. The limiting reactant is the one that produces the smallest amount of product. Malone and Dolter - Basic Concepts of Chemistry 9 e 17

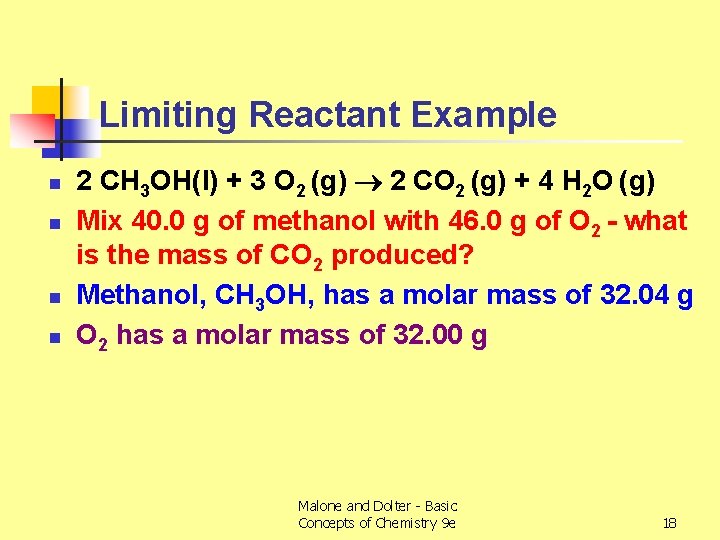
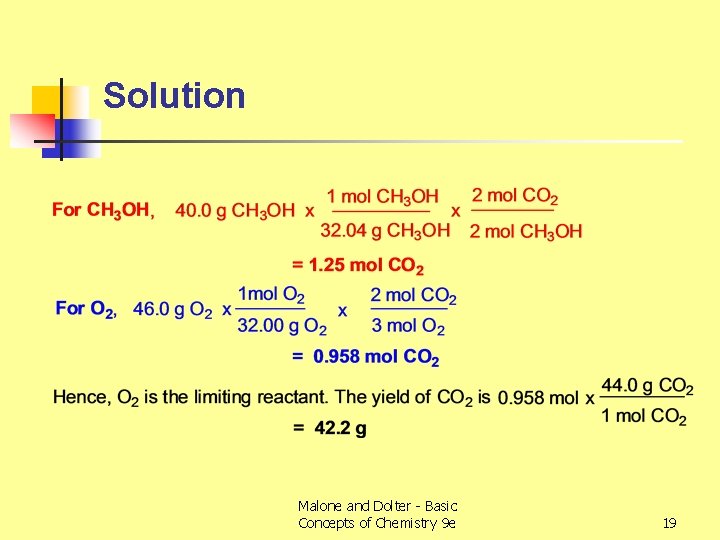
Limiting Reactant Example n n 2 CH 3 OH(l) + 3 O 2 (g) 2 CO 2 (g) + 4 H 2 O (g) Mix 40. 0 g of methanol with 46. 0 g of O 2 - what is the mass of CO 2 produced? Methanol, CH 3 OH, has a molar mass of 32. 04 g O 2 has a molar mass of 32. 00 g Malone and Dolter - Basic Concepts of Chemistry 9 e 18

Solution Malone and Dolter - Basic Concepts of Chemistry 9 e 19

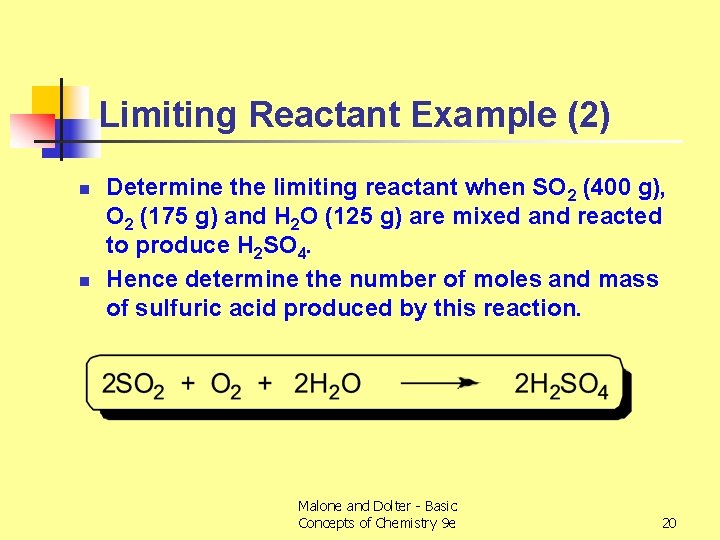
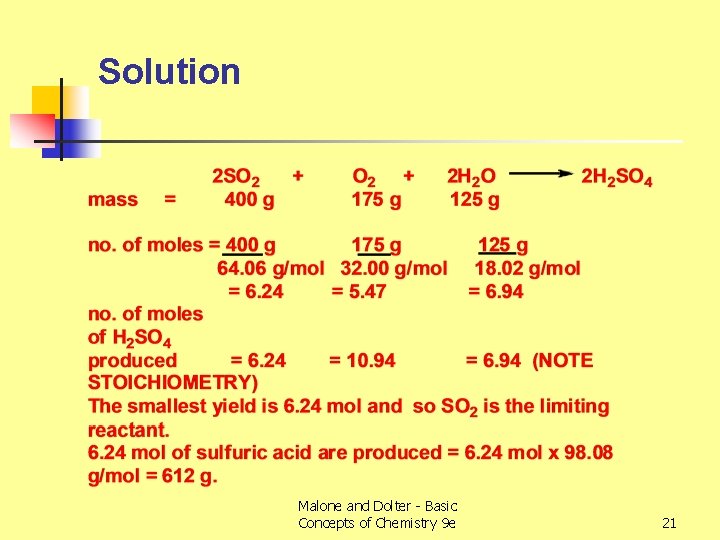
Limiting Reactant Example (2) n n Determine the limiting reactant when SO 2 (400 g), O 2 (175 g) and H 2 O (125 g) are mixed and reacted to produce H 2 SO 4. Hence determine the number of moles and mass of sulfuric acid produced by this reaction. Malone and Dolter - Basic Concepts of Chemistry 9 e 20

Solution Malone and Dolter - Basic Concepts of Chemistry 9 e 21

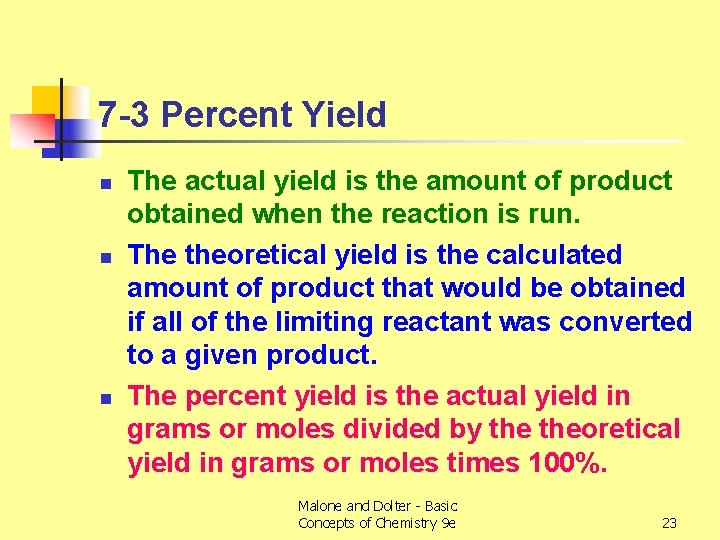
Objective for Section 7 -3 n Calculate the percent yield of a reaction from the measured actual yield and the calculated theoretical yield. Malone and Dolter - Basic Concepts of Chemistry 9 e 22

7 -3 Percent Yield n n n The actual yield is the amount of product obtained when the reaction is run. The theoretical yield is the calculated amount of product that would be obtained if all of the limiting reactant was converted to a given product. The percent yield is the actual yield in grams or moles divided by theoretical yield in grams or moles times 100%. Malone and Dolter - Basic Concepts of Chemistry 9 e 23

Incomplete Conversion n In some cases, a reverse reaction occurs whereby reactants are reformed from products. This limits the percent of reactants that are converted to products. Such reactions are known as reversible reactions. Malone and Dolter - Basic Concepts of Chemistry 9 e 24

Incomplete Conversion n The reaction between N 2 and H 2 to produce NH 3 is a reaction which is reversible. This has severe consequences for the commercial production of ammonia. Malone and Dolter - Basic Concepts of Chemistry 9 e 25

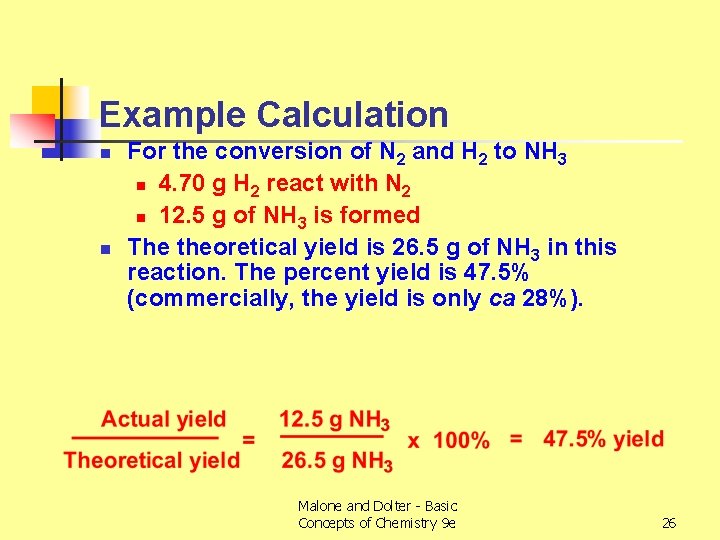
Example Calculation n n For the conversion of N 2 and H 2 to NH 3 n 4. 70 g H 2 react with N 2 n 12. 5 g of NH 3 is formed The theoretical yield is 26. 5 g of NH 3 in this reaction. The percent yield is 47. 5% (commercially, the yield is only ca 28%). Malone and Dolter - Basic Concepts of Chemistry 9 e 26

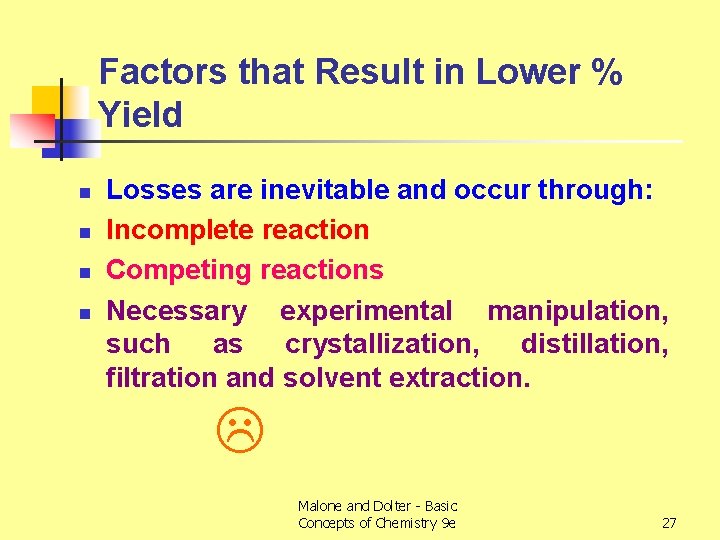
Factors that Result in Lower % Yield n n Losses are inevitable and occur through: Incomplete reaction Competing reactions Necessary experimental manipulation, such as crystallization, distillation, filtration and solvent extraction. Malone and Dolter - Basic Concepts of Chemistry 9 e 27

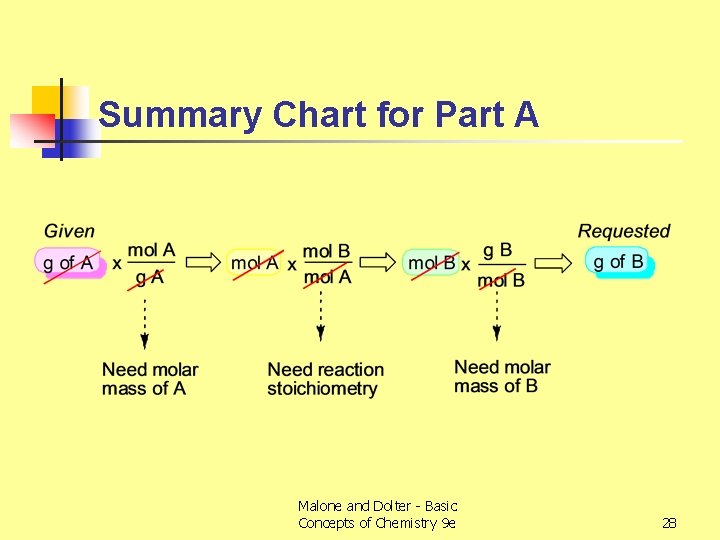
Summary Chart for Part A Malone and Dolter - Basic Concepts of Chemistry 9 e 28

Setting a Goal – Part B Energy Relationships in Chemical Reactions n You will extend your knowledge of stoichiometry to include amounts of heat involved in chemical reactions. Malone and Dolter - Basic Concepts of Chemistry 9 e 29

Objective for Section 7 -4 n Given the change in enthalpy for a reaction, calculate the amount of heat gained or released by a given mass of reactant. Malone and Dolter - Basic Concepts of Chemistry 9 e 30

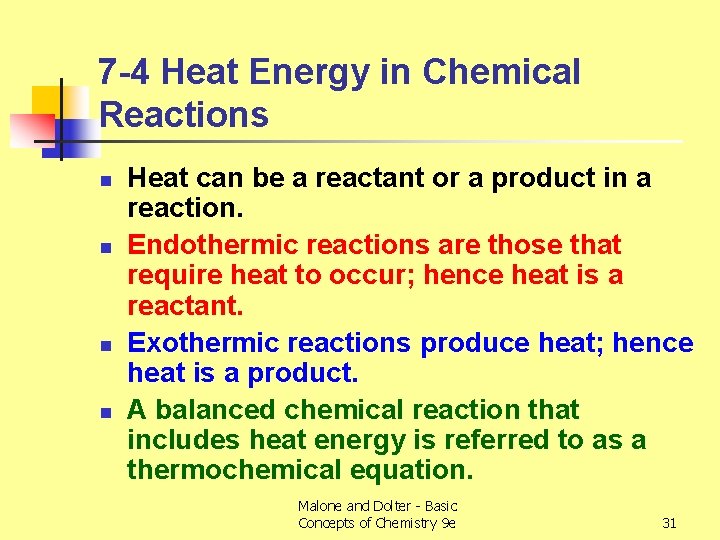
7 -4 Heat Energy in Chemical Reactions n n Heat can be a reactant or a product in a reaction. Endothermic reactions are those that require heat to occur; hence heat is a reactant. Exothermic reactions produce heat; hence heat is a product. A balanced chemical reaction that includes heat energy is referred to as a thermochemical equation. Malone and Dolter - Basic Concepts of Chemistry 9 e 31

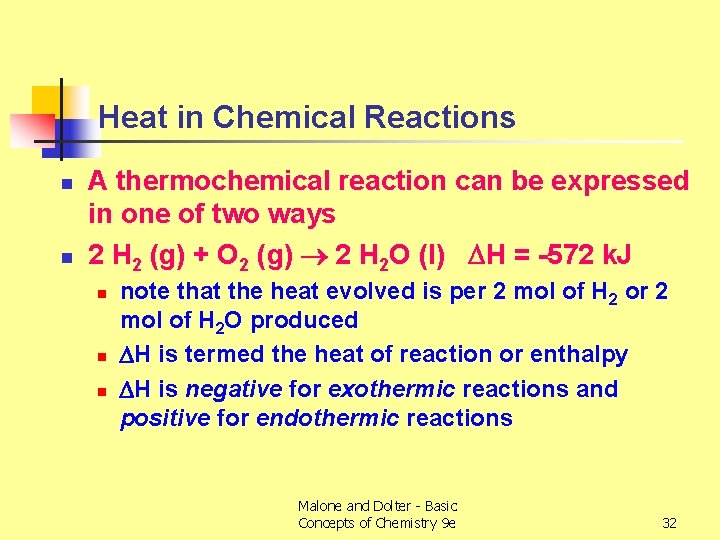
Heat in Chemical Reactions n n A thermochemical reaction can be expressed in one of two ways 2 H 2 (g) + O 2 (g) 2 H 2 O (l) H = -572 k. J n note that the heat evolved is per 2 mol of H 2 or 2 mol of H 2 O produced H is termed the heat of reaction or enthalpy H is negative for exothermic reactions and positive for endothermic reactions Malone and Dolter - Basic Concepts of Chemistry 9 e 32

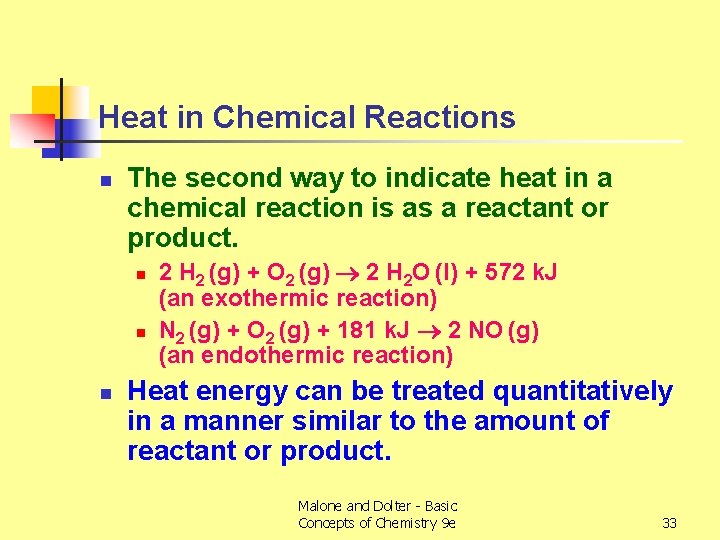
Heat in Chemical Reactions n The second way to indicate heat in a chemical reaction is as a reactant or product. n n n 2 H 2 (g) + O 2 (g) 2 H 2 O (l) + 572 k. J (an exothermic reaction) N 2 (g) + O 2 (g) + 181 k. J 2 NO (g) (an endothermic reaction) Heat energy can be treated quantitatively in a manner similar to the amount of reactant or product. Malone and Dolter - Basic Concepts of Chemistry 9 e 33

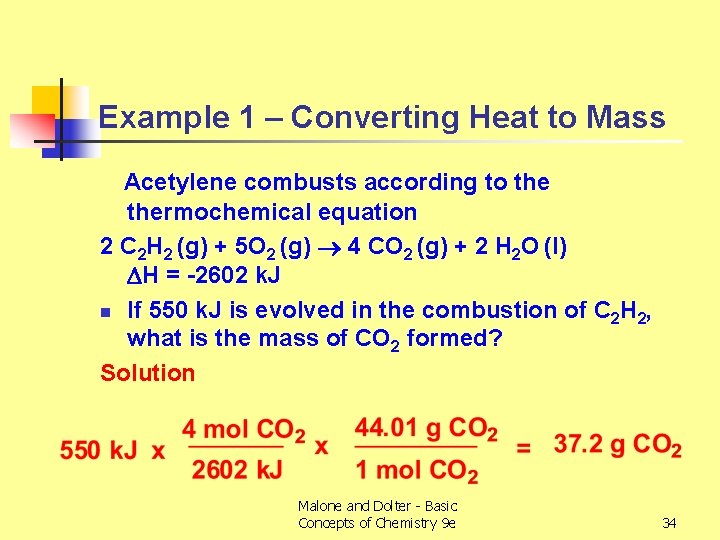
Example 1 – Converting Heat to Mass Acetylene combusts according to thermochemical equation 2 C 2 H 2 (g) + 5 O 2 (g) 4 CO 2 (g) + 2 H 2 O (l) H = -2602 k. J n If 550 k. J is evolved in the combustion of C 2 H 2, what is the mass of CO 2 formed? Solution Malone and Dolter - Basic Concepts of Chemistry 9 e 34

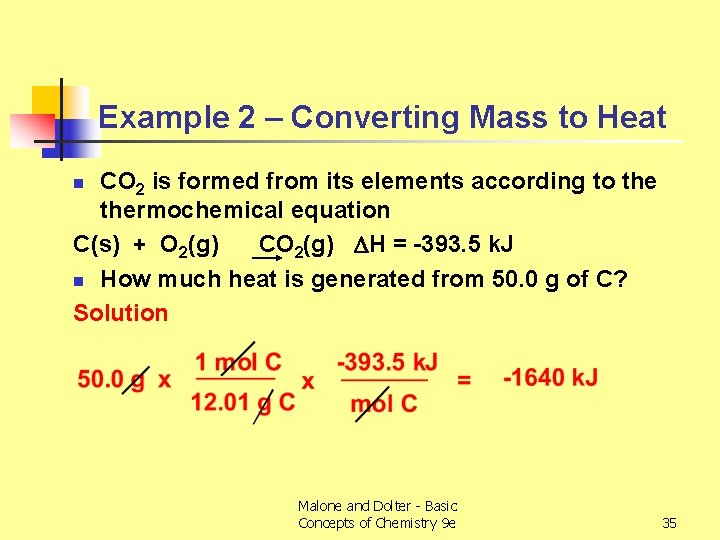
Example 2 – Converting Mass to Heat CO 2 is formed from its elements according to thermochemical equation C(s) + O 2(g) CO 2(g) H = -393. 5 k. J n How much heat is generated from 50. 0 g of C? Solution n Malone and Dolter - Basic Concepts of Chemistry 9 e 35

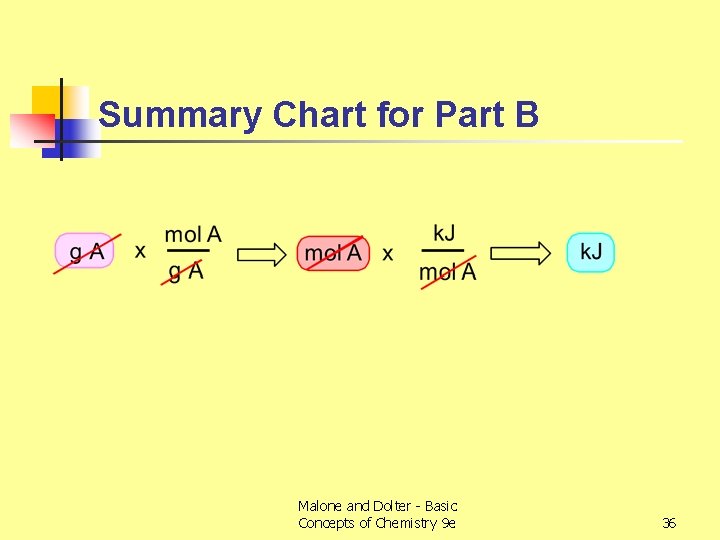
Summary Chart for Part B Malone and Dolter - Basic Concepts of Chemistry 9 e 36

Further Worked Example The complete combustion of 1 mole of octane (C 8 H 18), 1 mole of methanol, and 1 mole of methane (CH 4) evolves 5480 k. J, 1750 k. J and 890 k. J of heat, respectively. Calculate the heat evolved per gram by each of these fuels, and hence determine which is the most efficient fuel, in terms heat evolved per unit mass. Malone and Dolter - Basic Concepts of Chemistry 9 e 37

Malone and Dolter - Basic Concepts of Chemistry 9 e 38