CHAPTER 3 STOICHIOMETRY CHEMICAL EQUATIONS Stoichiometry The area
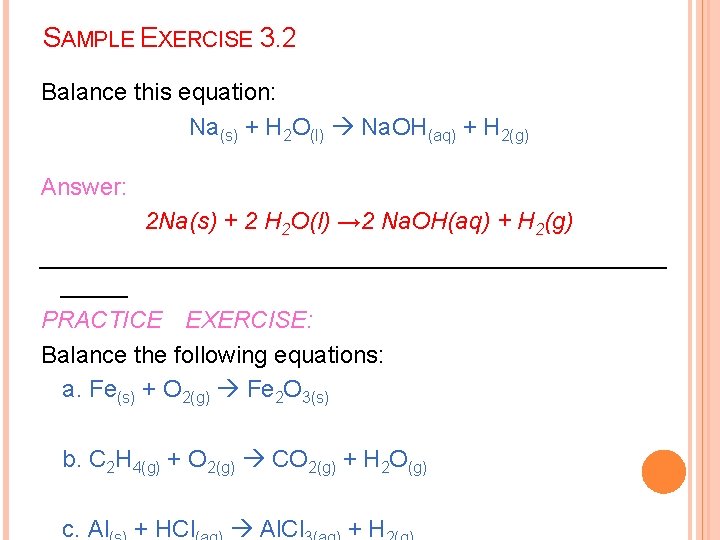













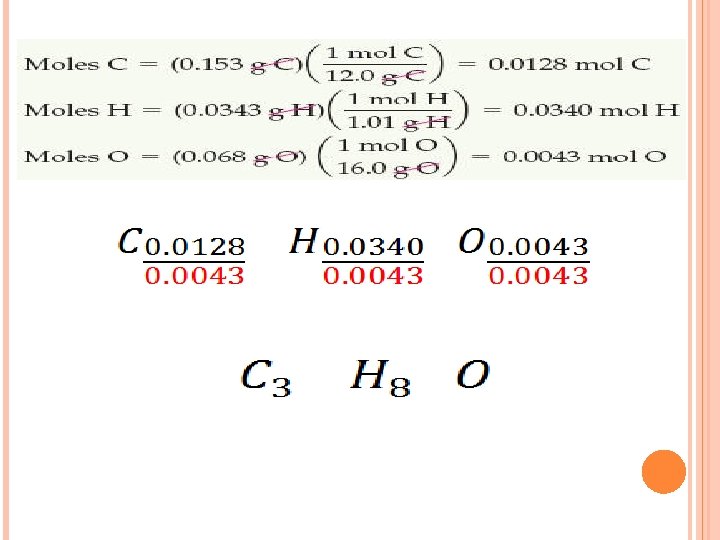






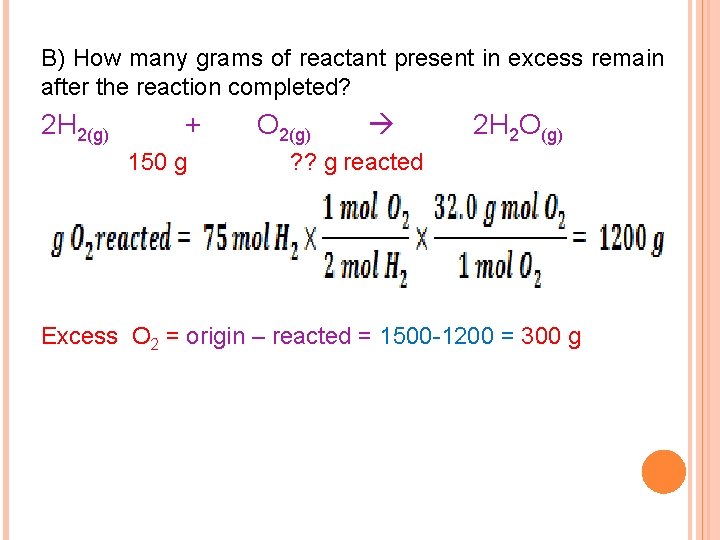


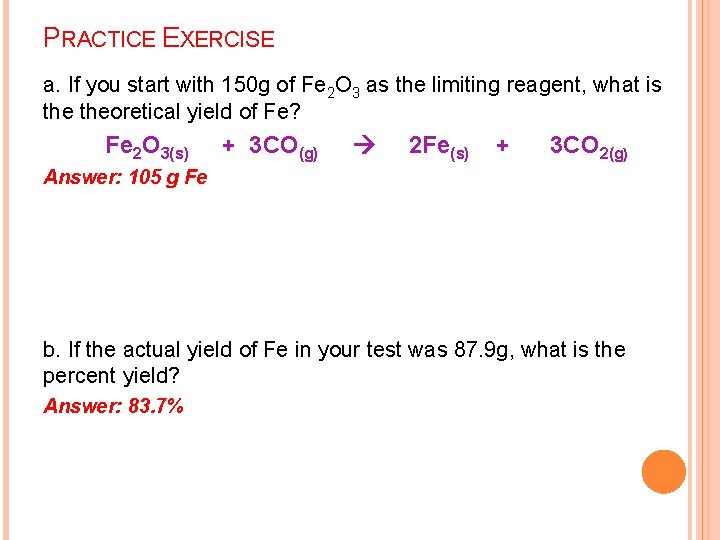


- Slides: 66

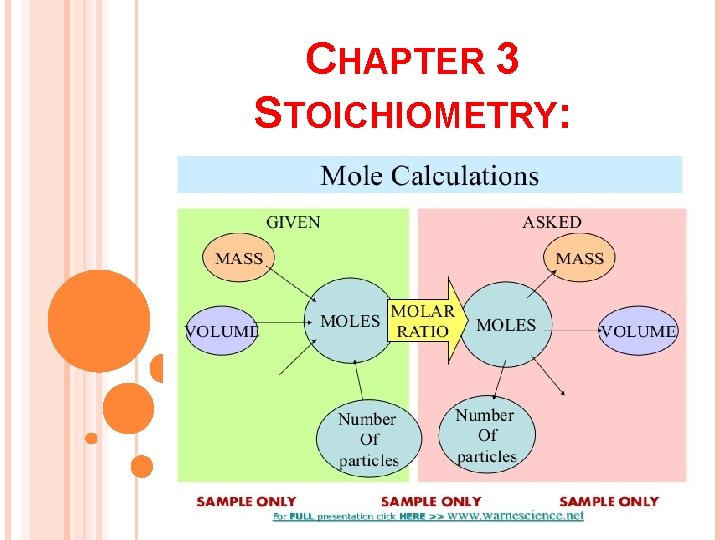
CHAPTER 3 STOICHIOMETRY:

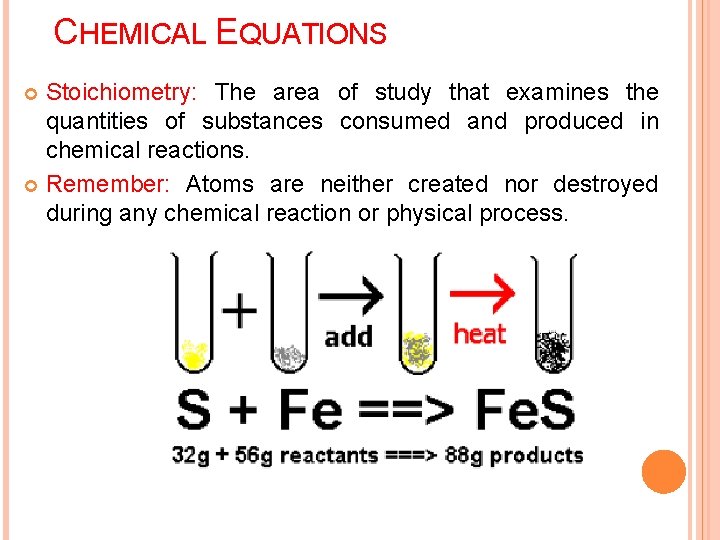
CHEMICAL EQUATIONS Stoichiometry: The area of study that examines the quantities of substances consumed and produced in chemical reactions. Remember: Atoms are neither created nor destroyed during any chemical reaction or physical process.

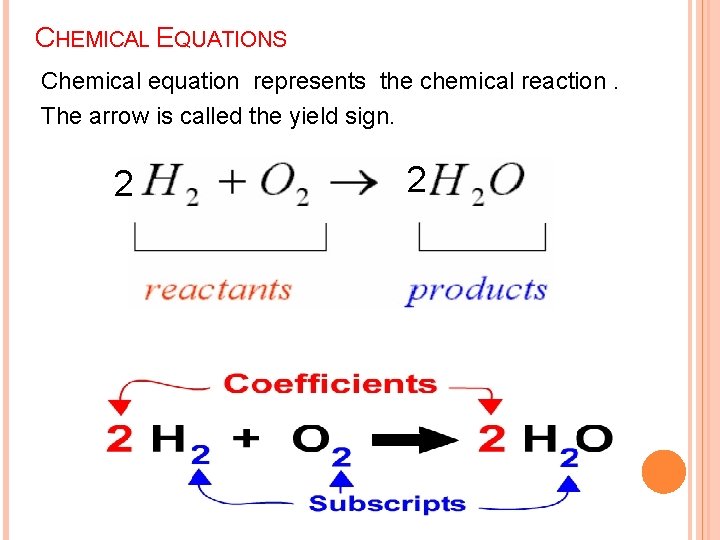
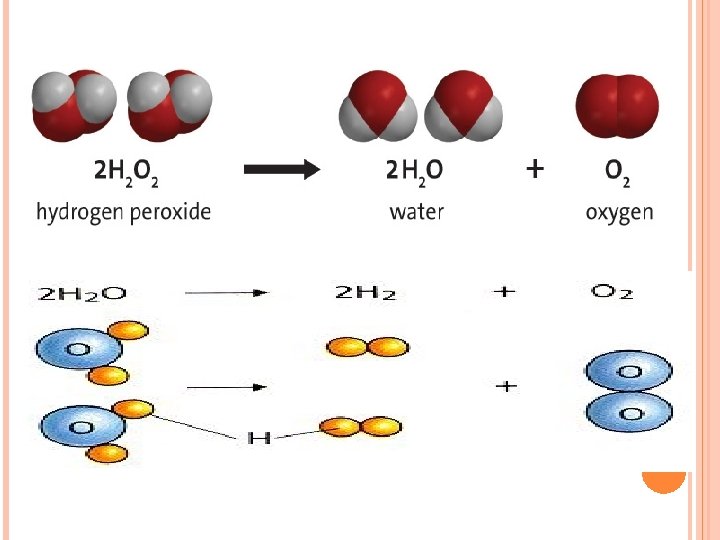
CHEMICAL EQUATIONS Chemical equation represents the chemical reaction. The arrow is called the yield sign. 2 2

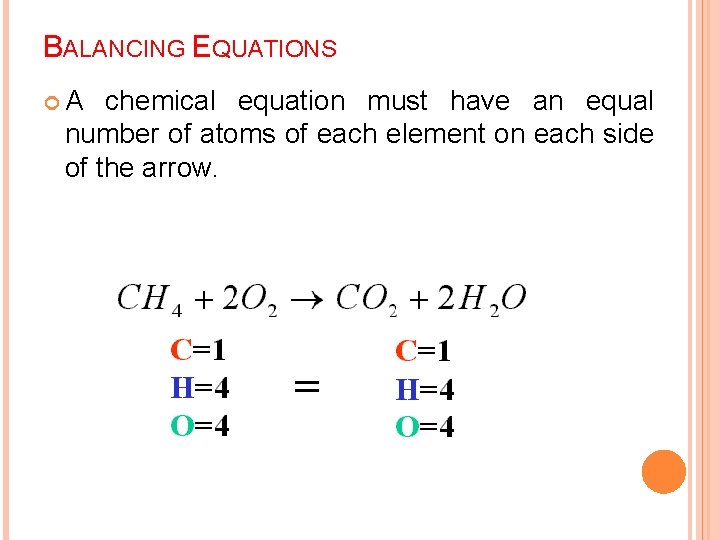
BALANCING EQUATIONS A chemical equation must have an equal number of atoms of each element on each side of the arrow.

RULES/HINTS FOR BALANCING EQUATIONS 1) Use correct formulas. 2) The coefficients must be the lowest whole-numbers possible. 3) Do not change or add subscripts (number of atoms of each element in a molecule). 4) Balance H and O last. 5) If a polyatomic ion stays together on both sides of the arrow, then keep it together when balancing. 2 HCl + Na 2 CO 3 2 Na. Cl + H 2 O + CO 2 C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O

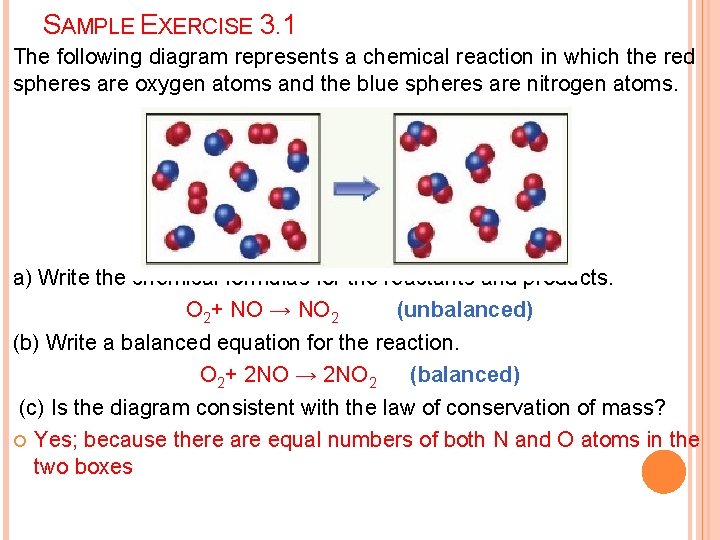
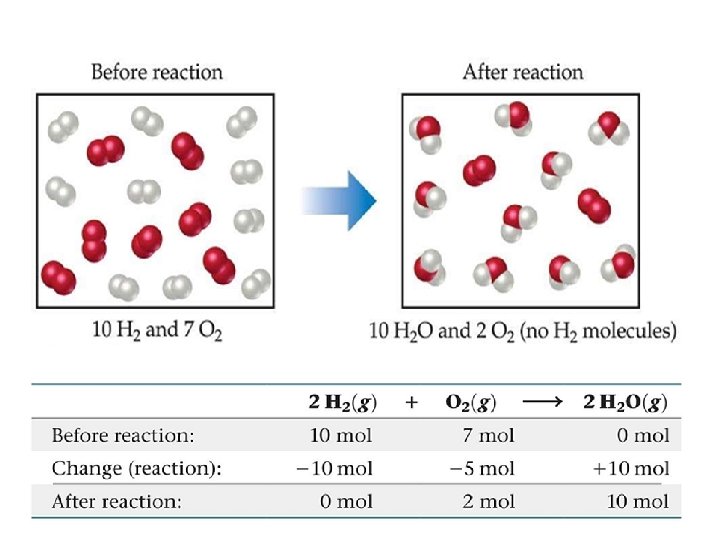
SAMPLE EXERCISE 3. 1 The following diagram represents a chemical reaction in which the red spheres are oxygen atoms and the blue spheres are nitrogen atoms. a) Write the chemical formulas for the reactants and products. O 2+ NO → NO 2 (unbalanced) (b) Write a balanced equation for the reaction. O 2+ 2 NO → 2 NO 2 (balanced) (c) Is the diagram consistent with the law of conservation of mass? Yes; because there are equal numbers of both N and O atoms in the two boxes

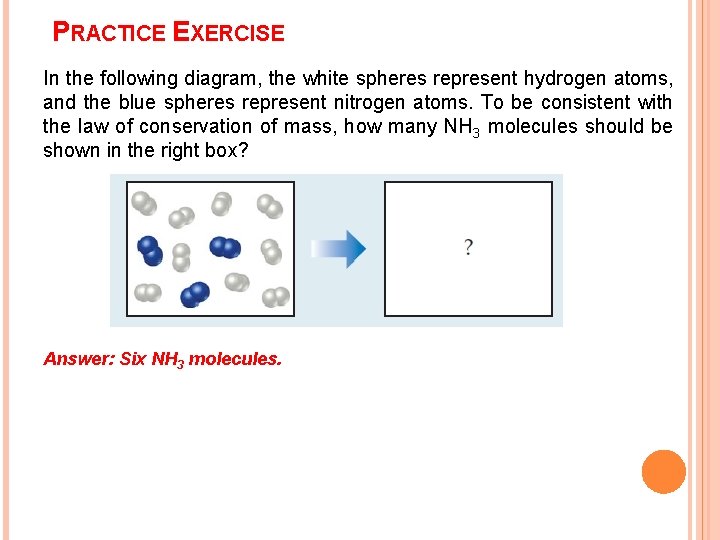
PRACTICE EXERCISE In the following diagram, the white spheres represent hydrogen atoms, and the blue spheres represent nitrogen atoms. To be consistent with the law of conservation of mass, how many NH 3 molecules should be shown in the right box? Answer: Six NH 3 molecules.

STATES OF MATTER v. We use the symbols (g) for gas, (l) for liquid, (s) for solid, and (aq) for aqueous to identify the states of matter of the reactants and products. v. Catalysts, photons (h ), or heat ( ) may stand above the reaction arrow ( ). Ca. CO 3(s) + 2 HCl(aq) Ca. Cl 2(aq) + CO 2(g) + H 2 O(l)
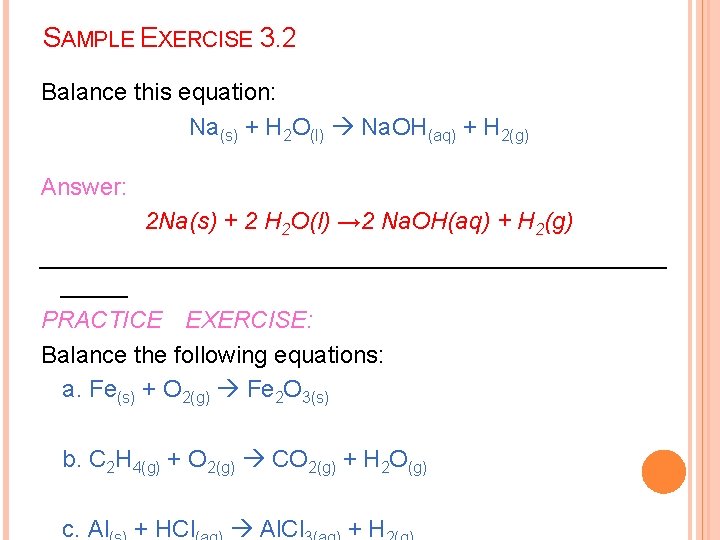
SAMPLE EXERCISE 3. 2 Balance this equation: Na(s) + H 2 O(l) Na. OH(aq) + H 2(g) Answer: 2 Na(s) + 2 H 2 O(l) → 2 Na. OH(aq) + H 2(g) ________________________ PRACTICE EXERCISE: Balance the following equations: a. Fe(s) + O 2(g) Fe 2 O 3(s) b. C 2 H 4(g) + O 2(g) CO 2(g) + H 2 O(g) c. Al + HCl Al. Cl +H

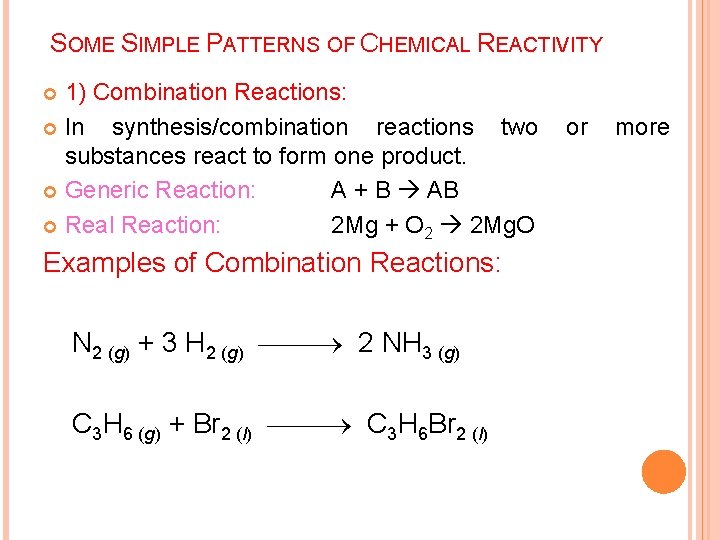
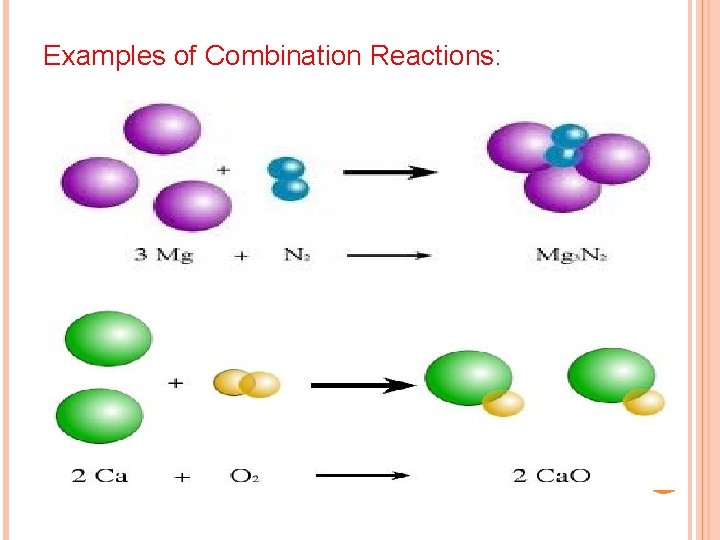
SOME SIMPLE PATTERNS OF CHEMICAL REACTIVITY 1) Combination Reactions: In synthesis/combination reactions two substances react to form one product. Generic Reaction: A + B AB Real Reaction: 2 Mg + O 2 2 Mg. O Examples of Combination Reactions: N 2 (g) + 3 H 2 (g) 2 NH 3 (g) C 3 H 6 (g) + Br 2 (l) C 3 H 6 Br 2 (l) or more

Examples of Combination Reactions:

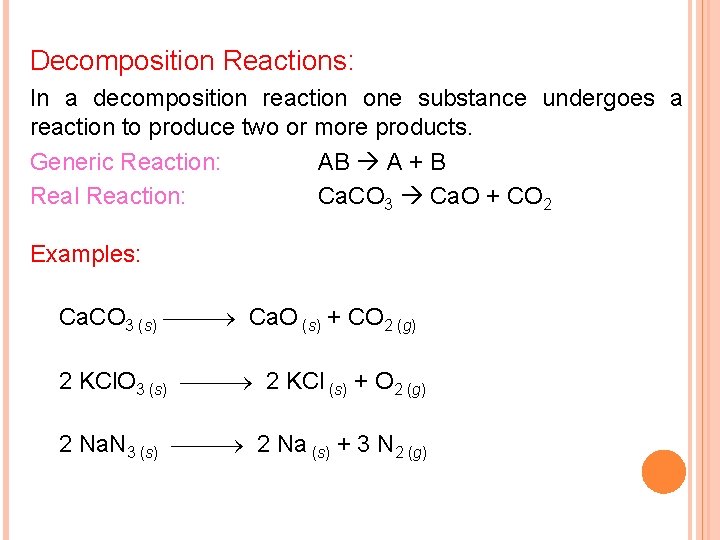
Decomposition Reactions: In a decomposition reaction one substance undergoes a reaction to produce two or more products. Generic Reaction: AB A + B Real Reaction: Ca. CO 3 Ca. O + CO 2 Examples: Ca. CO 3 (s) Ca. O (s) + CO 2 (g) 2 KCl. O 3 (s) 2 KCl (s) + O 2 (g) 2 Na. N 3 (s) 2 Na (s) + 3 N 2 (g)


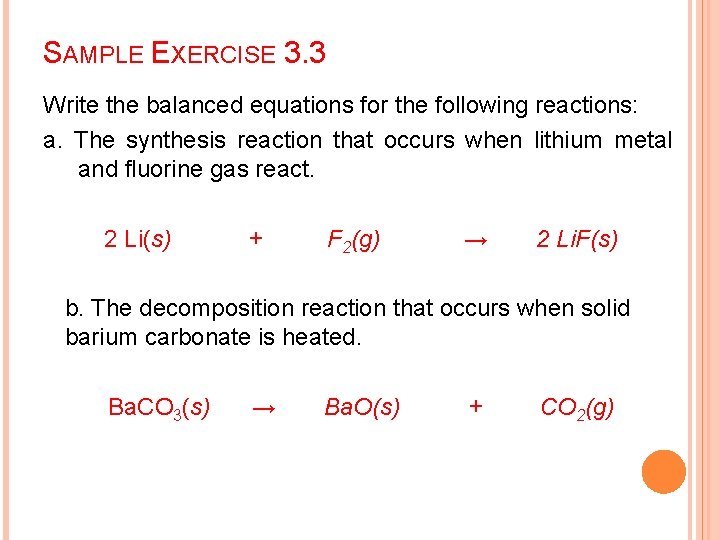
SAMPLE EXERCISE 3. 3 Write the balanced equations for the following reactions: a. The synthesis reaction that occurs when lithium metal and fluorine gas react. 2 Li(s) + F 2(g) → 2 Li. F(s) b. The decomposition reaction that occurs when solid barium carbonate is heated. Ba. CO 3(s) → Ba. O(s) + CO 2(g)

PRACTICE EXERCISE Write the balance equation for the following reactions: a. Solid mercury (II) sulfide decomposes into its elements when heated. b. The surface of aluminum metal undergoes a combination reaction with oxygen in the air. Answer: (a) Hg. S(s) → Hg(l) + S(s) (b)4 Al(s) + 3 O 2(g) → 2 Al 2 O 3(s)

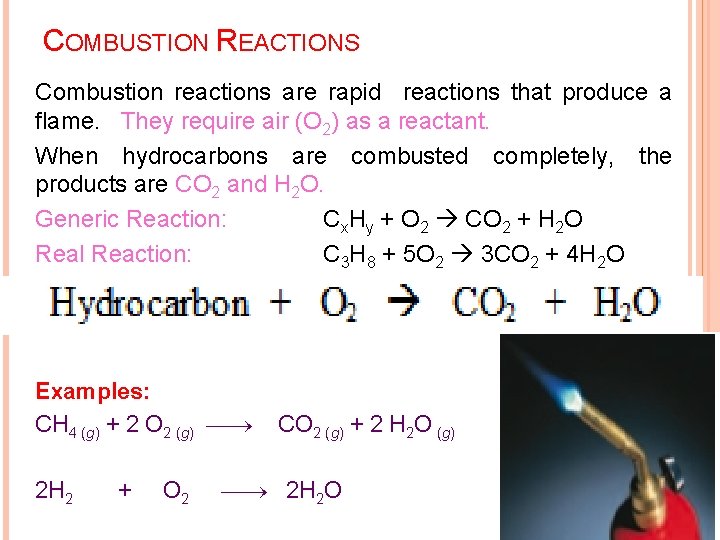
COMBUSTION REACTIONS Combustion reactions are rapid reactions that produce a flame. They require air (O 2) as a reactant. When hydrocarbons are combusted completely, the products are CO 2 and H 2 O. Generic Reaction: Cx. Hy + O 2 CO 2 + H 2 O Real Reaction: C 3 H 8 + 5 O 2 3 CO 2 + 4 H 2 O Examples: CH 4 (g) + 2 O 2 (g) 2 H 2 + O 2 CO 2 (g) + 2 H 2 O (g) 2 H 2 O

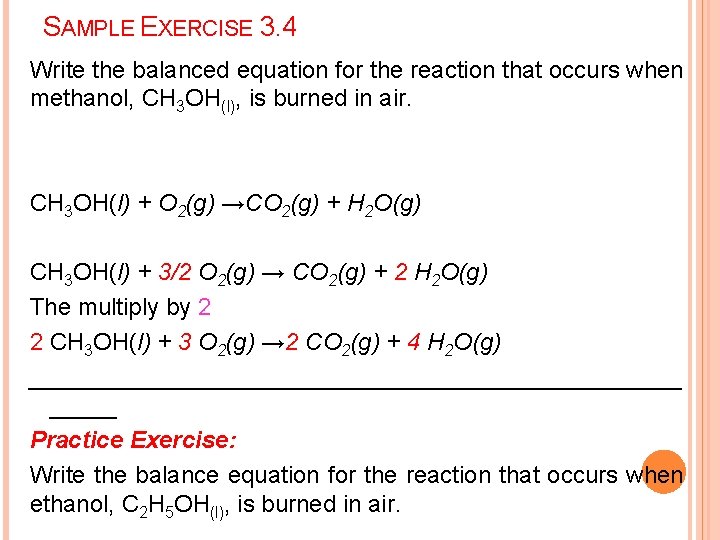
SAMPLE EXERCISE 3. 4 Write the balanced equation for the reaction that occurs when methanol, CH 3 OH(l), is burned in air. CH 3 OH(l) + O 2(g) →CO 2(g) + H 2 O(g) CH 3 OH(l) + 3/2 O 2(g) → CO 2(g) + 2 H 2 O(g) The multiply by 2 2 CH 3 OH(l) + 3 O 2(g) → 2 CO 2(g) + 4 H 2 O(g) _________________________ Practice Exercise: Write the balance equation for the reaction that occurs when ethanol, C 2 H 5 OH(l), is burned in air.

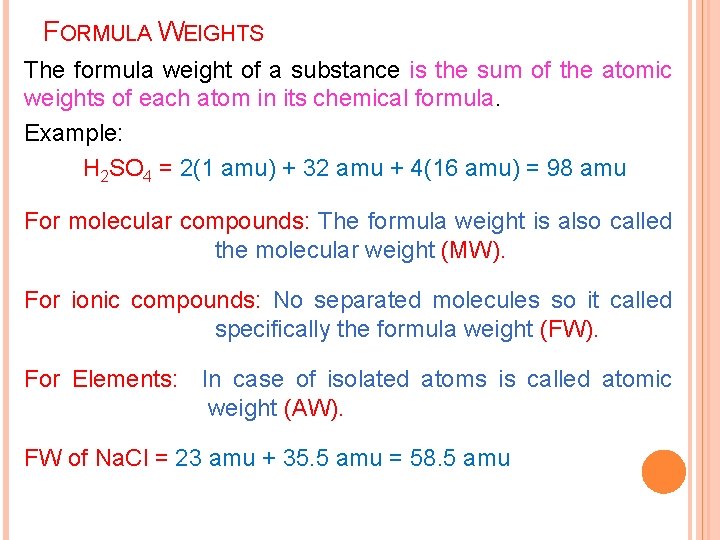
FORMULA WEIGHTS The formula weight of a substance is the sum of the atomic weights of each atom in its chemical formula. Example: H 2 SO 4 = 2(1 amu) + 32 amu + 4(16 amu) = 98 amu For molecular compounds: The formula weight is also called the molecular weight (MW). For ionic compounds: No separated molecules so it called specifically the formula weight (FW). For Elements: In case of isolated atoms is called atomic weight (AW). FW of Na. Cl = 23 amu + 35. 5 amu = 58. 5 amu

SAMPLE EXERCISE 3. 5 Calculate the formula weight of a. sucrose (C 12 H 22 O 11) MW or (FW) of sucrose (C 12 H 22 O 11) = 12(12 amu) + 22(1 amu) + 11(16 amu) = 342 amu b. Calcium nitrate. FW Ca(NO 3)2 = 40. 1 amu) + 2[14 amu+3(16 amu)] = 164. 1 amu ___________________________ Practice Exercise: Calculate the formula weight of (a) Al(OH)3 and (b) CH 3 OH. Answer: (a) 78. 0 amu, (b) 32. 0 amu

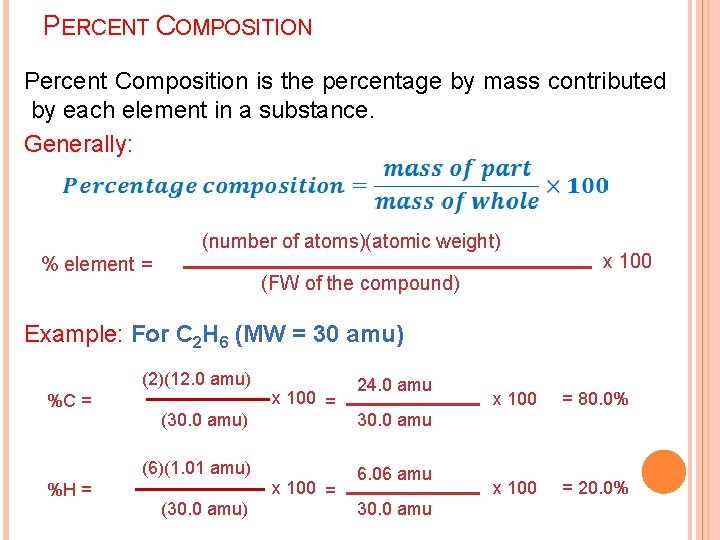
PERCENT COMPOSITION Percent Composition is the percentage by mass contributed by each element in a substance. Generally: (number of atoms)(atomic weight) % element = (FW of the compound) x 100 Example: For C 2 H 6 (MW = 30 amu) (2)(12. 0 amu) %C = (30. 0 amu) (6)(1. 01 amu) %H = (30. 0 amu) x 100 = 24. 0 amu x 100 = 80. 0% x 100 = 20. 0% 30. 0 amu 6. 06 amu 30. 0 amu

SAMPLE EXERCISE 3. 6 Calculate the percent of carbon, hydrogen, and oxygen (by mass) in C 12 H 22 O 11. Solution: __________________________ Practice Exercise: Calculate the percentage of nitrogen, by mass, in calcium nitrate. Answer: 17. 1%

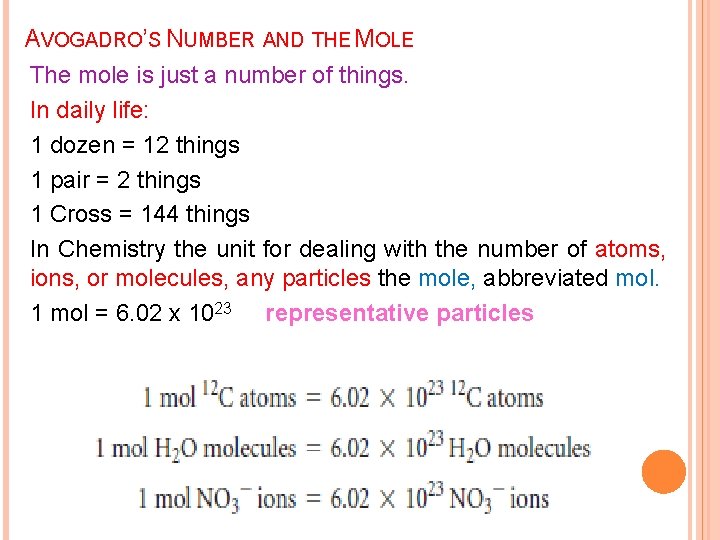
AVOGADRO’S NUMBER AND THE MOLE The mole is just a number of things. In daily life: 1 dozen = 12 things 1 pair = 2 things 1 Cross = 144 things In Chemistry the unit for dealing with the number of atoms, ions, or molecules, any particles the mole, abbreviated mol. 1 mol = 6. 02 x 1023 representative particles

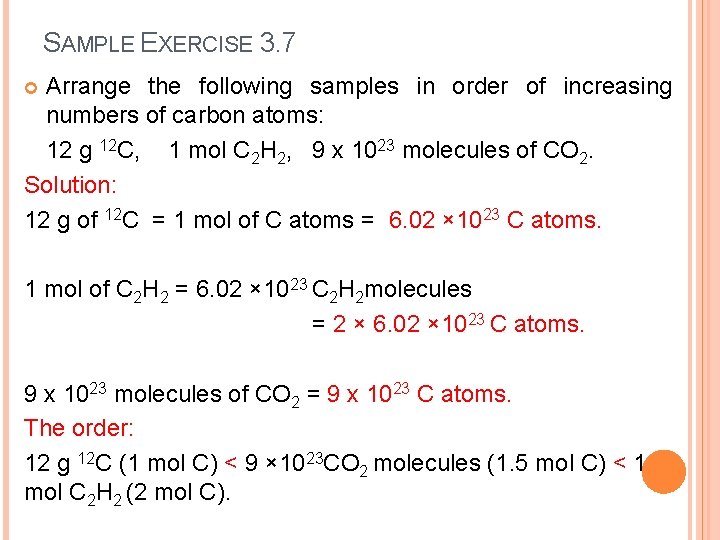
SAMPLE EXERCISE 3. 7 Arrange the following samples in order of increasing numbers of carbon atoms: 12 g 12 C, 1 mol C 2 H 2, 9 x 1023 molecules of CO 2. Solution: 12 g of 12 C = 1 mol of C atoms = 6. 02 × 1023 C atoms. 1 mol of C 2 H 2 = 6. 02 × 1023 C 2 H 2 molecules = 2 × 6. 02 × 1023 C atoms. 9 x 1023 molecules of CO 2 = 9 x 1023 C atoms. The order: 12 g 12 C (1 mol C) < 9 × 1023 CO 2 molecules (1. 5 mol C) < 1 mol C 2 H 2 (2 mol C).

PRACTICE EXERCISE Arrange the following samples in order of increasing number of O atoms: 1 mol H 2 O, 1 mol CO 2, 3 x 1023 molecules O 3. Answer: 1 mol H 2 O (6 × 1023 O atoms) < 3 × 1023 molecules O 3(9 × 1023 O atoms) < 1 mol CO 2(12 × 1023 O atoms)

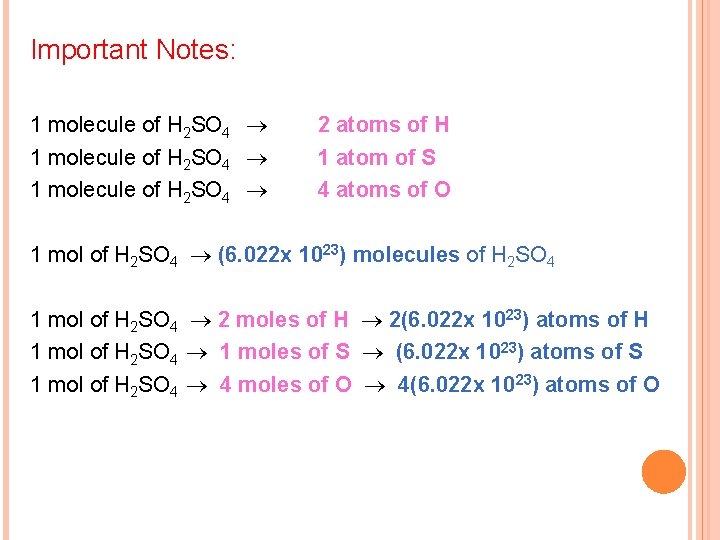
Important Notes: 1 molecule of H 2 SO 4 2 atoms of H 1 atom of S 4 atoms of O 1 mol of H 2 SO 4 (6. 022 x 1023) molecules of H 2 SO 4 1 mol of H 2 SO 4 2 moles of H 2(6. 022 x 1023) atoms of H 1 mol of H 2 SO 4 1 moles of S (6. 022 x 1023) atoms of S 1 mol of H 2 SO 4 4 moles of O 4(6. 022 x 1023) atoms of O

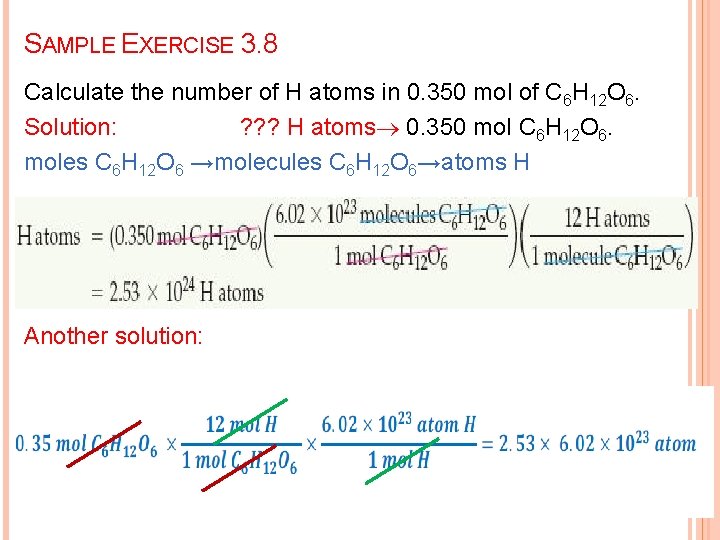
SAMPLE EXERCISE 3. 8 Calculate the number of H atoms in 0. 350 mol of C 6 H 12 O 6. Solution: ? ? ? H atoms 0. 350 mol C 6 H 12 O 6. moles C 6 H 12 O 6 →molecules C 6 H 12 O 6→atoms H Another solution:

PRACTICE EXERCISE How many oxygen atoms are in: a. 0. 25 mol of calcium nitrate? Answer: 9. 0 × 1023. b. 1. 50 mol of sodium carbonate? Answer: 2. 71 × 1024

MOLAR MASS A mole is always the same number (6. 02 x 1023). v But 1 mole sample of different substances will have different masses. v A mol of an element = AW (amu) in grams of that element. v The mole in grams of substance called the molar mass of the substance (g/mol). v Each is 1 mole of different elements have different masses but all contain same number of atoms = 6. 02 x 1023

SAMPLE EXERCISE 3. 9 What is the mass in grams of 1. 000 mol of glucose, C 6 H 12 O 6? Solution: MW glucose C 6 H 12 O 6 = 6(12 amu) + 12(1 amu) + 6(16 amu) = 180 amu So 1 mole of glucose = 180 g And the molar mass = 180 g/mol _________________________ Practice Exercise: Calculate the molar mass of calcium nitrate. Answer: 164. 1 g/mol

SAMPLE EXERCISE 3. 10 Calculate the number of moles of glucose in 5. 380 g of glucose. ? ? mol Glucose 5. 380 g Glucose Conversion factor: 1 mol Gl 180 g Gl _________________________ Practice Exercise: How many moles of sodium bicarbonate are in 508 g of sodium bicarbonate (also known as sodium hydrogen carbonate)? Answer: 6. 05 mol Na. HCO 3

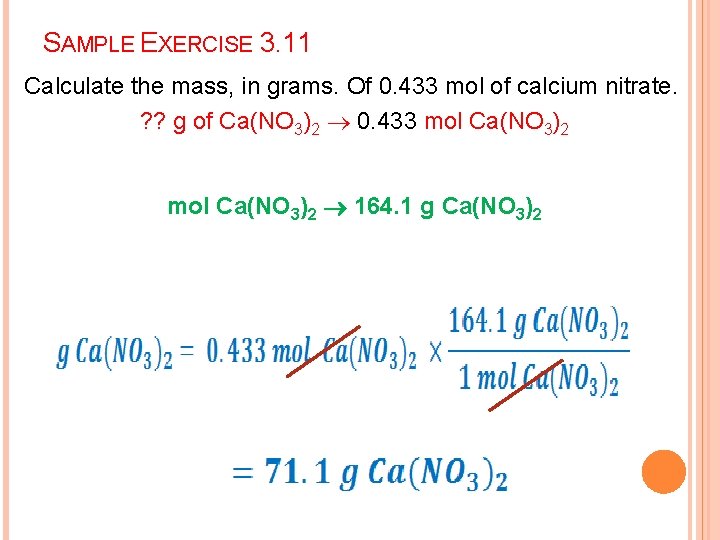
SAMPLE EXERCISE 3. 11 Calculate the mass, in grams. Of 0. 433 mol of calcium nitrate. ? ? g of Ca(NO 3)2 0. 433 mol Ca(NO 3)2 164. 1 g Ca(NO 3)2

PRACTICE EXERCISE What is the mass, in grams, of a. 6. 33 mol sodium bicarbonate? b. 3. 0 x 10 -5 mol of sulfuric acid? Answer: 532 g Answer: 2. 9 × 10– 3 g

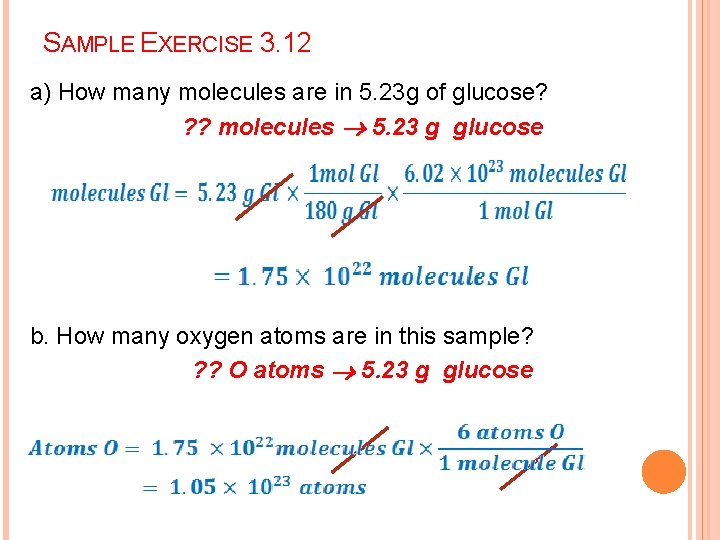
SAMPLE EXERCISE 3. 12 a) How many molecules are in 5. 23 g of glucose? ? ? molecules 5. 23 g glucose b. How many oxygen atoms are in this sample? ? ? O atoms 5. 23 g glucose

PRACTICE EXERCISE a) How many molecules are in 4. 20 g of nitric acid? Answer: 4. 01 × 1022 molecules HNO 3 b) How many O atoms are in this sample? Answer: 1. 20 × 1023 atoms O

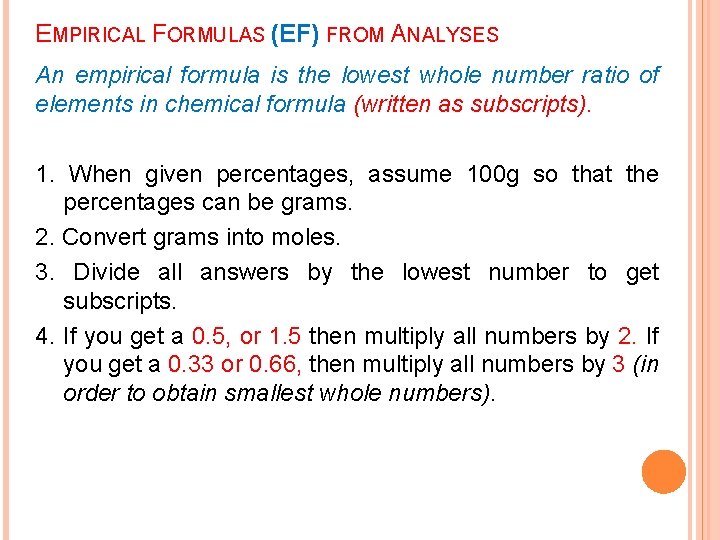
EMPIRICAL FORMULAS (EF) FROM ANALYSES An empirical formula is the lowest whole number ratio of elements in chemical formula (written as subscripts). 1. When given percentages, assume 100 g so that the percentages can be grams. 2. Convert grams into moles. 3. Divide all answers by the lowest number to get subscripts. 4. If you get a 0. 5, or 1. 5 then multiply all numbers by 2. If you get a 0. 33 or 0. 66, then multiply all numbers by 3 (in order to obtain smallest whole numbers).

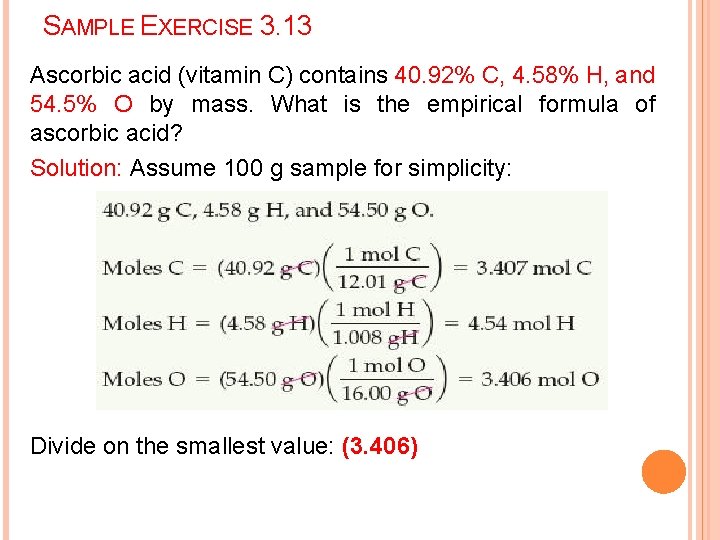
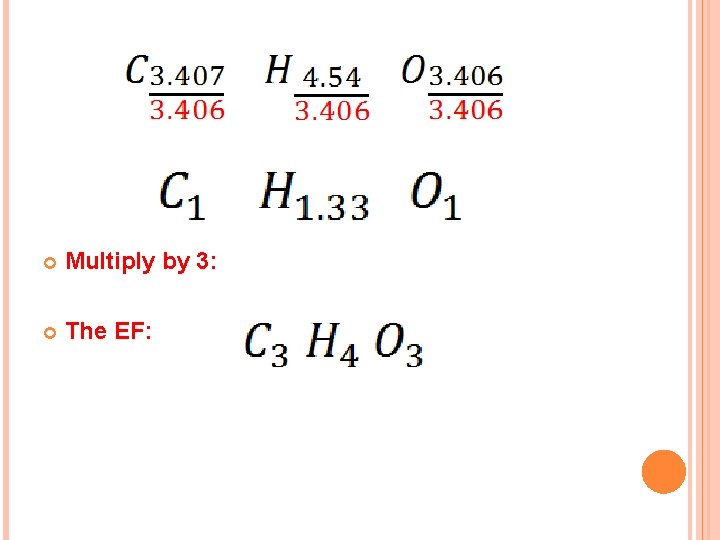
SAMPLE EXERCISE 3. 13 Ascorbic acid (vitamin C) contains 40. 92% C, 4. 58% H, and 54. 5% O by mass. What is the empirical formula of ascorbic acid? Solution: Assume 100 g sample for simplicity: Divide on the smallest value: (3. 406)

Multiply by 3: The EF:

PRACTICE EXERCISE A 5. 325 g sample of methyl benzoate, a compound used in perfumes, contains 3. 758 g C, 0. 316 g H, and 1. 251 g of O. What is the empirical formula of this substance? Answer: C 4 H 4 O

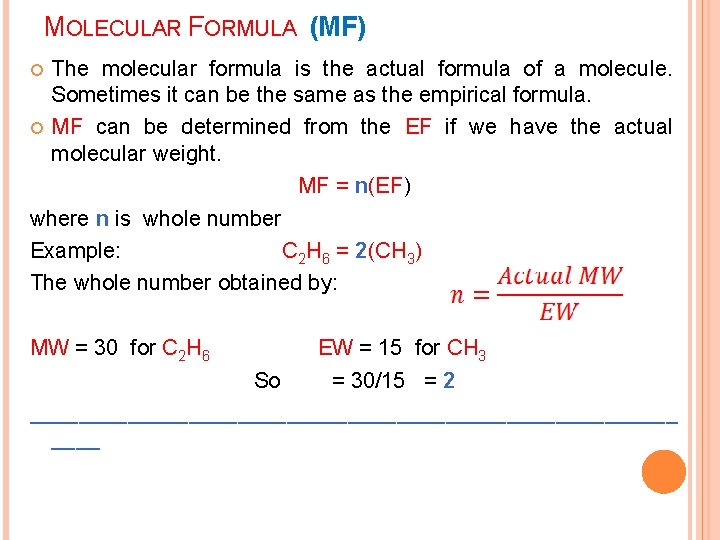
MOLECULAR FORMULA (MF) The molecular formula is the actual formula of a molecule. Sometimes it can be the same as the empirical formula. MF can be determined from the EF if we have the actual molecular weight. MF = n(EF) where n is whole number Example: C 2 H 6 = 2(CH 3) The whole number obtained by: MW = 30 for C 2 H 6 EW = 15 for CH 3 So = 30/15 = 2 ___________________________

In the previous example 3. 13: If the actual MW of ascorbic acid = 176 what is the MF of the acid? EF was : C 3 H 4 O 3 EW [3(12) +4(1)+3(16) ]= 88 Actual MW 176 n = 176/88 = 2 MF = 2 (C 3 H 4 O 3) = C 6 H 8 O 6 ________________________ ___

SAMPLE EXERCISE 3. 14 Mesitylene, a hydrocarbon in crude oil, has an empirical formula of C 3 H 4. The molecular weight of the substance is 121 amu. What is the molecular formula of mesitylene? EF : C 3 H 4 EW [3(12) +4(1)]=40 Actual MW 121 n = 121/40 = 3. 02 MF = 3 (C 3 H 4) = C 9 H 12

PRACTICE EXERCISE Ethylene glycol, the substance used in antifreeze, is composed of 38. 7%C, 9. 7% H, and 51. 6% O by mass. Its molar mass is 62. 1 g/mol. a) What is the empirical formula of ethylene glycol? Answers: CH 3 O b. What is the molecular formula for the previous question? Answers: C 2 H 6 O 2

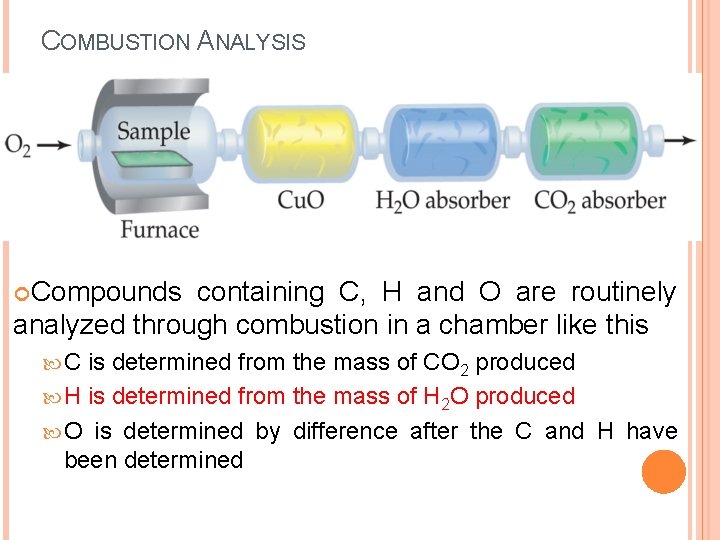
COMBUSTION ANALYSIS Compounds containing C, H and O are routinely analyzed through combustion in a chamber like this C is determined from the mass of CO 2 produced H is determined from the mass of H 2 O produced O is determined by difference after the C and H have been determined

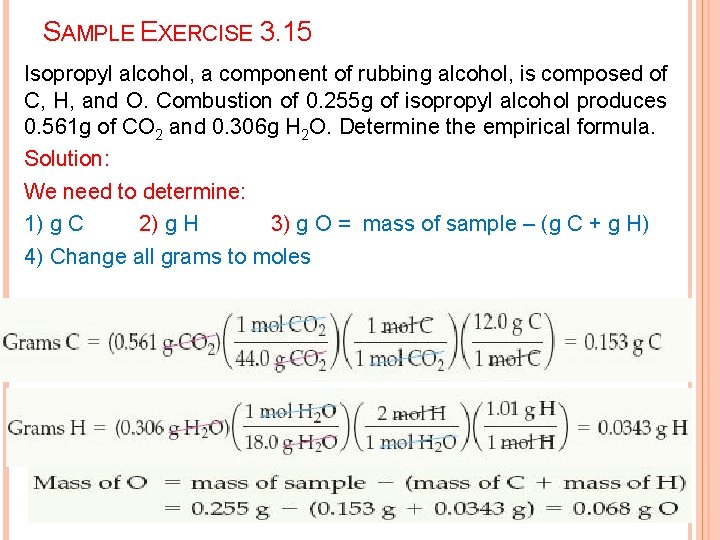
SAMPLE EXERCISE 3. 15 Isopropyl alcohol, a component of rubbing alcohol, is composed of C, H, and O. Combustion of 0. 255 g of isopropyl alcohol produces 0. 561 g of CO 2 and 0. 306 g H 2 O. Determine the empirical formula. Solution: We need to determine: 1) g C 2) g H 3) g O = mass of sample – (g C + g H) 4) Change all grams to moles
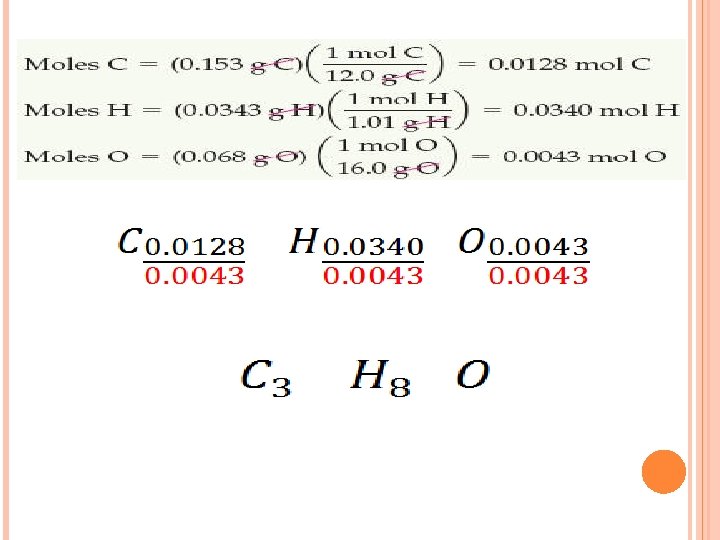

PRACTICE EXERCISE (b) Caproic acid, the smell in dirty socks, is composed of C, H, and O atoms. Combustion of a 0. 225 g sample of this compound produces 0. 512 g CO 2, 0. 209 g H 2 O. What is the empirical formula of caproic acid? Answers: C 3 H 6 O (b) Caproic acid has a molar mass of 116 g/mol. What is the molecular formula? Answer: C 6 H 12 O 2

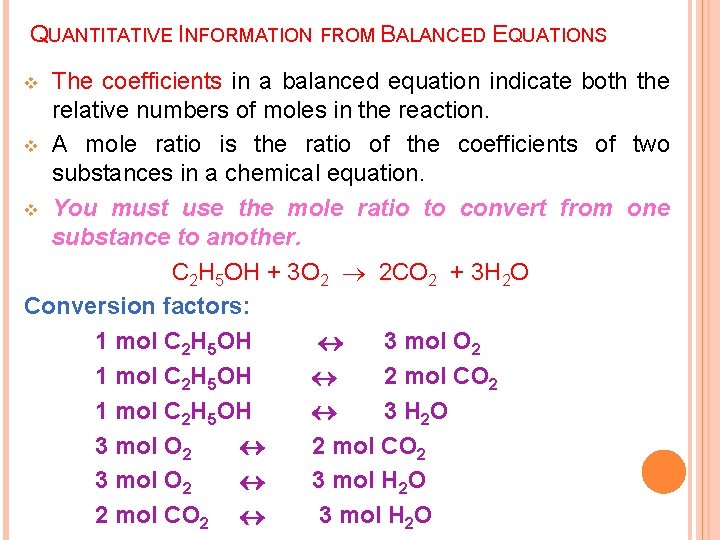
QUANTITATIVE INFORMATION FROM BALANCED EQUATIONS The coefficients in a balanced equation indicate both the relative numbers of moles in the reaction. v A mole ratio is the ratio of the coefficients of two substances in a chemical equation. v You must use the mole ratio to convert from one substance to another. C 2 H 5 OH + 3 O 2 2 CO 2 + 3 H 2 O Conversion factors: 1 mol C 2 H 5 OH 3 mol O 2 1 mol C 2 H 5 OH 2 mol CO 2 1 mol C 2 H 5 OH 3 H 2 O 3 mol O 2 2 mol CO 2 3 mol O 2 3 mol H 2 O 2 mol CO 2 3 mol H 2 O v

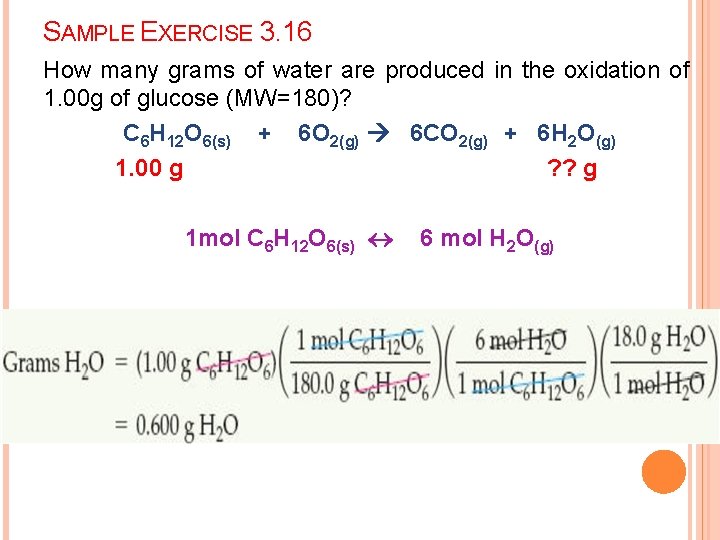
SAMPLE EXERCISE 3. 16 How many grams of water are produced in the oxidation of 1. 00 g of glucose (MW=180)? C 6 H 12 O 6(s) + 6 O 2(g) 6 CO 2(g) + 6 H 2 O(g) 1. 00 g ? ? g 1 mol C 6 H 12 O 6(s) 6 mol H 2 O(g)

PRACTICE EXERCISE How many grams of O 2 can be prepared from 4. 50 g KCl. O 3? 2 KCl. O 3(s) 2 KCl(s) + 3 O 2(g) Answer: 1. 77 g

SAMPLE EXERCISE 3. 17 Lithium hydroxide reacts with carbon dioxide to form lithium carbonate and water. How many grams of carbon dioxide (MW = 44) can be absorbed by 1. 00 g of lithium hydroxide (FW = 23. 95)? Solution: 2 Li. OH(s) 1. 00 g + CO 2(g) → Li 2 CO 3(s) + H 2 O(l) ? ? g 2 mol Li. OH(s) 1 mol CO 2(g) Grams Li. OH →moles CO 2 →grams CO 2

PRACTICE EXERCISE What mass of O 2 is consumed in the combustion of 1. 00 g of propane (C 3 H 8)? Answer: 3. 64 g

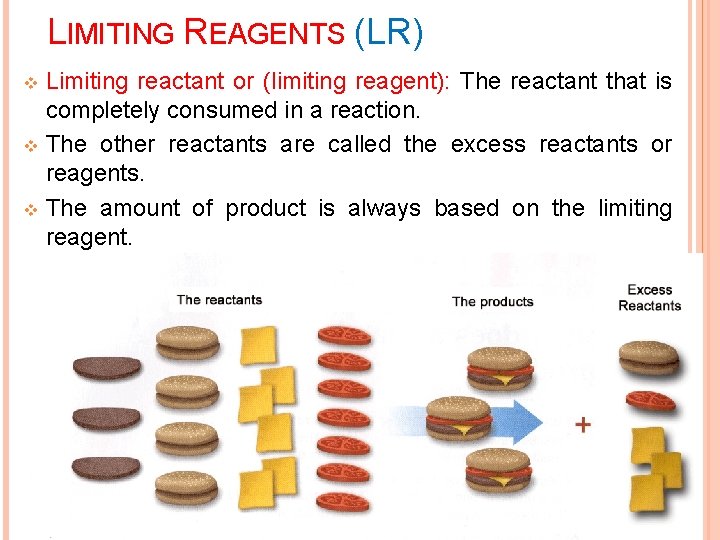
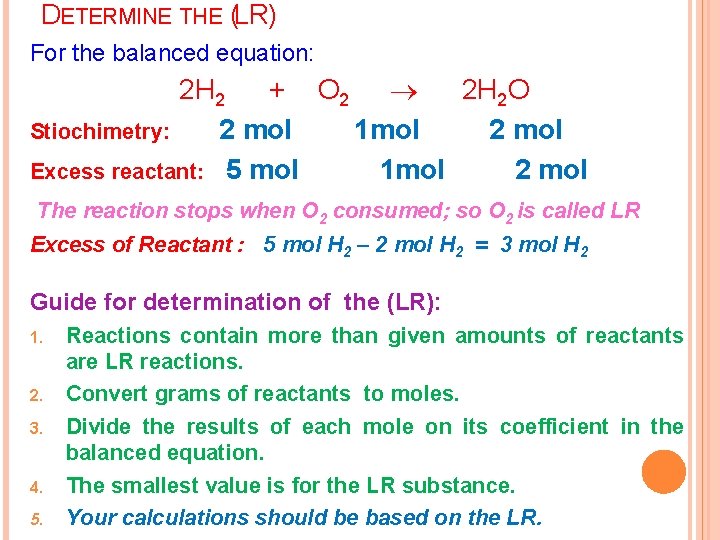
LIMITING REAGENTS (LR) Limiting reactant or (limiting reagent): The reactant that is completely consumed in a reaction. v The other reactants are called the excess reactants or reagents. v The amount of product is always based on the limiting reagent. v


DETERMINE THE (LR) For the balanced equation: 2 H 2 + O 2 2 H 2 O Stiochimetry: 2 mol 1 mol 2 mol Excess reactant: 5 mol 1 mol 2 mol The reaction stops when O 2 consumed; so O 2 is called LR Excess of Reactant : 5 mol H 2 – 2 mol H 2 = 3 mol H 2 Guide for determination of the (LR): 1. 2. 3. 4. 5. Reactions contain more than given amounts of reactants are LR reactions. Convert grams of reactants to moles. Divide the results of each mole on its coefficient in the balanced equation. The smallest value is for the LR substance. Your calculations should be based on the LR.

SAMPLE EXERCISE 3. 18 How many moles of NH 3 can be formed from 3. 0 mol of N 2 and 6. 0 mol of H 2? N 2(g) Given: + 3 H 2(g) 3 mol 6 mol 3. 0/1 = 3 6/3 = 2 LR 2 NH 3(g)

PRACTICE EXERCISE A mixture of 1. 50 mol of Al and 3. 00 mol of Cl 2 is allowed to react. 2 Al(s) + 3 Cl 2(g) 2 Al. Cl 3(s) a) Which is the limiting reagent? Answer: Al b) How many moles of Al. Cl 3 are formed? Answer: 1. 50 mol How many moles of the excess reactant remain at the end of the reaction? Answer: 0. 75 mol Cl 2

SAMPLE EXERCISE 3. 19 Suppose a fuel cell is set up with 150 g of hydrogen gas and 1500 g of oxygen gas. A)How many grams of water can be formed? 2 H 2(g) 150 g For H 2 : 75/2 = 37. 5 H 2 is LR + O 2(g) 1500 g 2 H 2 O(g) ? ? g For O 2: 47/1 =47
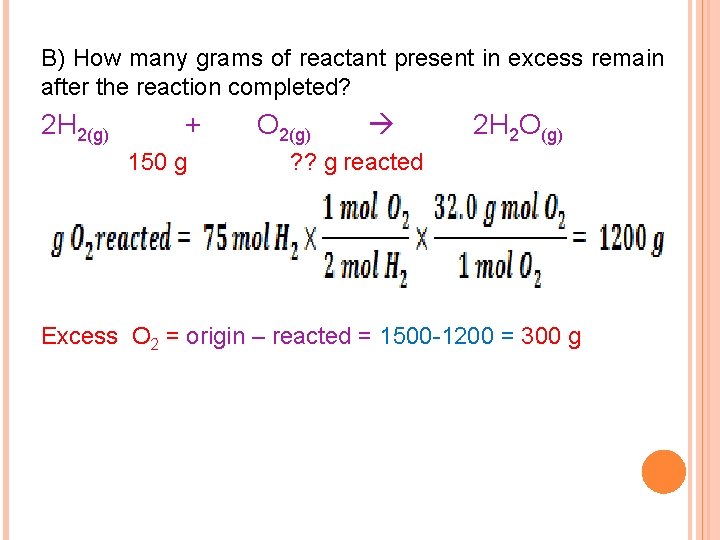
B) How many grams of reactant present in excess remain after the reaction completed? 2 H 2(g) + 150 g O 2(g) 2 H 2 O(g) ? ? g reacted Excess O 2 = origin – reacted = 1500 -1200 = 300 g

PRACTICE EXERCISE A strip of zinc metal with a mass of 2. 00 g is placed in an aqueous solution containing 2. 50 g of silver nitrate, causing the following reaction to occur: Zn(s) + 2 Ag. NO 3(aq) a. Which reactant is limiting? 2 Ag(s) + Zn(NO 3)2(aq)

b. How many grams of Ag will form? c. How many grams of Zn(NO 3)2 will form? d. How many grams of the excess reagent will be left?

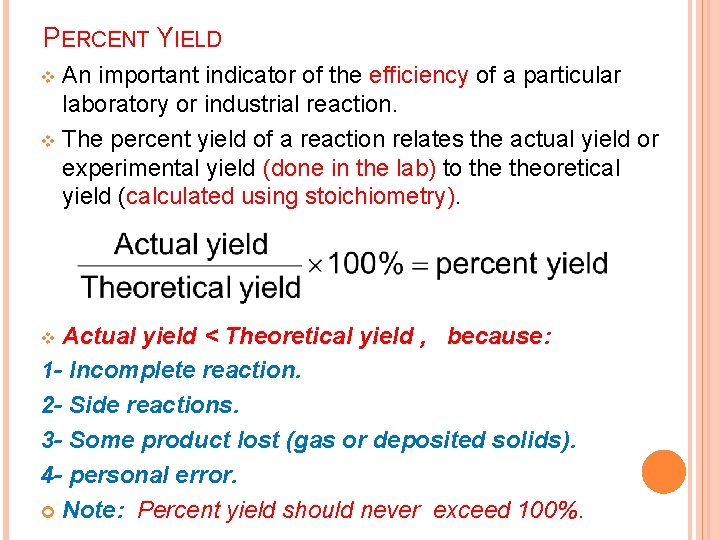
PERCENT YIELD An important indicator of the efficiency of a particular laboratory or industrial reaction. v The percent yield of a reaction relates the actual yield or experimental yield (done in the lab) to theoretical yield (calculated using stoichiometry). v Actual yield < Theoretical yield , because: 1 - Incomplete reaction. 2 - Side reactions. 3 - Some product lost (gas or deposited solids). 4 - personal error. Note: Percent yield should never exceed 100%. v

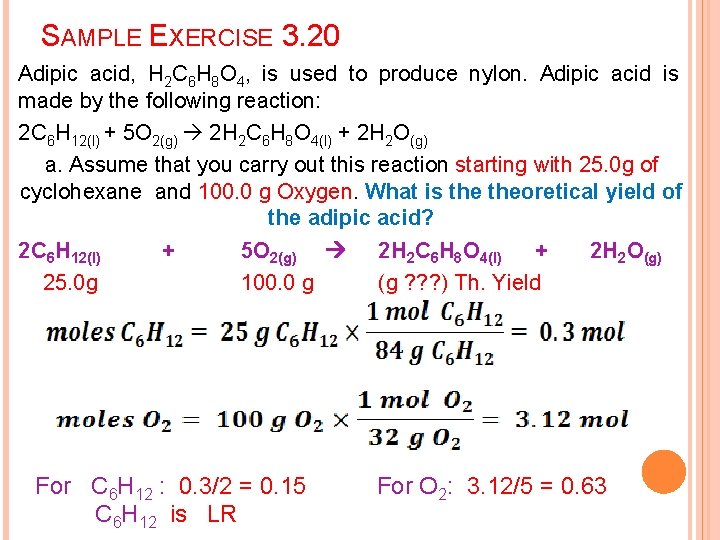
SAMPLE EXERCISE 3. 20 Adipic acid, H 2 C 6 H 8 O 4, is used to produce nylon. Adipic acid is made by the following reaction: 2 C 6 H 12(l) + 5 O 2(g) 2 H 2 C 6 H 8 O 4(l) + 2 H 2 O(g) a. Assume that you carry out this reaction starting with 25. 0 g of cyclohexane and 100. 0 g Oxygen. What is theoretical yield of the adipic acid? 2 C 6 H 12(l) + 5 O 2(g) 2 H 2 C 6 H 8 O 4(l) + 2 H 2 O(g) 25. 0 g 100. 0 g (g ? ? ? ) Th. Yield For C 6 H 12 : 0. 3/2 = 0. 15 C 6 H 12 is LR For O 2: 3. 12/5 = 0. 63

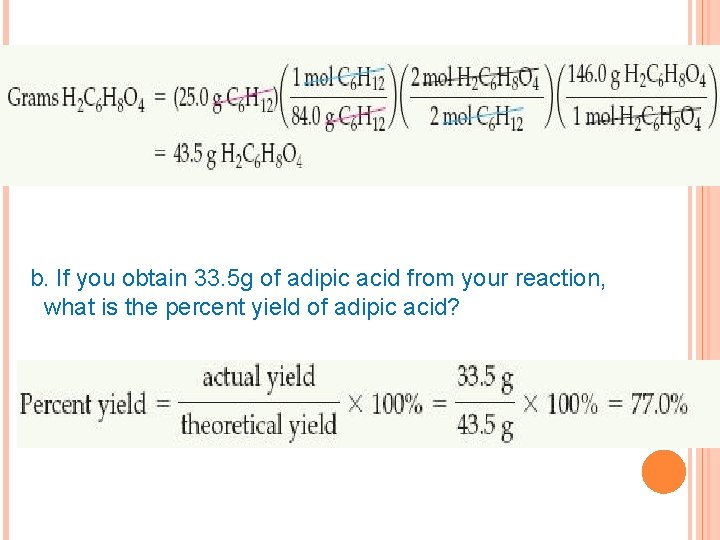
b. If you obtain 33. 5 g of adipic acid from your reaction, what is the percent yield of adipic acid?
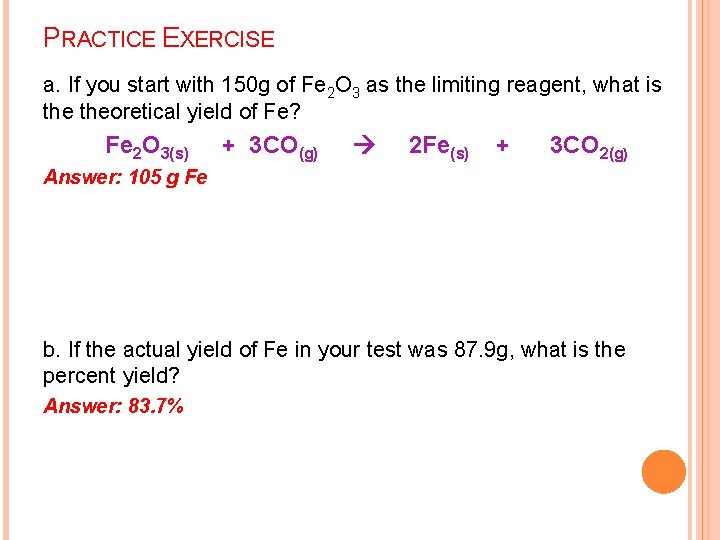
PRACTICE EXERCISE a. If you start with 150 g of Fe 2 O 3 as the limiting reagent, what is theoretical yield of Fe? Fe 2 O 3(s) + 3 CO(g) 2 Fe(s) + 3 CO 2(g) Answer: 105 g Fe b. If the actual yield of Fe in your test was 87. 9 g, what is the percent yield? Answer: 83. 7%

HW PROBLEMS 1, 4, 6, 8, 11, 15, 21, 25, 27, 32, 35, 38, 41, 47, 49, 52, 53, 55, 59, 63, 65, 70, 80, 81, 83, 85.
