Section 12 4 Structure of Molecules Section 12




- Slides: 60

Section 12. 4 Structure of Molecules

Section 12. 4 Structure of Molecules Objectives

Section 12. 4 Structure of Molecules Objectives 1. To understand the VSEPR theory model 2. To learn to predict electronic geometries from the number of regions of high e- density 1. Download VSEPR Charts Here 3. To understand electronic structure and bond angles OK State summary page http: //intro. chem. okstate. edu/1314 F 97/Chapter 9/VSEPR. html

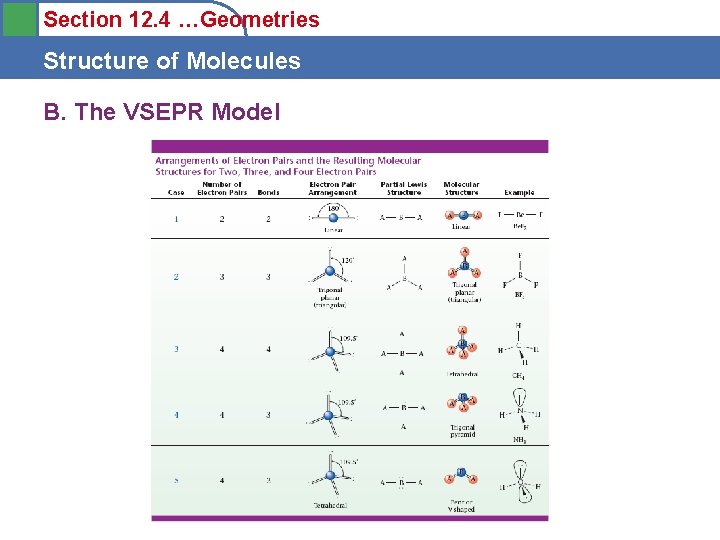
Section 12. 4 Structure of Molecules A. The VSEPR Model • Valence shell electron pair repulsion (VSEPR) model – Each pair of e-s in a valence shell are significant. – e- pairs about the central atom repel each other. – They arrange to minimize repulsion forces. – Electronic geometry is determined by the number of - pairs (RHED) about a central atom. – Region of high e- density – (RHED) any e- pair or bond I, II, or III – Central atom – any atom bonded to > 1 other atom – Bonded atom – any atom bonded to a central atom e

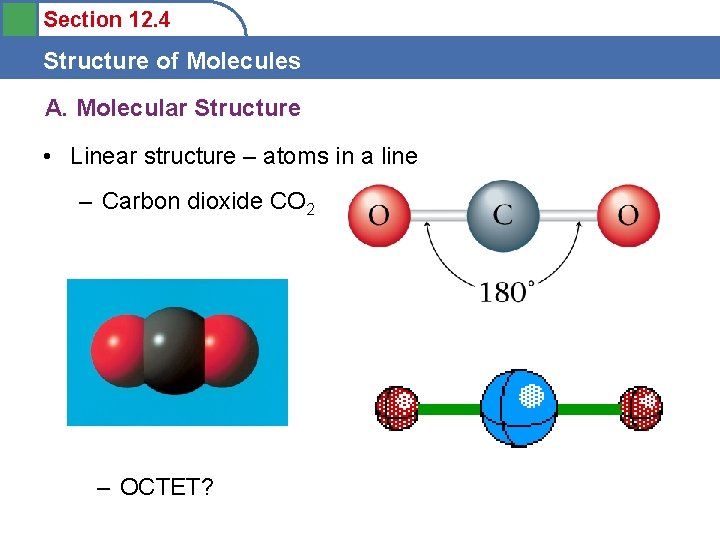
Section 12. 4 Structure of Molecules A. Molecular Structure • Linear structure – atoms in a line – Carbon dioxide CO 2 – OCTET?

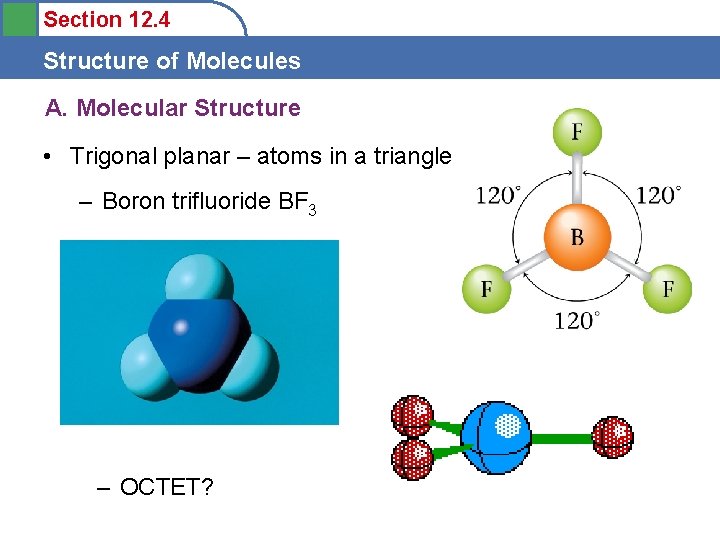
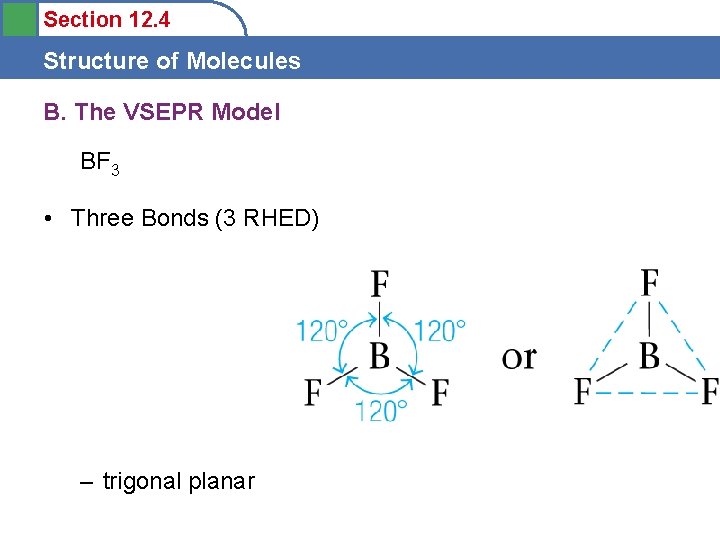
Section 12. 4 Structure of Molecules A. Molecular Structure • Trigonal planar – atoms in a triangle – Boron trifluoride BF 3 – OCTET?

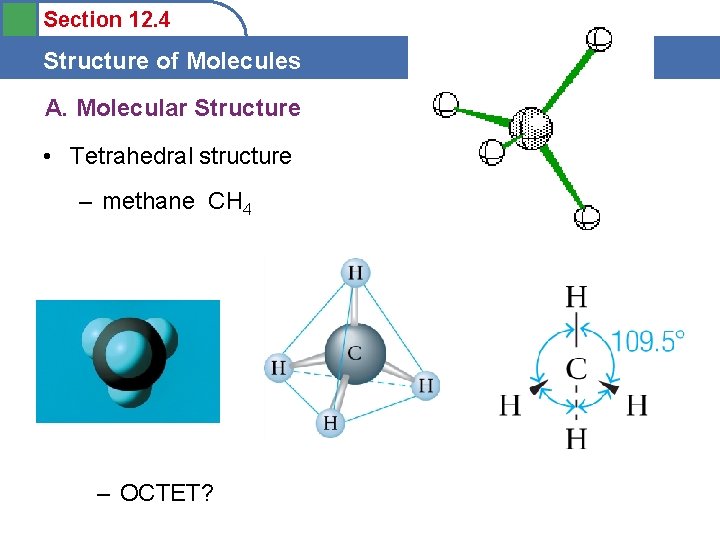
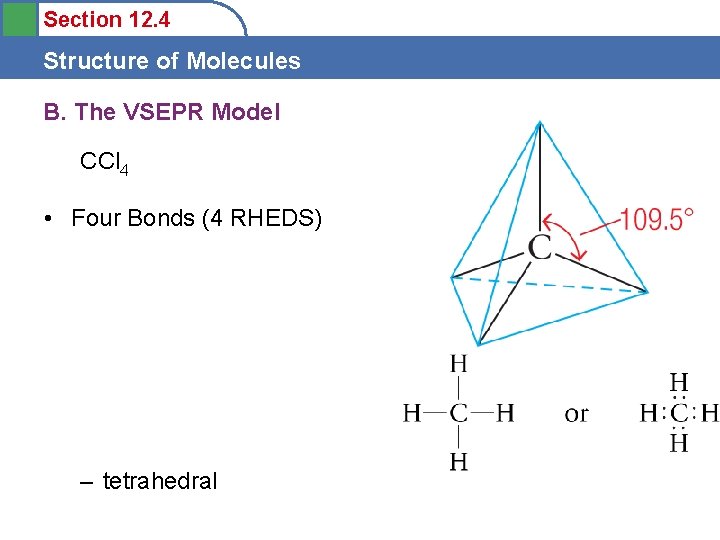
Section 12. 4 Structure of Molecules A. Molecular Structure • Tetrahedral structure – methane CH 4 – OCTET?

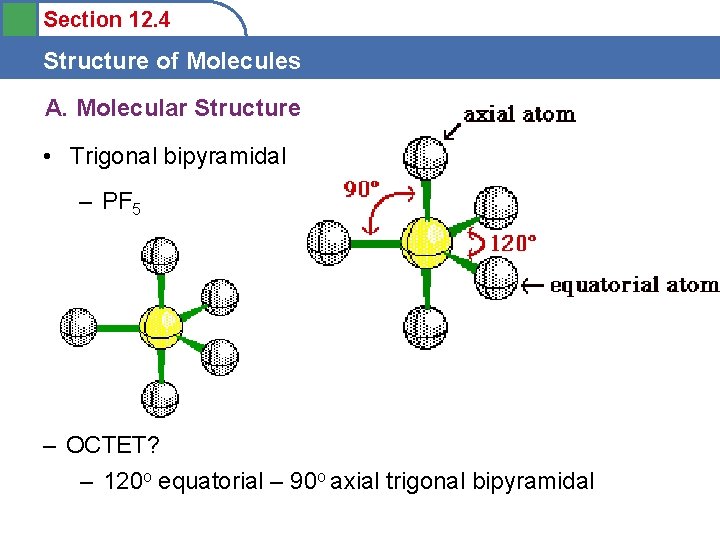
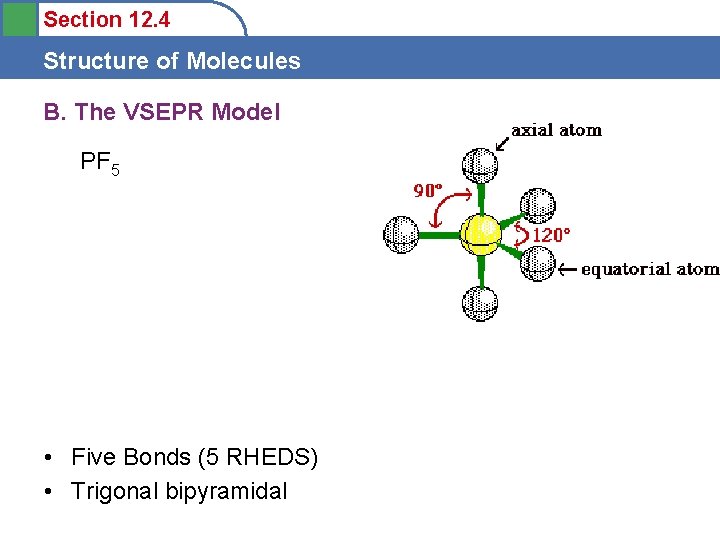
Section 12. 4 Structure of Molecules A. Molecular Structure • Trigonal bipyramidal – PF 5 – OCTET? – 120 o equatorial – 90 o axial trigonal bipyramidal

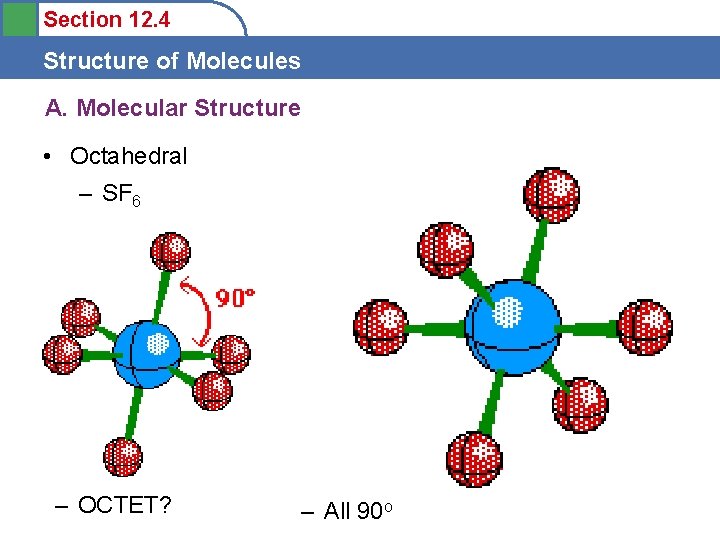
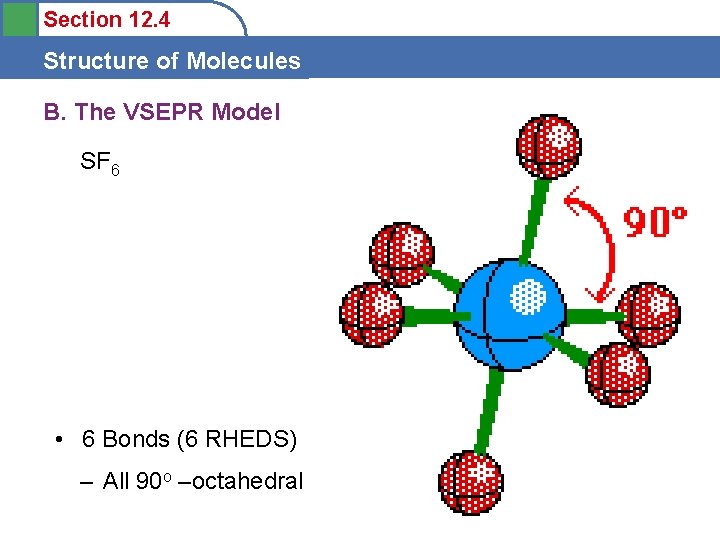
Section 12. 4 Structure of Molecules A. Molecular Structure • Octahedral – SF 6 OK State Website – OCTET? – All 90 o

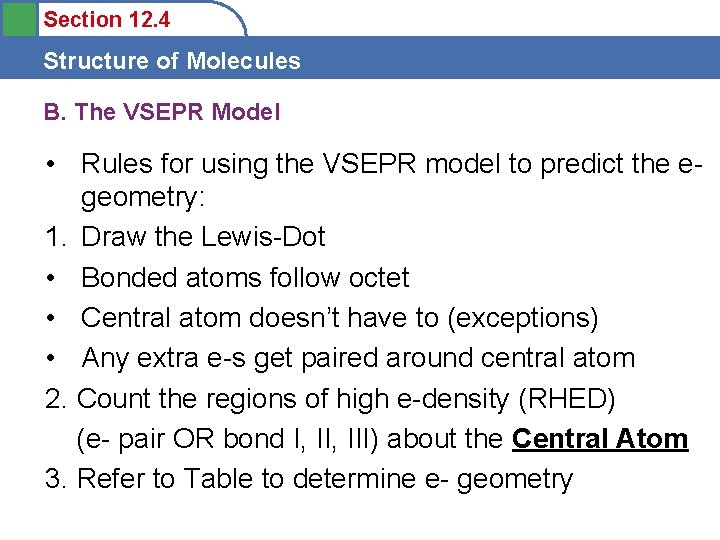
Section 12. 4 Structure of Molecules B. The VSEPR Model • Rules for using the VSEPR model to predict the egeometry: 1. Draw the Lewis-Dot • Bonded atoms follow octet • Central atom doesn’t have to (exceptions) • Any extra e-s get paired around central atom 2. Count the regions of high e-density (RHED) (e- pair OR bond I, III) about the Central Atom 3. Refer to Table to determine e- geometry

Section 12. 4 Structure of Molecules B. The VSEPR Model Be. Cl 2 • Two Bonds (2 RHED) – linear

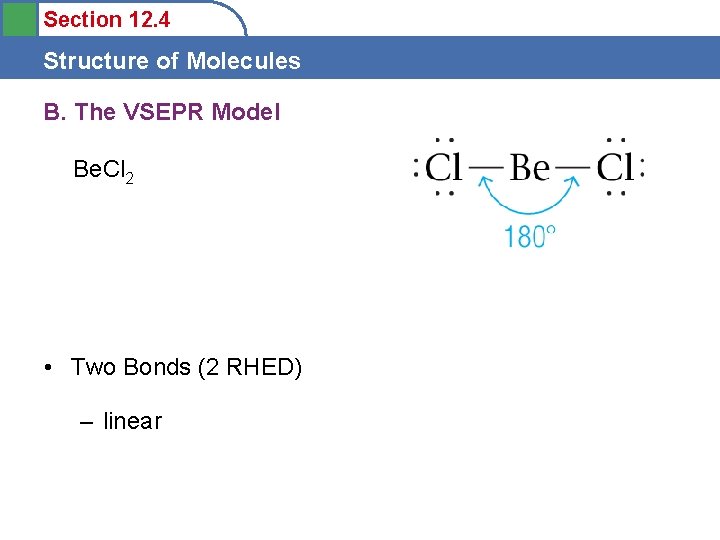
Section 12. 4 Structure of Molecules B. The VSEPR Model BF 3 • Three Bonds (3 RHED) – trigonal planar

Section 12. 4 Structure of Molecules B. The VSEPR Model CCl 4 • Four Bonds (4 RHEDS) – tetrahedral

Section 12. 4 Structure of Molecules B. The VSEPR Model PF 5 • Five Bonds (5 RHEDS) • Trigonal bipyramidal

Section 12. 4 Structure of Molecules B. The VSEPR Model SF 6 • 6 Bonds (6 RHEDS) – All 90 o –octahedral

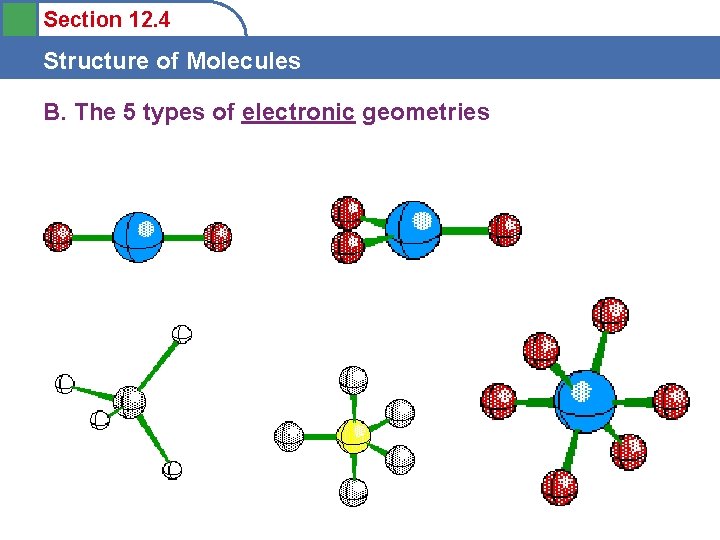
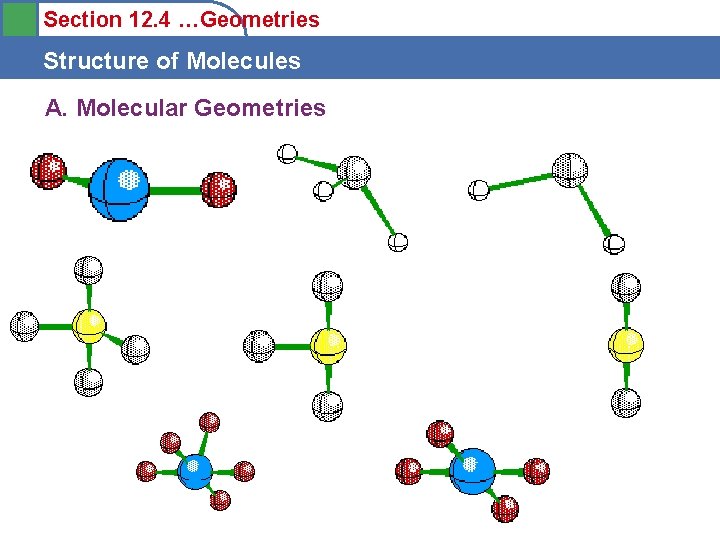
Section 12. 4 Structure of Molecules B. The 5 types of electronic geometries

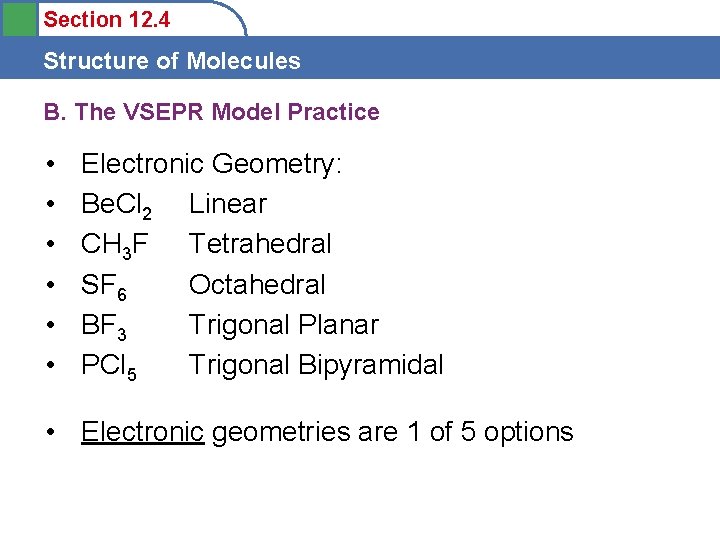
Section 12. 4 Structure of Molecules B. The VSEPR Model Practice • Draw the Lewis Dot Structure and predict the Electronic Geometry of the following: • Be. Cl 2 • CH 3 F • SF 6 • BF 3 • PCl 5 • Electronic geometries are 1 of 5 options

Section 12. 4 Structure of Molecules B. The VSEPR Model Practice • • • Electronic Geometry: Be. Cl 2 Linear CH 3 F Tetrahedral SF 6 Octahedral BF 3 Trigonal Planar PCl 5 Trigonal Bipyramidal • Electronic geometries are 1 of 5 options

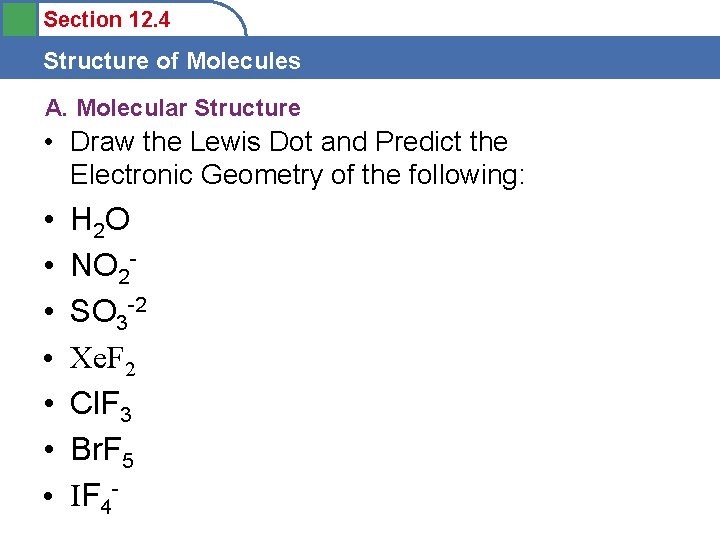
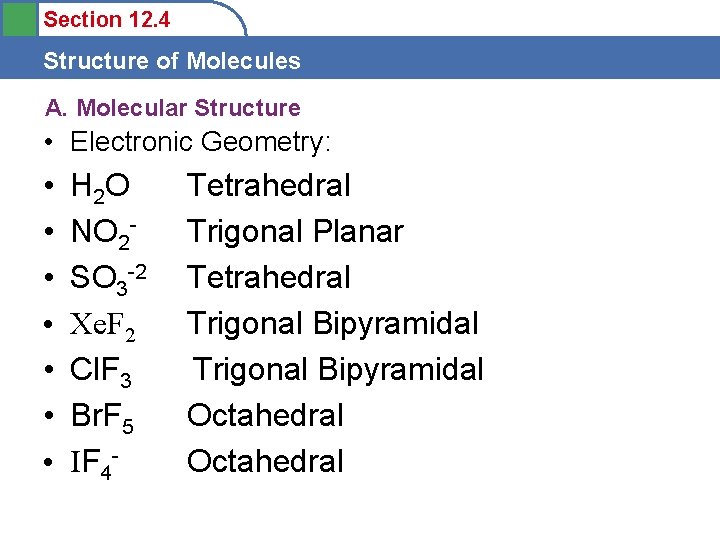
Section 12. 4 Structure of Molecules A. Molecular Structure • Draw the Lewis Dot and Predict the Electronic Geometry of the following: • • H 2 O NO 2 SO 3 -2 Xe. F 2 Cl. F 3 Br. F 5 IF 4 -

Section 12. 4 Structure of Molecules A. Molecular Structure • Electronic Geometry: • • H 2 O NO 2 SO 3 -2 Xe. F 2 Cl. F 3 Br. F 5 IF 4 - Tetrahedral Trigonal Planar Tetrahedral Trigonal Bipyramidal Octahedral

Section 12. 4 Structure of Molecules Objectives Review 1. To understand the VSEPR theory model 2. To learn to predict electronic geometries from the number of regions of high e- density 3. To understand electronic structure and bond angles 4. Work Session: Review Page 433 # 1, 4, 5 (egeometry)

Section 12. 4 …Geometries Structure of Molecules Objectives • To learn to predict MOLECULAR geometries from the number of paired and unpaired e-s • To predict the polarity of a molecule • To understand the effect of an unshared pair on bond angles OK State summary page

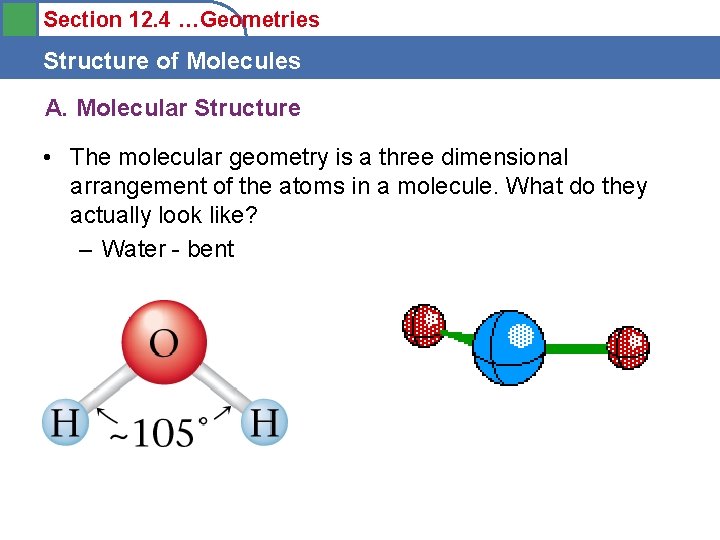
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • The molecular geometry is a three dimensional arrangement of the atoms in a molecule. What do they actually look like? – Water - bent

Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • How do we predict the Molecular Geometry? • Same as Electronic Geometry, only more options… • 1. Draw the Lewis Dot • 2. Count the RHEDS • 3. Determine the General Formula (ABx. Uy) • 4. Refer to Table

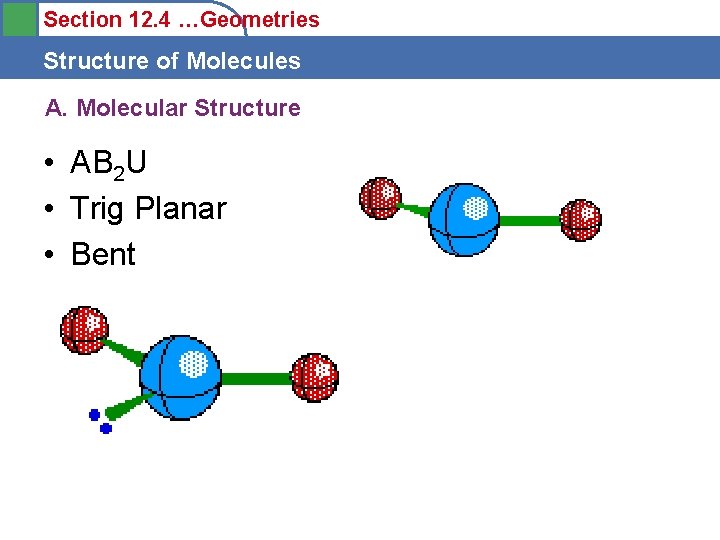
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • AB 2 U • Trig Planar • Bent

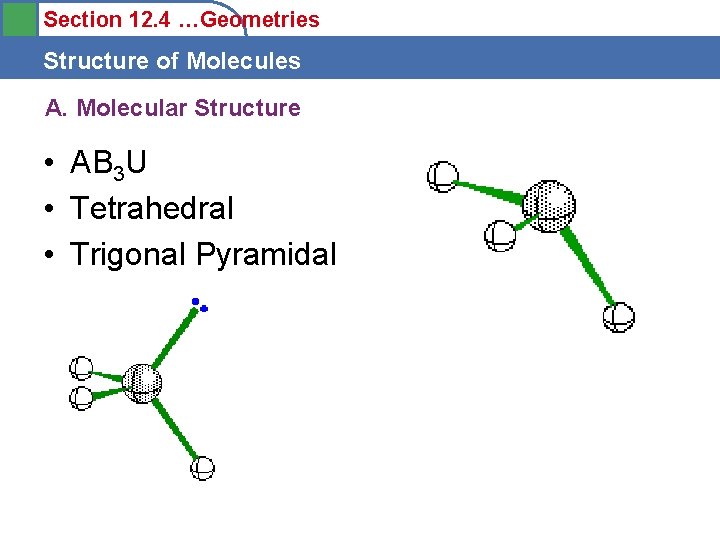
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • AB 3 U • Tetrahedral • Trigonal Pyramidal

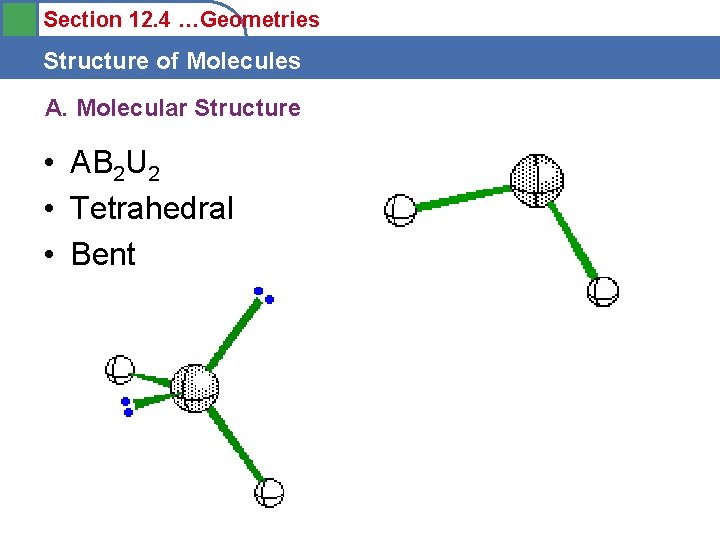
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • AB 2 U 2 • Tetrahedral • Bent

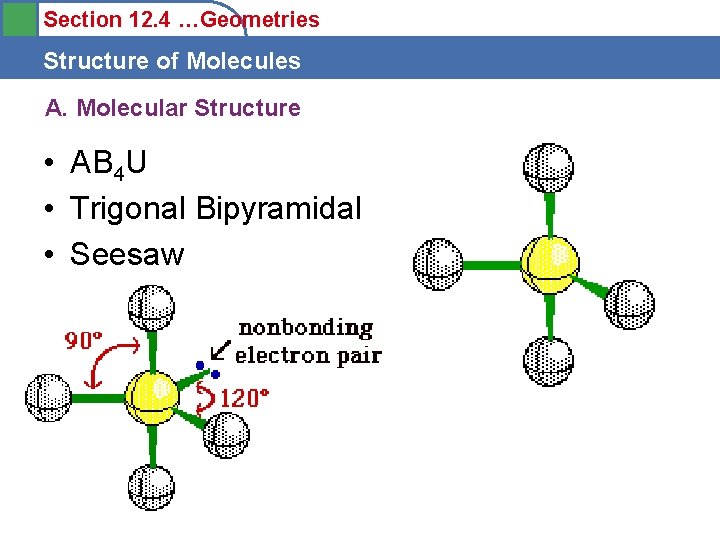
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • AB 4 U • Trigonal Bipyramidal • Seesaw

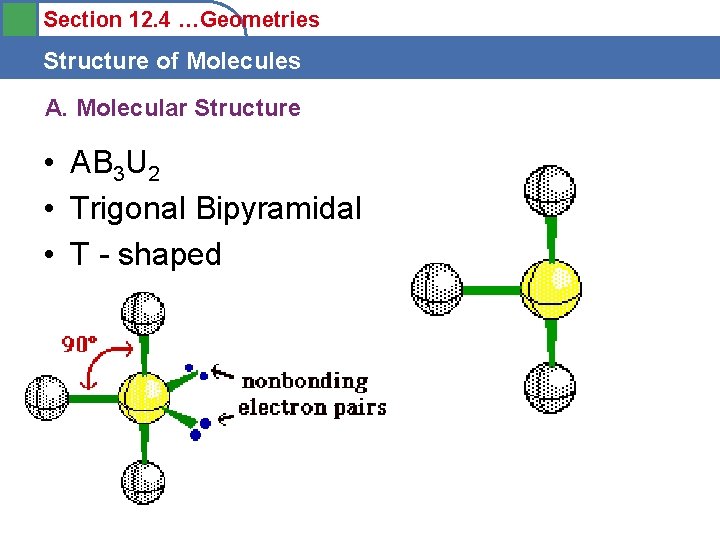
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • AB 3 U 2 • Trigonal Bipyramidal • T - shaped

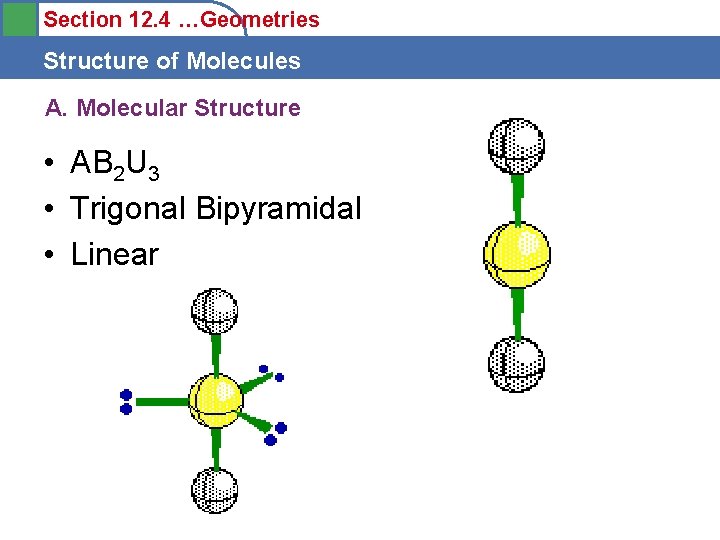
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • AB 2 U 3 • Trigonal Bipyramidal • Linear

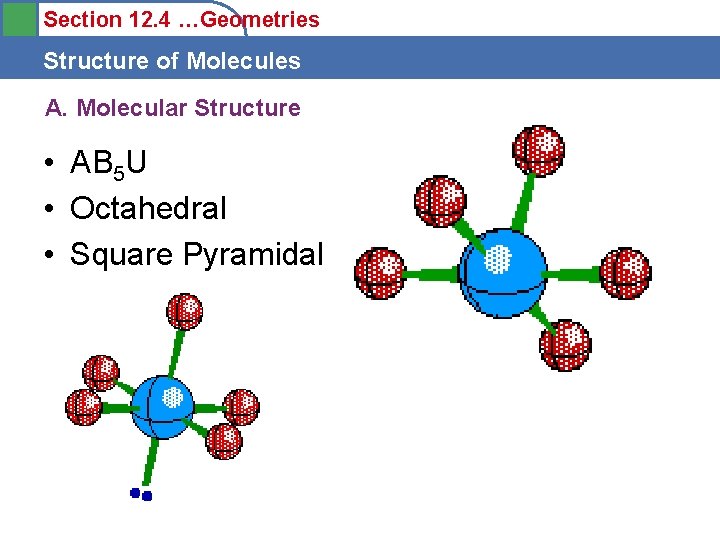
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • AB 5 U • Octahedral • Square Pyramidal

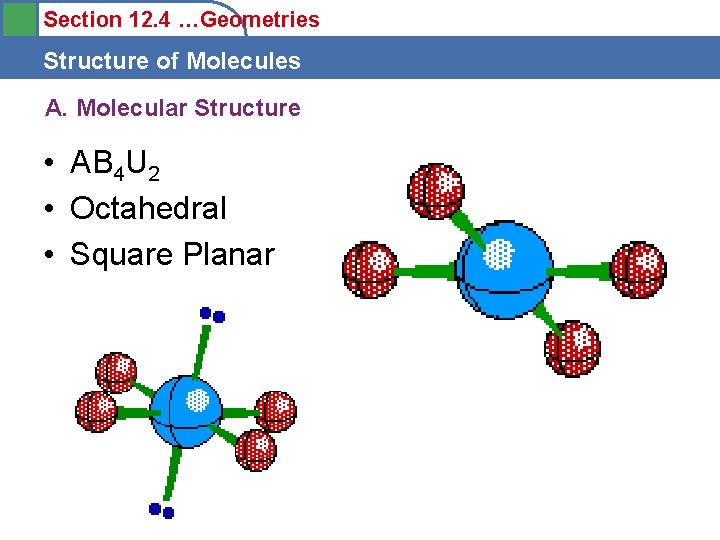
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • AB 4 U 2 • Octahedral • Square Planar

Section 12. 4 …Geometries Structure of Molecules A. Molecular Geometries Seesaw

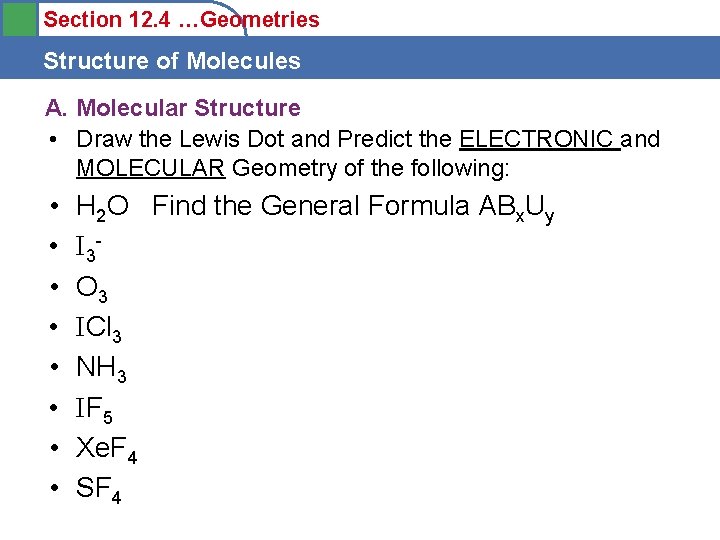
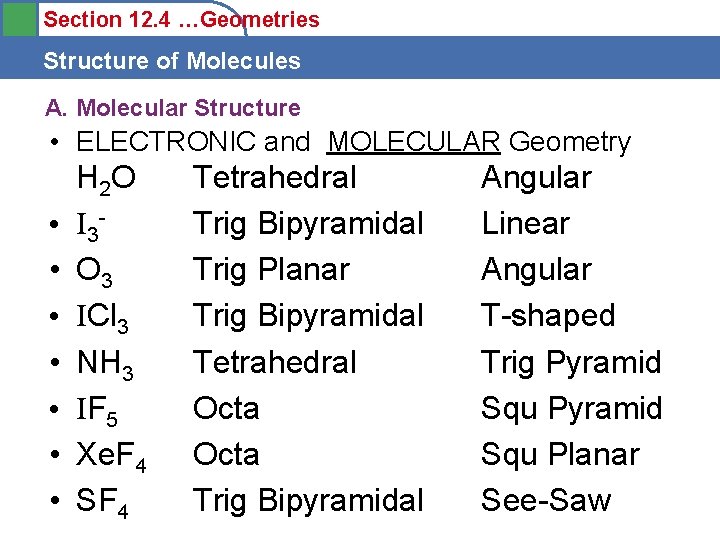
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • Draw the Lewis Dot and Predict the ELECTRONIC and MOLECULAR Geometry of the following: • • H 2 O Find the General Formula ABx. Uy I 3 O 3 ICl 3 NH 3 IF 5 Xe. F 4 SF 4

Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • ELECTRONIC and MOLECULAR Geometry • • H 2 O I 3 O 3 ICl 3 NH 3 IF 5 Xe. F 4 SF 4 Tetrahedral Trig Bipyramidal Trig Planar Trig Bipyramidal Tetrahedral Octa Trig Bipyramidal Angular Linear Angular T-shaped Trig Pyramid Squ Planar See-Saw

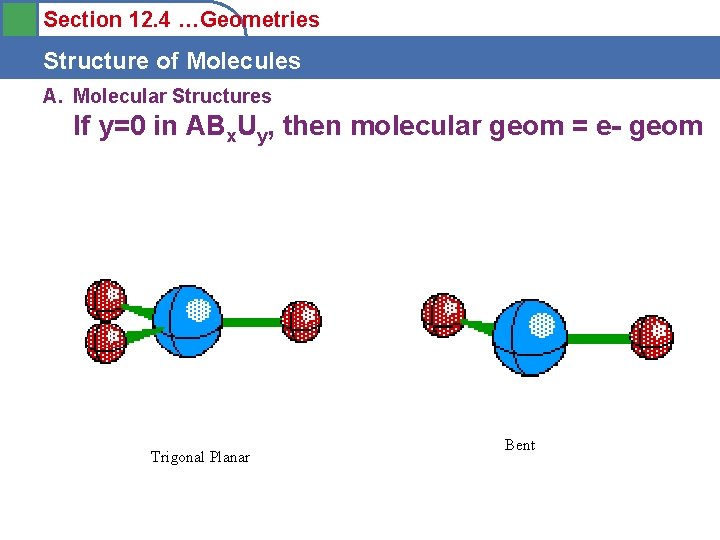
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structures If y=0 in ABx. Uy, then molecular geom = e- geom Trigonal Planar Bent

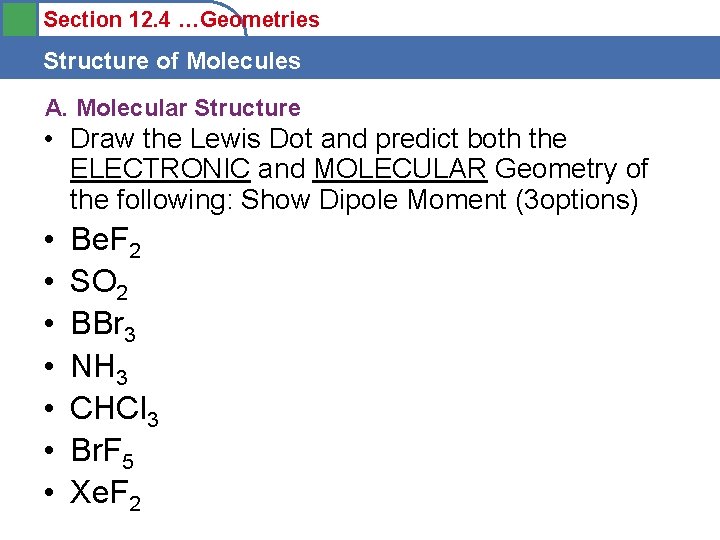
Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • Draw the Lewis Dot and predict both the ELECTRONIC and MOLECULAR Geometry of the following: Show Dipole Moment (3 options) • • Be. F 2 SO 2 BBr 3 NH 3 CHCl 3 Br. F 5 Xe. F 2

Section 12. 4 …Geometries Structure of Molecules A. Molecular Structure • Draw the Lewis Dot and predict both the ELECTRONIC and MOLECULAR Geometry of the following: Show the dipole if needed. • • SF 4 Cl. F 3 Xe. F 4 CH 2 Cl. Br Sb. Cl 5 Se. F 6 BI 3

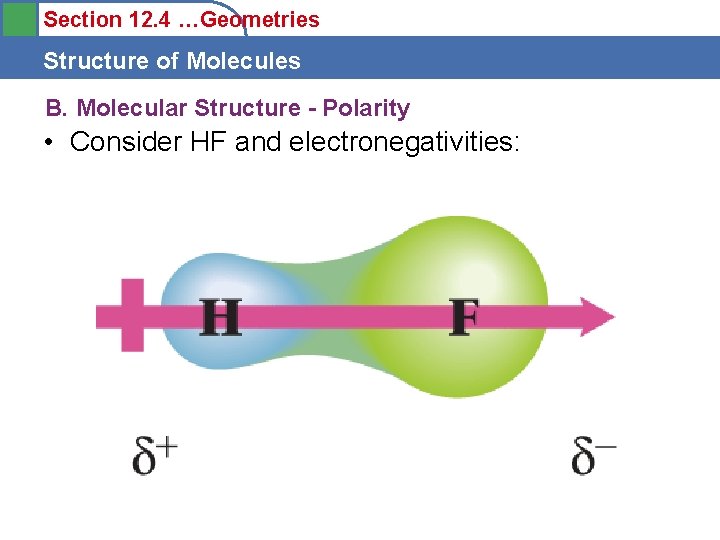
Section 12. 4 …Geometries Structure of Molecules B. Molecular Structure - Polarity • Consider HF and electronegativities:

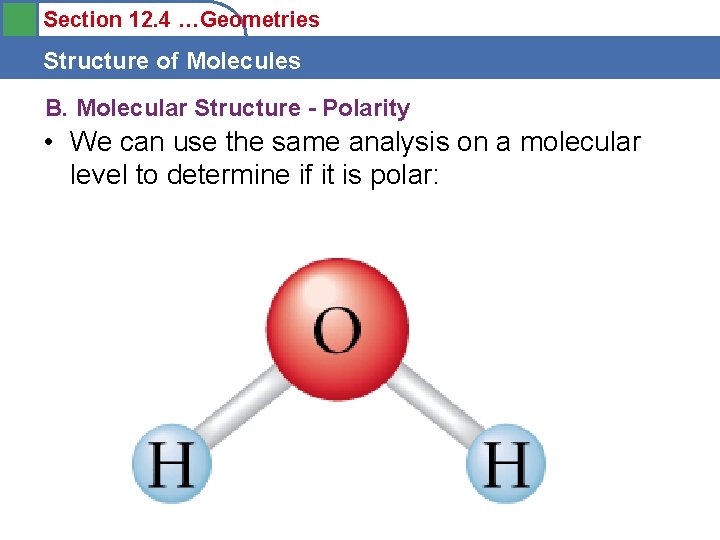
Section 12. 4 …Geometries Structure of Molecules B. Molecular Structure - Polarity • We can use the same analysis on a molecular level to determine if it is polar:

Section 12. 4 …Geometries Structure of Molecules B. Molecular Structure - Polarity • Draw the Lewis Dot, analyze each bond, and predict the overall polarity of the molecule: • • Be. Br 2 Xe. Br 2 Be. FI CH 3 Cl Sb. Cl 5 Se. F 5 Br BFI 2

Section 12. 4 …Geometries Structure of Molecules B. Molecular Structure - Polarity • Draw or look over the Lewis Dot and predict the overall polarity of the molecule: • • SF 4 Cl. F 3 Xe. F 4 CH 3 Br Sb. Cl 5 NH 3 Br. I 5

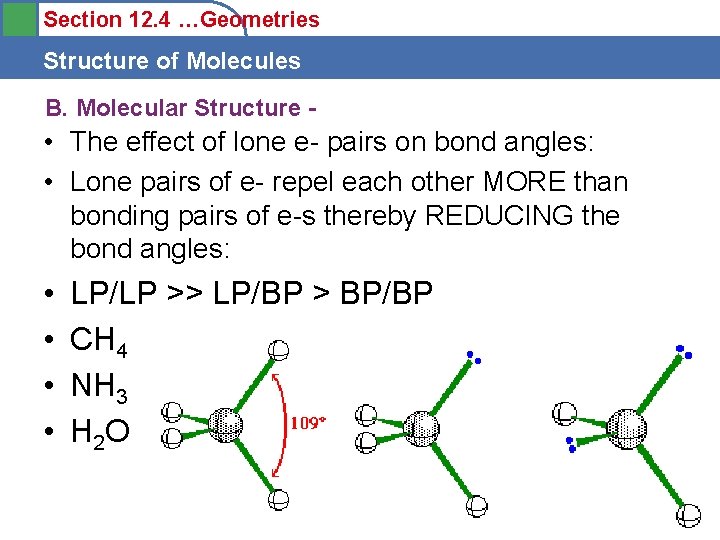
Section 12. 4 …Geometries Structure of Molecules B. Molecular Structure - • The effect of lone e- pairs on bond angles: • Lone pairs of e- repel each other MORE than bonding pairs of e-s thereby REDUCING the bond angles: • • LP/LP >> LP/BP > BP/BP CH 4 NH 3 H 2 O

Section 12. 4 …Geometries Structure of Molecules Objectives Review • • To learn to predict MOLECULAR geometries from the number of paired and unpaired e-s To predict the polarity of a molecule To understand the effect of an unshared pair on bond angles Work Session: Review Page 433 # 2, 3, #5 Predict the molecular geometry AND tell if the molecule is polar. Give an example of one non-polar molecule. OK State summary page

Section 12. 4 …VB Theory Structure of Molecules Objectives • To understand the Valence Bond (VB) Theory • To describe HOW the exceptions to the octet rule occur • To use the VB theory to predict the orbital hybridization about a central atom

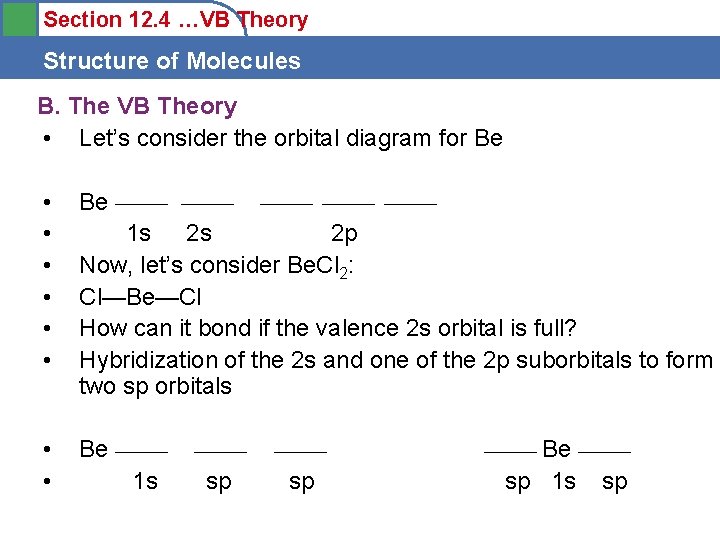
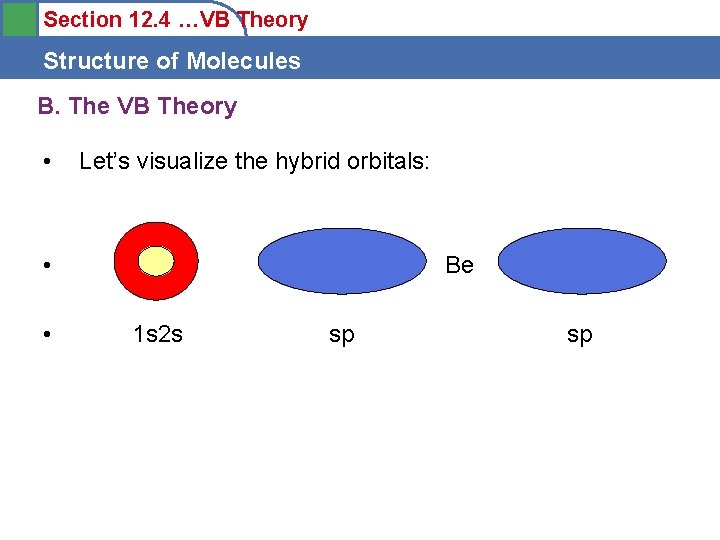
Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory • Let’s consider the orbital diagram for Be • • • Be ______ ______ 1 s 2 s 2 p Now, let’s consider Be. Cl 2: Cl—Be—Cl How can it bond if the valence 2 s orbital is full? Hybridization of the 2 s and one of the 2 p suborbitals to form two sp orbitals • • Be ______ 1 s ______ sp sp Be ______ sp 1 s sp ______

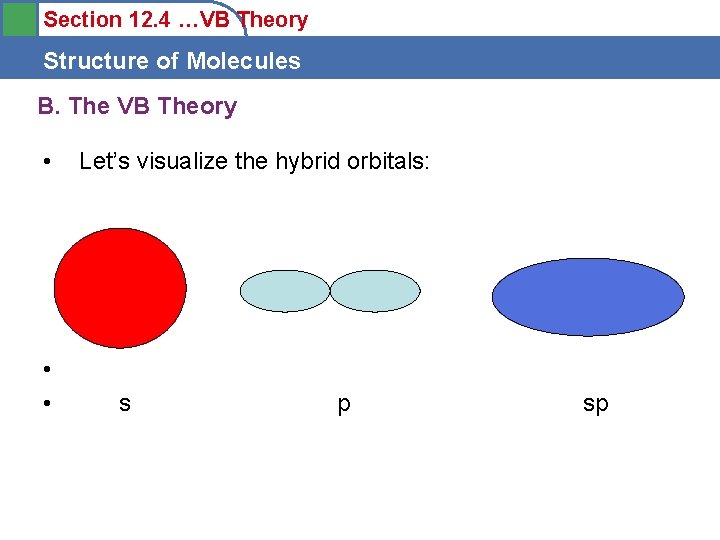
Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory • • • Let’s visualize the hybrid orbitals: s p sp

Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory • Let’s visualize the hybrid orbitals: • • Be 1 s 2 s sp sp

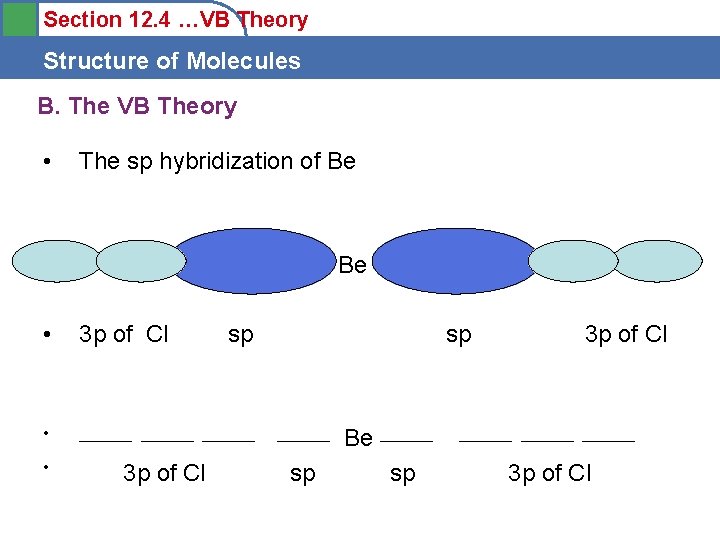
Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory • The sp hybridization of Be • 3 p of Cl • ______ • 3 p of Cl sp sp sp Be ______ sp 3 p of Cl ______ 3 p of Cl

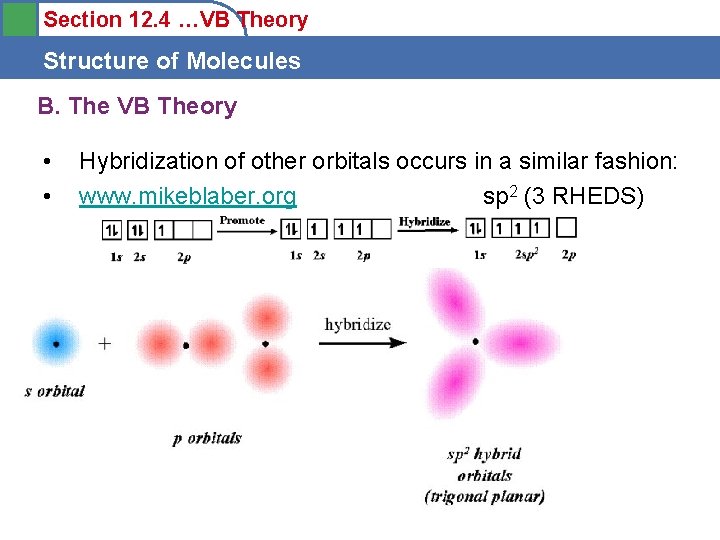
Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory • • Hybridization of other orbitals occurs in a similar fashion: www. mikeblaber. org sp 2 (3 RHEDS)

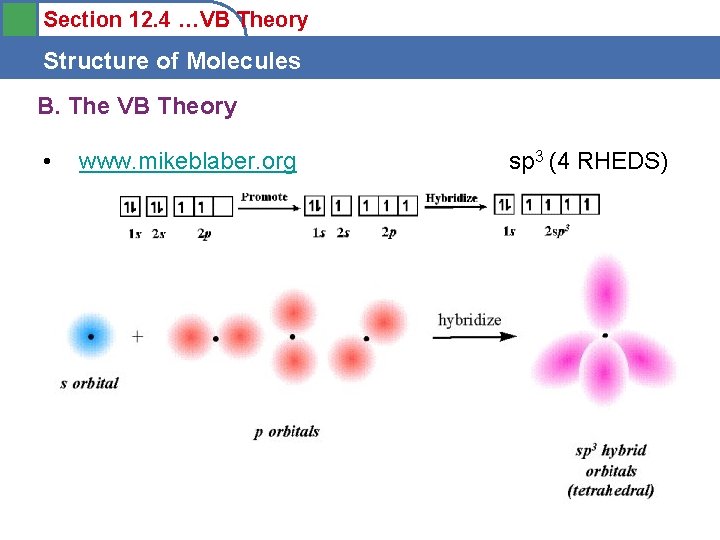
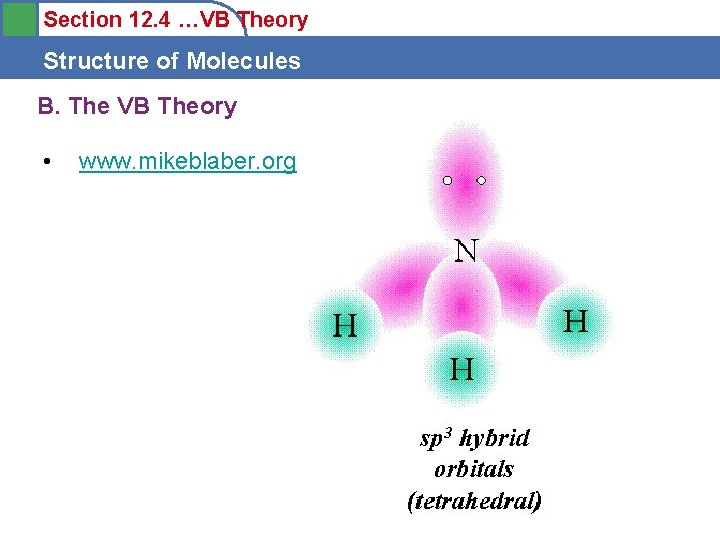
Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory • www. mikeblaber. org sp 3 (4 RHEDS)

Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory • www. mikeblaber. org

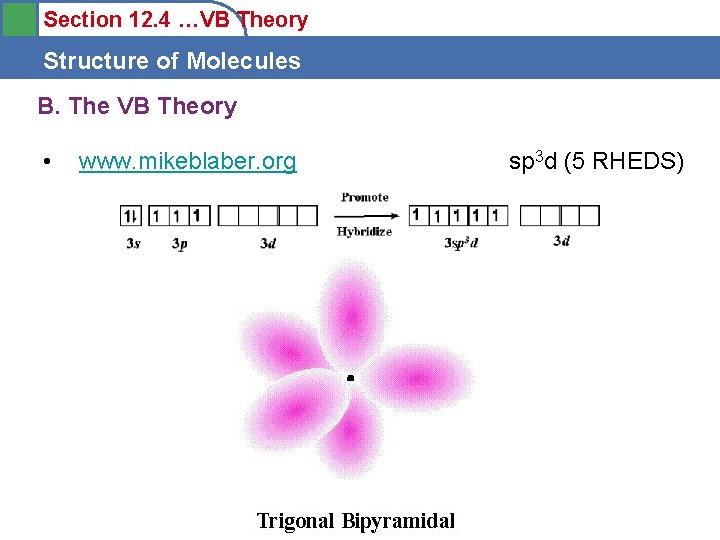
Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory • www. mikeblaber. org sp 3 d (5 RHEDS)

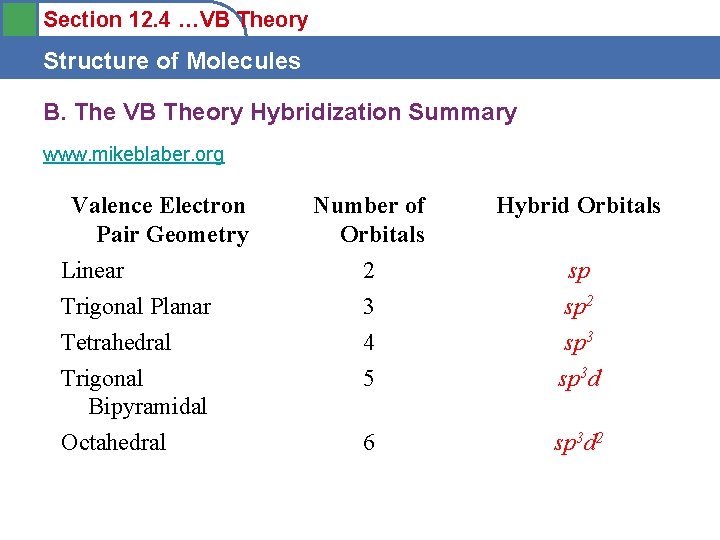
Section 12. 4 …VB Theory Structure of Molecules B. The VB Theory Hybridization Summary www. mikeblaber. org Valence Electron Pair Geometry Number of Orbitals Hybrid Orbitals Linear 2 sp Trigonal Planar 3 sp 2 Tetrahedral 4 sp 3 Trigonal Bipyramidal 5 sp 3 d Octahedral 6 sp 3 d 2

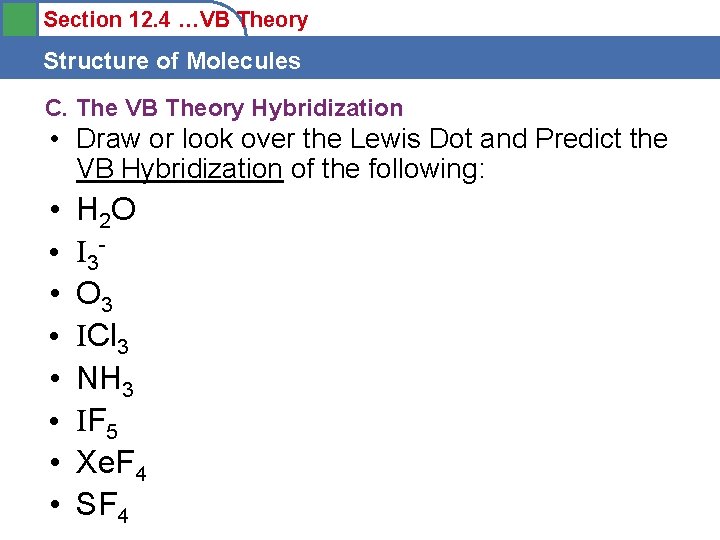
Section 12. 4 …VB Theory Structure of Molecules C. The VB Theory Hybridization • Draw or look over the Lewis Dot and Predict the VB Hybridization of the following: • • H 2 O I 3 O 3 ICl 3 NH 3 IF 5 Xe. F 4 SF 4

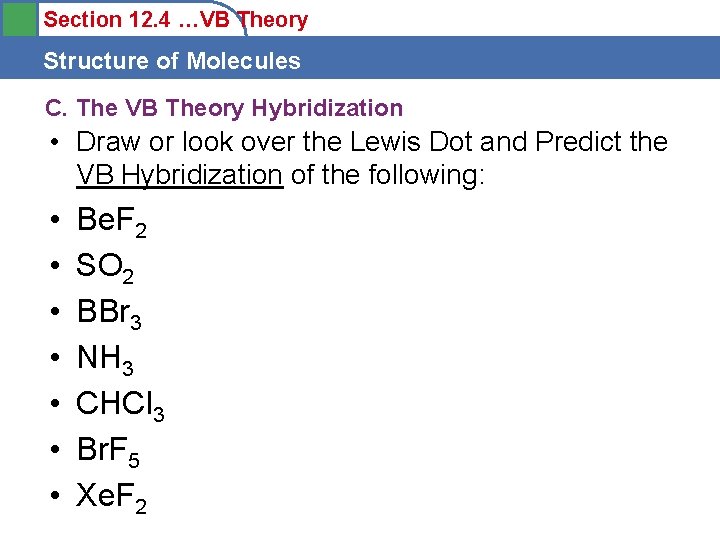
Section 12. 4 …VB Theory Structure of Molecules C. The VB Theory Hybridization • Draw or look over the Lewis Dot and Predict the VB Hybridization of the following: • • Be. F 2 SO 2 BBr 3 NH 3 CHCl 3 Br. F 5 Xe. F 2

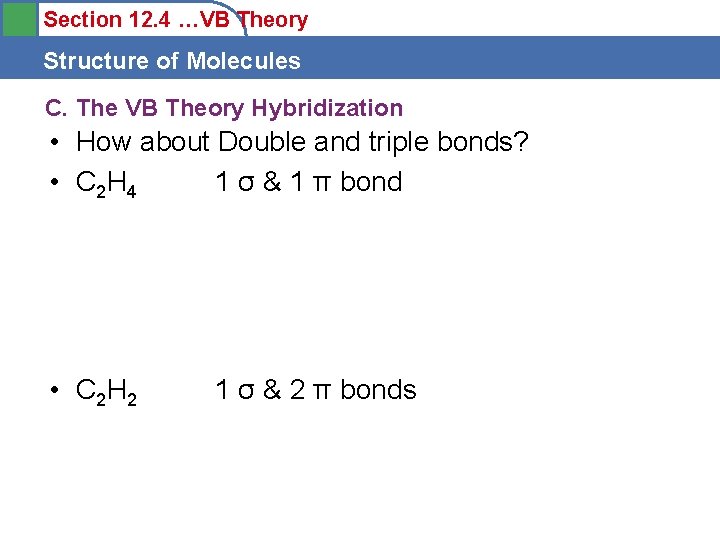
Section 12. 4 …VB Theory Structure of Molecules C. The VB Theory Hybridization • How about Double and triple bonds? • C 2 H 4 1 σ & 1 π bond • C 2 H 2 1 σ & 2 π bonds

Section 12. 4 …VB Theory Structure of Molecules C. The VB Theory Hybridization • C 2 H 2 1 σ & 2 π bonds

Section 12. 4 …VB Theory Structure of Molecules Objectives Review • • To understand the Valence Bond (VB) Theory To describe HOW the exceptions to the octet rule occur To use the VB theory to predict the orbital hybridization about a central atom Work Session: Draw the Lewis Dot and predict the hybridization about the central atom: Al. Cl 3, NCl 3, Si. H 4, SF 6, Mg. Cl 2, IF 4 -, Al. H 4 -, NH 4+, PCI 3, CIO 3 -

Section 12. 4 …Geometries Structure of Molecules B. The VSEPR Model