Nitrogen containing compounds Nitrocompounds Amines Diazo and azocompounds









![Hoffman reaction: NH 3 + CH 3 I → [CH 3 NH 3+]I− ↔ Hoffman reaction: NH 3 + CH 3 I → [CH 3 NH 3+]I− ↔](https://slidetodoc.com/presentation_image/8014489fe77ea2802fa35d39dee1779a/image-26.jpg)





![NH 2 NO + [O] H 2 SO 5 nitrozobenzene NH 2 NO 2 NH 2 NO + [O] H 2 SO 5 nitrozobenzene NH 2 NO 2](https://slidetodoc.com/presentation_image/8014489fe77ea2802fa35d39dee1779a/image-59.jpg)












- Slides: 90

Nitrogen containing compounds. Nitrocompounds. Amines. Diazo- and azocompounds. Prepared by ass. Medvid I. I. , ass. Burmas N. I.

Outline 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. Nitroderivates of hydrocarbons. The methods of extraction of nitroalkanes. Chemical properties of nitroalkanes. The aromatic nitrocompounds. Amines. Isomery of amines. Structure and bonding of amines. Physical properties of amines. The methods of extraction of amines. Chemical properties of amines. Synthetically useful transformations involving aryl diazonium ions. The medico-biological importance of amines. Aminoalcohols. The methods of extraction of aminoalcohols. Chemical properties of aminoalcohols. Arylamines. The methods of extraction of aromatic amines. Physical properties of aromatic amines Comparative structure of aromatic and aliphatic amines Sulphanilic acid The synthesis of streptocide Sulphanylamidic preparations Medicinal preparations (derivates of p-aminobenzoic acid (p. ABA ). (p. ABA). Diazocompounds The methods of extraction of aromatic diazocompounds Chemical properties aromatic diazocompounds Azocompounds The methods of extraction of aromatic azocompounds. Chemical properties of aromatic azocompounds Physical bases of theory of colouration Azo-dyes.

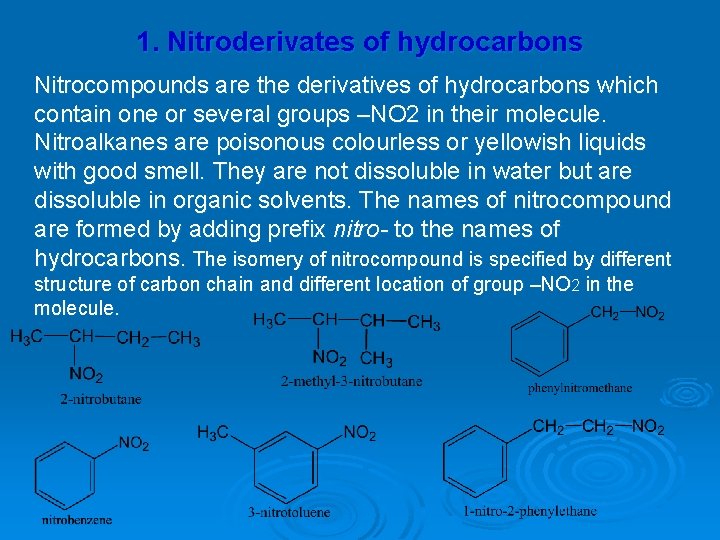
1. Nitroderivates of hydrocarbons Nitrocompounds are the derivatives of hydrocarbons which contain one or several groups –NO 2 in their molecule. Nitroalkanes are poisonous colourless or yellowish liquids with good smell. They are not dissoluble in water but are dissoluble in organic solvents. The names of nitrocompound are formed by adding prefix nitro- to the names of hydrocarbons. The isomery of nitrocompound is specified by different structure of carbon chain and different location of group –NO 2 in the molecule.

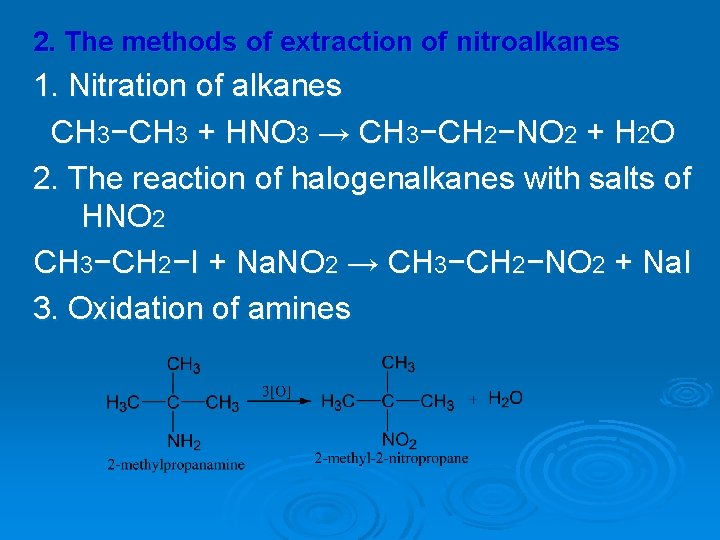
2. The methods of extraction of nitroalkanes 1. Nitration of alkanes CH 3−CH 3 + HNO 3 → CH 3−CH 2−NO 2 + H 2 O 2. The reaction of halogenalkanes with salts of HNO 2 CH 3−CH 2−I + Na. NO 2 → CH 3−CH 2−NO 2 + Na. I 3. Oxidation of amines

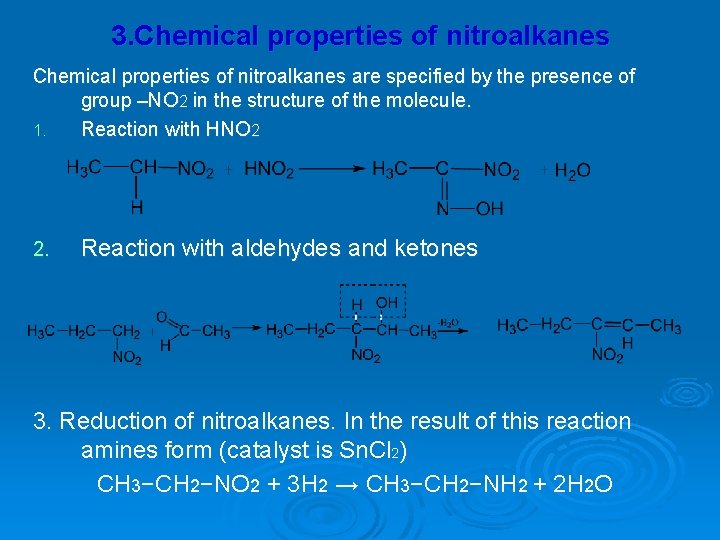
3. Chemical properties of nitroalkanes are specified by the presence of group –NO 2 in the structure of the molecule. 1. Reaction with HNO 2 2. Reaction with aldehydes and ketones 3. Reduction of nitroalkanes. In the result of this reaction amines form (catalyst is Sn. Cl 2) CH 3−CH 2−NO 2 + 3 H 2 → CH 3−CH 2−NH 2 + 2 H 2 O


4. The aromatic nitrocompounds The simplest aromatic nitro compound, having the molecular formula C 6 H 5 NO 2. Nitrobenzene, also known as nitrobenzol or oil of mirbane, is an organic compound with the chemical formula C 6 H 5 NO 2. Nitrobenzene is a water-insoluble oil which exhibits a pale yellow to yellow-brown coloration in liquid form (at room temperature and pressure) with an almondlike odor. When frozen, it appears as a greenish-yellow crystal. Although occasionally used as a flavoring or perfume additive, nitrobenzene is highly toxic in large quantities and is mainly produced as a precursor to aniline. In the laboratory, it is occasionally used as a solvent, especially for electrophilic reagents.

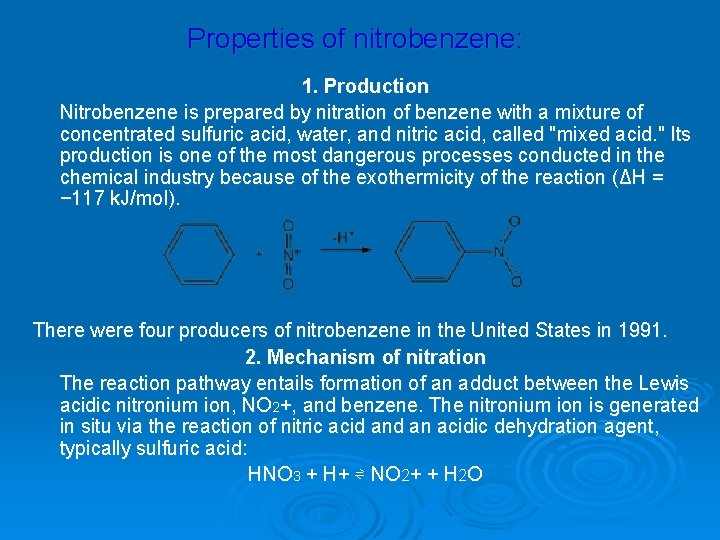
Properties of nitrobenzene: 1. Production Nitrobenzene is prepared by nitration of benzene with a mixture of concentrated sulfuric acid, water, and nitric acid, called "mixed acid. " Its production is one of the most dangerous processes conducted in the chemical industry because of the exothermicity of the reaction (ΔH = − 117 k. J/mol). There were four producers of nitrobenzene in the United States in 1991. 2. Mechanism of nitration The reaction pathway entails formation of an adduct between the Lewis acidic nitronium ion, NO 2+, and benzene. The nitronium ion is generated in situ via the reaction of nitric acid an acidic dehydration agent, typically sulfuric acid: HNO 3 + H+ ⇌ NO 2+ + H 2 O

Zinin’s reaction:



3. Uses Approximately 95% of nitrobenzene is consumed in the production of aniline. 4. Specialized applications More specialized applications include the use of nitrobenzene as a precursor to rubber chemicals, pesticides, dyes, explosives, and pharmaceuticals. Nitrobenzene is also used in shoe and floor polishes, leather dressings, paint solvents, and other materials to mask unpleasant odors. Redistilled, as oil of mirbane, nitrobenzene has been used as an inexpensive perfume for soaps. A significant merchant market for nitrobenzene is its use in the production of the analgesic paracetamol (also known as acetaminophen) (Mannsville 1991). Nitrobenzene is also used in Kerr cells, as it has an unusually large Kerr constant.

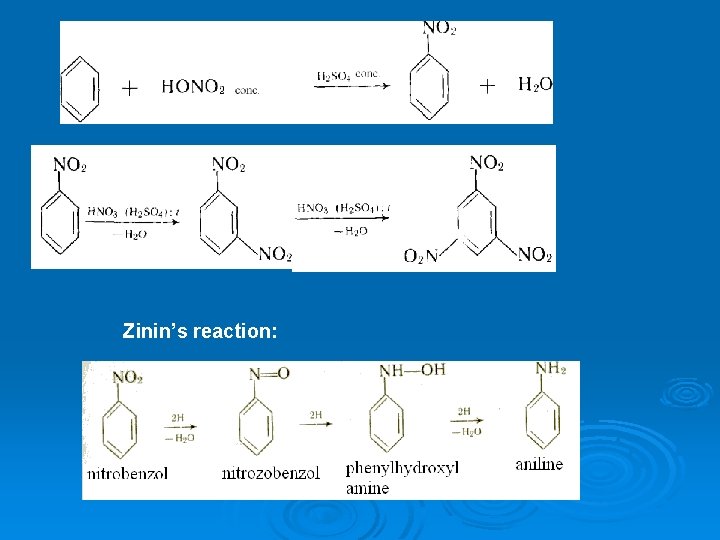
5. Organic reactions Aside from its conversion to aniline, nitrobenzene is readily converted to related derivatives such as azobenzene, nitrosobenzene, and phenylhydroxylamine. The nitro- group is deactivating, thus substitution tends to occur at the meta-position. 6. Safety Nitrobenzene is highly toxic (TLV 5 mg/m 3) and readily absorbed through the skin. Although nitrobenzene is not currently known to be a carcinogen, prolonged exposure may cause serious damage to the central nervous system, impair vision, cause liver or kidney damage, anemia and lung irritation. Inhalation of fumes may induce headache, nausea, fatigue, dizziness, cyanosis, weakness in the arms and legs, and in rare cases may be fatal. The oil is readily absorbed through the skin and may increase heart rate, cause convulsions or rarely death. Ingestion may similarly cause headaches, dizziness, nausea, vomiting and gastrointestinal irritation.

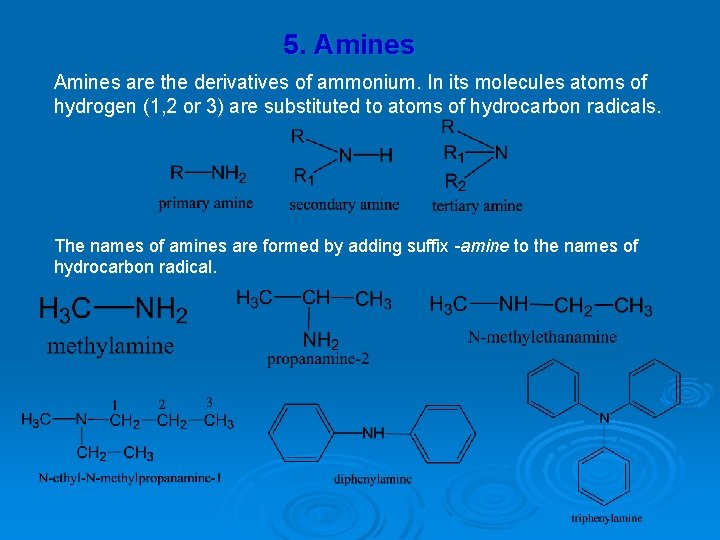
5. Amines are the derivatives of ammonium. In its molecules atoms of hydrogen (1, 2 or 3) are substituted to atoms of hydrocarbon radicals. The names of amines are formed by adding suffix -amine to the names of hydrocarbon radical.

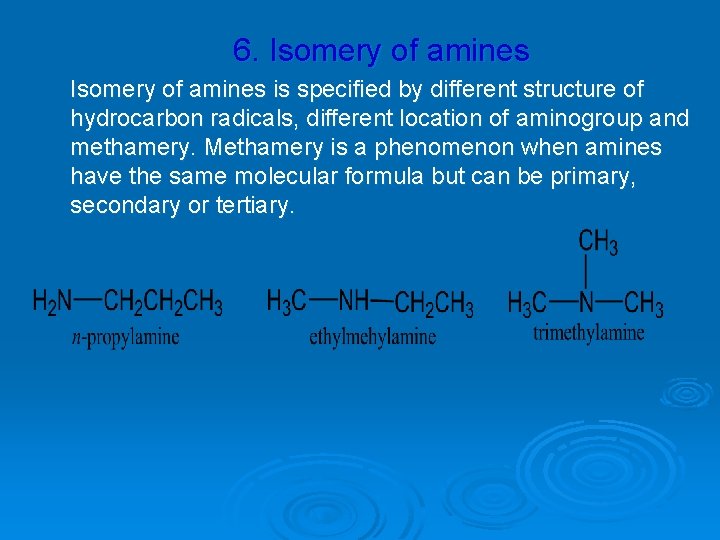
6. Isomery of amines is specified by different structure of hydrocarbon radicals, different location of aminogroup and methamery. Methamery is a phenomenon when amines have the same molecular formula but can be primary, secondary or tertiary.

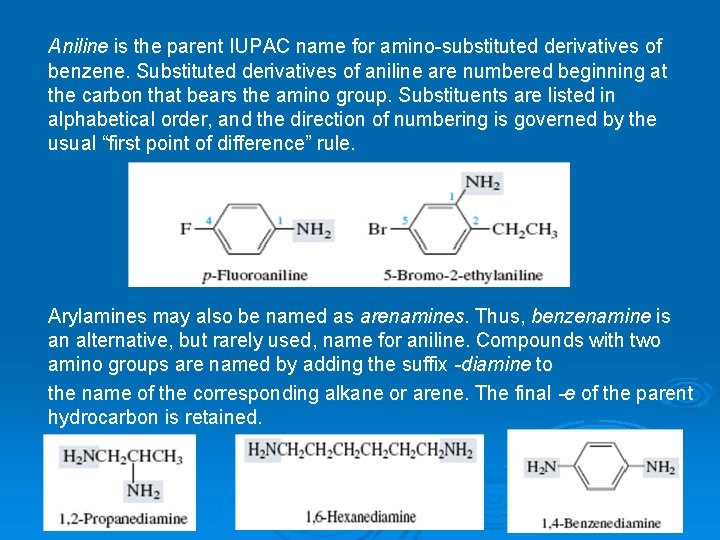
Aniline is the parent IUPAC name for amino-substituted derivatives of benzene. Substituted derivatives of aniline are numbered beginning at the carbon that bears the amino group. Substituents are listed in alphabetical order, and the direction of numbering is governed by the usual “first point of difference” rule. Arylamines may also be named as arenamines. Thus, benzenamine is an alternative, but rarely used, name for aniline. Compounds with two amino groups are named by adding the suffix -diamine to the name of the corresponding alkane or arene. The final -e of the parent hydrocarbon is retained.

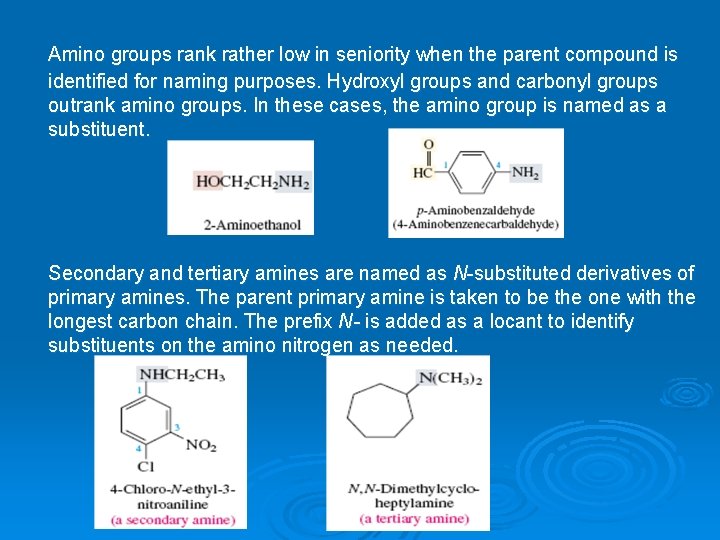
Amino groups rank rather low in seniority when the parent compound is identified for naming purposes. Hydroxyl groups and carbonyl groups outrank amino groups. In these cases, the amino group is named as a substituent. Secondary and tertiary amines are named as N-substituted derivatives of primary amines. The parent primary amine is taken to be the one with the longest carbon chain. The prefix N- is added as a locant to identify substituents on the amino nitrogen as needed.

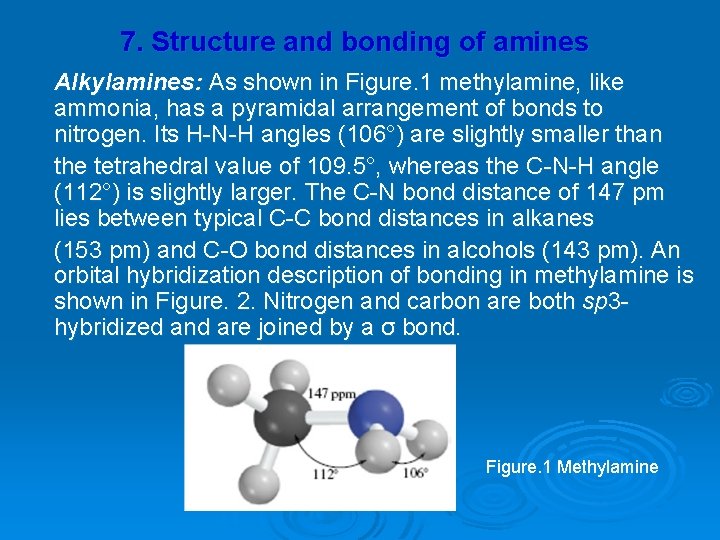
7. Structure and bonding of amines Alkylamines: As shown in Figure. 1 methylamine, like ammonia, has a pyramidal arrangement of bonds to nitrogen. Its H-N-H angles (106°) are slightly smaller than the tetrahedral value of 109. 5°, whereas the C-N-H angle (112°) is slightly larger. The C-N bond distance of 147 pm lies between typical C-C bond distances in alkanes (153 pm) and C-O bond distances in alcohols (143 pm). An orbital hybridization description of bonding in methylamine is shown in Figure. 2. Nitrogen and carbon are both sp 3 hybridized and are joined by a σ bond. Figure. 1 Methylamine

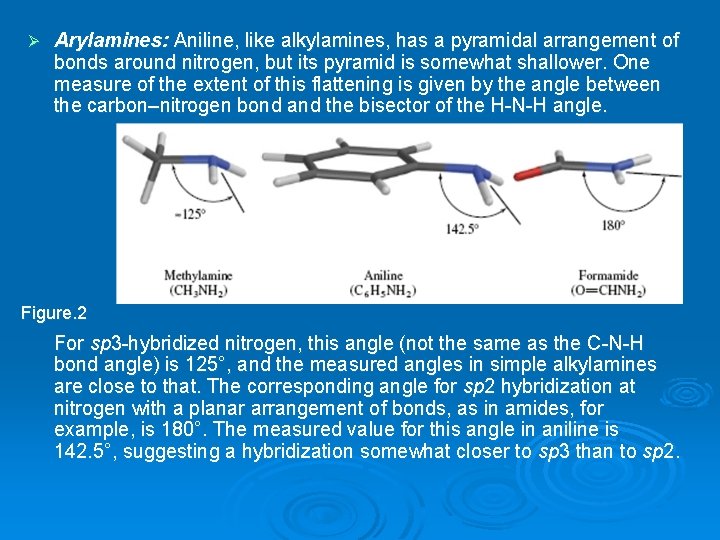
Ø Arylamines: Aniline, like alkylamines, has a pyramidal arrangement of bonds around nitrogen, but its pyramid is somewhat shallower. One measure of the extent of this flattening is given by the angle between the carbon–nitrogen bond and the bisector of the H-N-H angle. Figure. 2 For sp 3 -hybridized nitrogen, this angle (not the same as the C-N-H bond angle) is 125°, and the measured angles in simple alkylamines are close to that. The corresponding angle for sp 2 hybridization at nitrogen with a planar arrangement of bonds, as in amides, for example, is 180°. The measured value for this angle in aniline is 142. 5°, suggesting a hybridization somewhat closer to sp 3 than to sp 2.

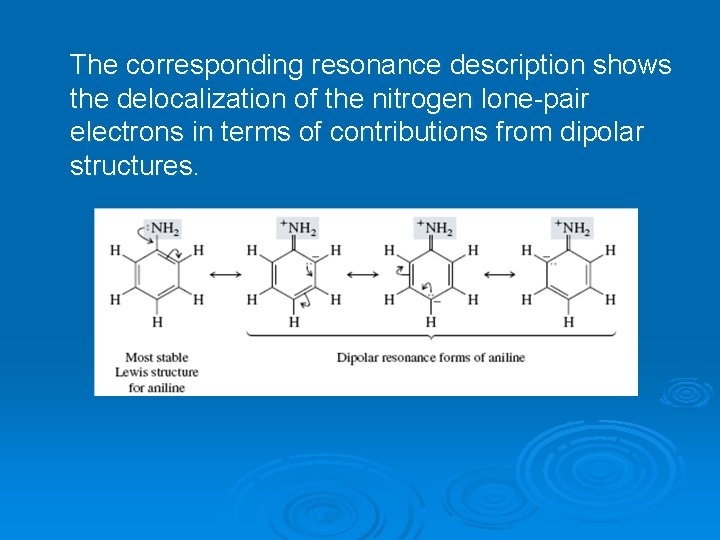
The corresponding resonance description shows the delocalization of the nitrogen lone-pair electrons in terms of contributions from dipolar structures.

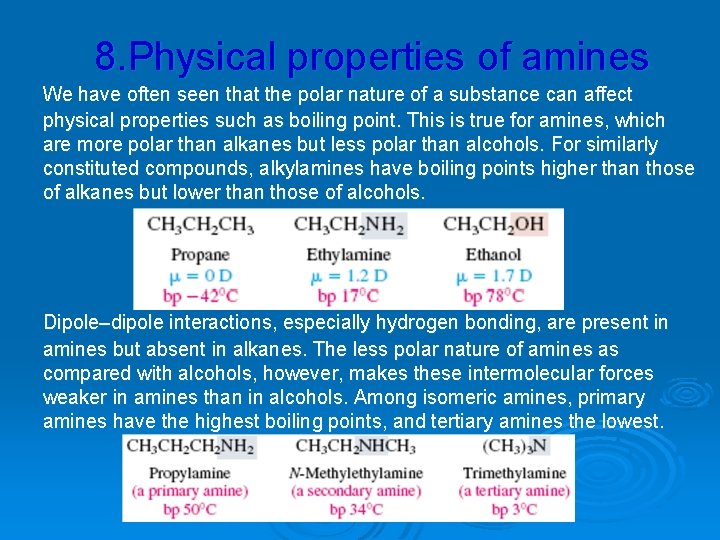
8. Physical properties of amines We have often seen that the polar nature of a substance can affect physical properties such as boiling point. This is true for amines, which are more polar than alkanes but less polar than alcohols. For similarly constituted compounds, alkylamines have boiling points higher than those of alkanes but lower than those of alcohols. Dipole–dipole interactions, especially hydrogen bonding, are present in amines but absent in alkanes. The less polar nature of amines as compared with alcohols, however, makes these intermolecular forces weaker in amines than in alcohols. Among isomeric amines, primary amines have the highest boiling points, and tertiary amines the lowest.

Primary and secondary amines can participate in intermolecular hydrogen bonding, but tertiary amines cannot. Amines that have fewer than six or seven carbon atoms are soluble in water. All amines, even tertiary amines, can act as proton acceptors in hydrogen bonding to water molecules. The simplest arylamine, aniline, is a liquid at room temperature and has a boiling point of 184°C. Almost all other arylamines have higher boiling points. Aniline is only slightly soluble in water (3 g/100 m. L). Substituted derivatives of aniline tend to be even less watersoluble.

9. The methods of extraction of amines


![Hoffman reaction NH 3 CH 3 I CH 3 NH 3I Hoffman reaction: NH 3 + CH 3 I → [CH 3 NH 3+]I− ↔](https://slidetodoc.com/presentation_image/8014489fe77ea2802fa35d39dee1779a/image-26.jpg)
Hoffman reaction: NH 3 + CH 3 I → [CH 3 NH 3+]I− ↔ CH 3 NH 2 + NH 4 I NH 3 CH 3 NH 2 + CH 3 I → [(CH 3)2 NH 2+]I− ↔ (CH 3)2 NH + NH 4 I NH 3 (CH 3)2 NH + CH 3 I → [(CH 3)3 NH+]I− ↔ (CH 3)3 N + NH 4 I NH 3 (CH 3)3 N + CH 3 I → [(CH 3)4 N+]I− Gabriele synthesis:


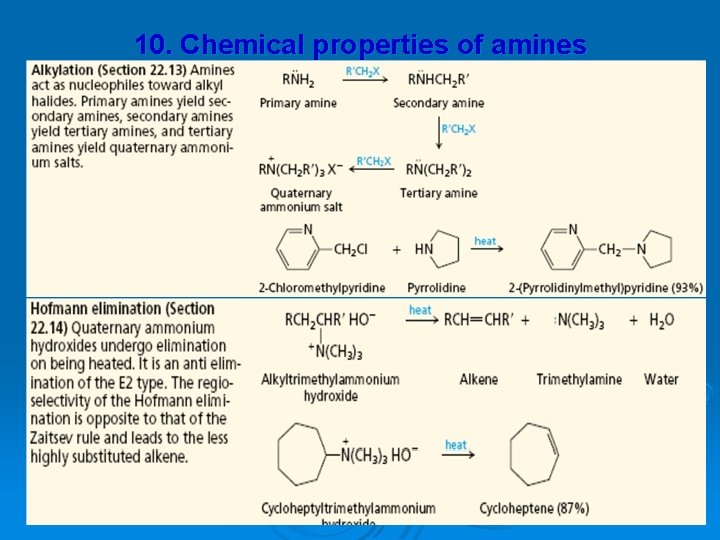
10. Chemical properties of amines

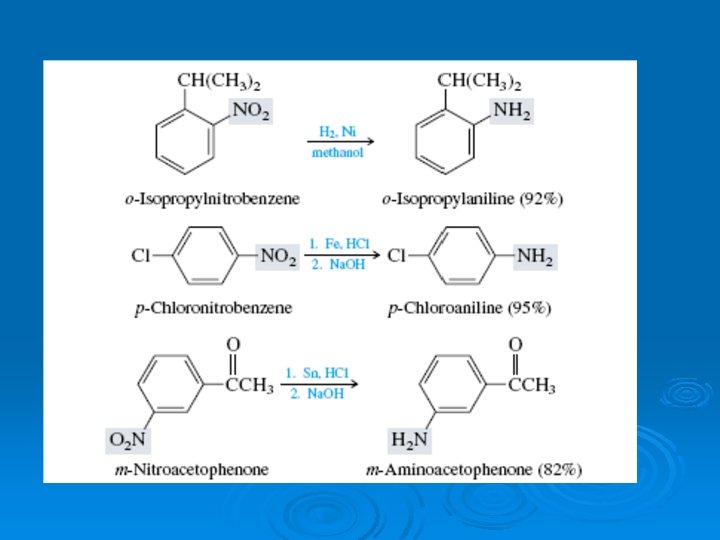
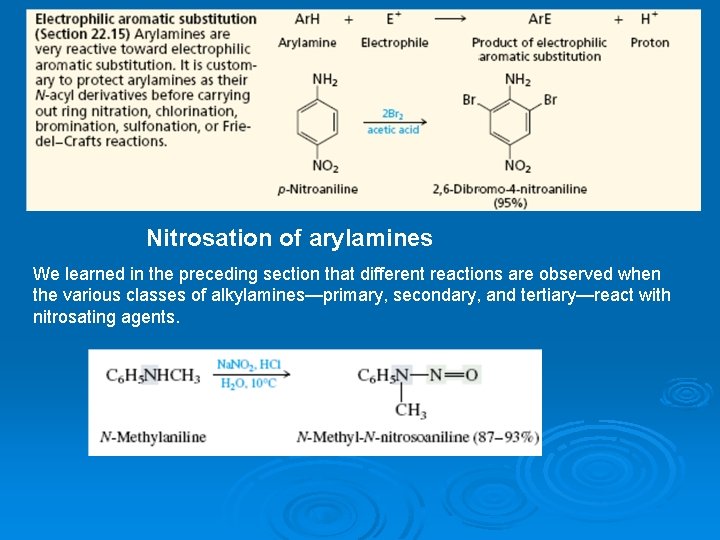
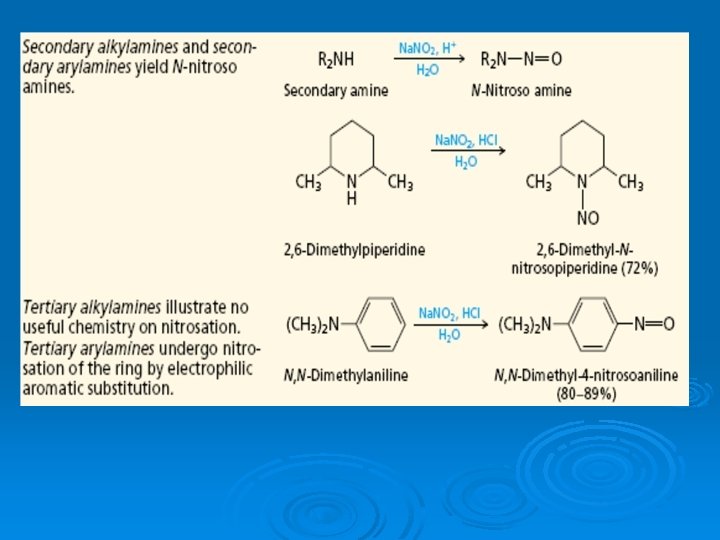
Nitrosation of arylamines We learned in the preceding section that different reactions are observed when the various classes of alkylamines—primary, secondary, and tertiary—react with nitrosating agents.

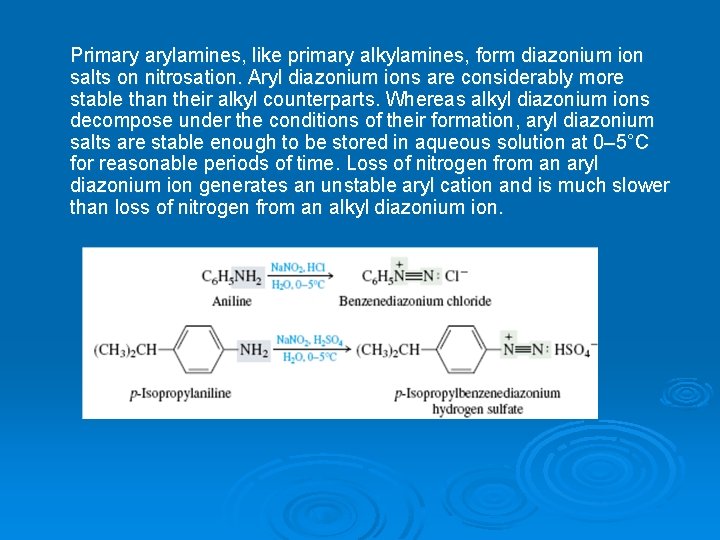
Primary arylamines, like primary alkylamines, form diazonium ion salts on nitrosation. Aryl diazonium ions are considerably more stable than their alkyl counterparts. Whereas alkyl diazonium ions decompose under the conditions of their formation, aryl diazonium salts are stable enough to be stored in aqueous solution at 0– 5°C for reasonable periods of time. Loss of nitrogen from an aryl diazonium ion generates an unstable aryl cation and is much slower than loss of nitrogen from an alkyl diazonium ion.


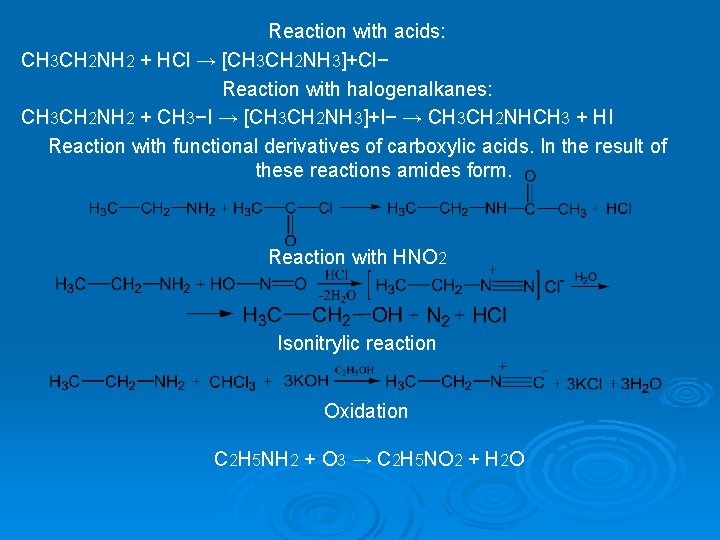
Reaction with acids: CH 3 CH 2 NH 2 + HCl → [CH 3 CH 2 NH 3]+Cl− Reaction with halogenalkanes: CH 3 CH 2 NH 2 + CH 3−I → [CH 3 CH 2 NH 3]+I− → CH 3 CH 2 NHCH 3 + HI Reaction with functional derivatives of carboxylic acids. In the result of these reactions amides form. Reaction with HNO 2 Isonitrylic reaction Oxidation C 2 H 5 NH 2 + O 3 → C 2 H 5 NO 2 + H 2 O

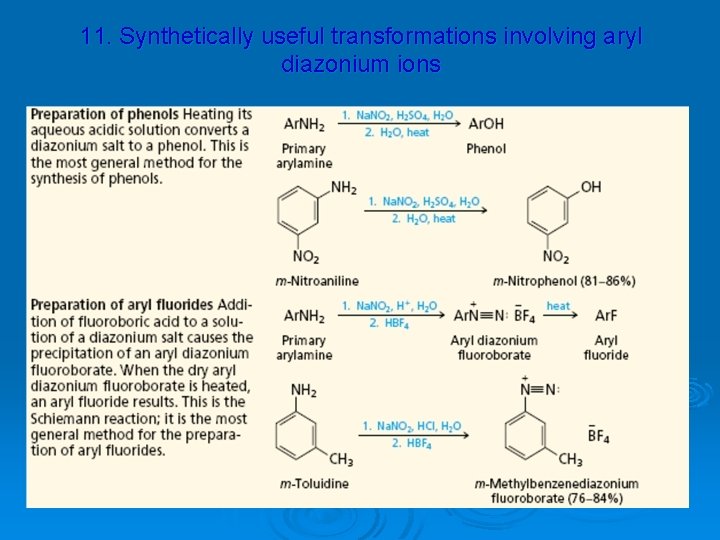
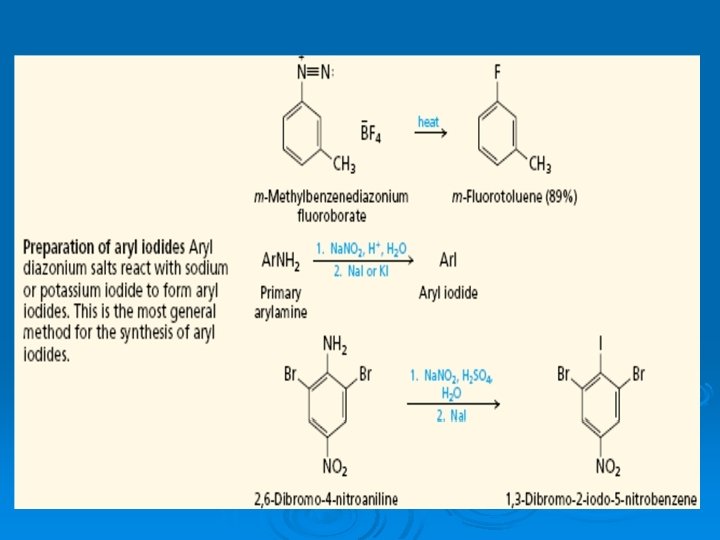
11. Synthetically useful transformations involving aryl diazonium ions




Ø Ø Ø 12. The medico-biological importance of amines Methylamine CH 3 NH 2. It is a gas with the smell of ammonium. Methylamine is used in the production of medicines, dyes, insecticides and fungicides. Putrescin NH 2 CH 2 CH 2 NH 2 (tetramethylendiamine). It is crystal solid. It is formed in the process of rotting of corpses. In the human organism it is used for synthesis of biologically active polyamines which take part in the biosynthesis of DNA and RNA. Cadaverine NH 2 CH 2 CH 2 CH 2 NH 2 (pentamethylendiamine). It is liquid. It is formed in the process of rotting of corpses like putrescin. Aniline C 6 H 5 NH 2. It is colourless liquid with peculiar smell. It is poisonous. It is used in the process of synthesis of dyes, medicines, plastic materials. Phenamine C 6 H 5 CH 2 CH(NH 2)CH 3 (1 phenylpropanamine-2). It is white crystal solid. It is used as stimulator of CNS.

13. Aminoalcohols are the derivatives of hydrocarbons which contain aminogroup in their molecule. For aminoalcohols it is used the nomenclature according to which the location of aminogroup is denoted by number or Greek letter. 2 -aminoethanol or β-aminoethyl alkohol 2 -N-methylaminoethanol Isomery of aminoalcohols is similar to isomery of disubstituted hydrocarbons.

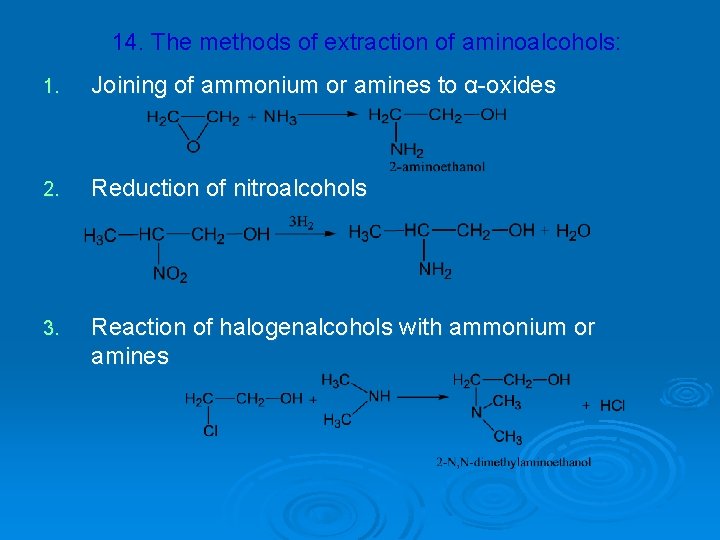
14. The methods of extraction of aminoalcohols: 1. Joining of ammonium or amines to α-oxides 2. Reduction of nitroalcohols 3. Reaction of halogenalcohols with ammonium or amines

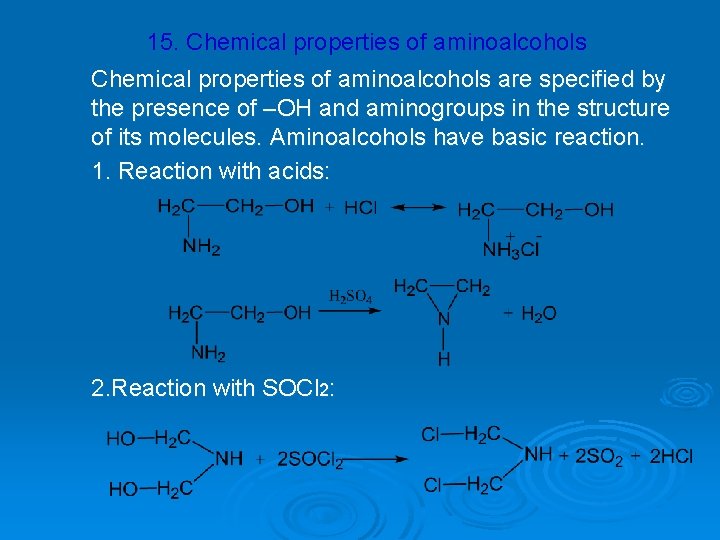
15. Chemical properties of aminoalcohols are specified by the presence of –OH and aminogroups in the structure of its molecules. Aminoalcohols have basic reaction. 1. Reaction with acids: 2. Reaction with SOCl 2:

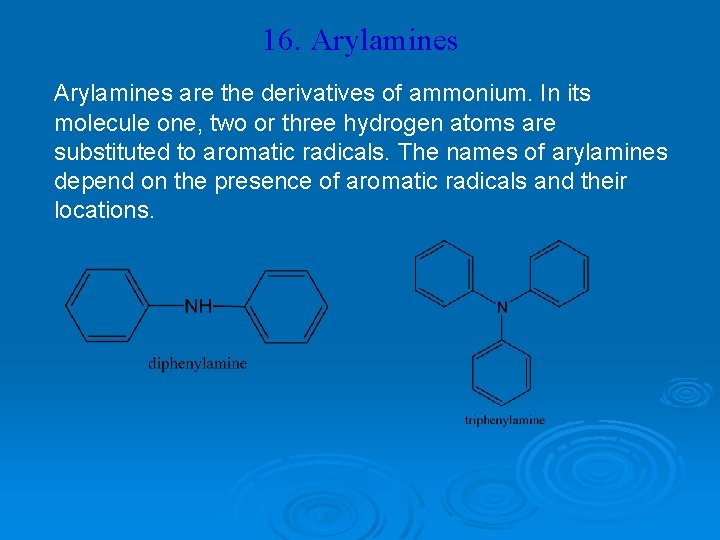
16. Arylamines are the derivatives of ammonium. In its molecule one, two or three hydrogen atoms are substituted to aromatic radicals. The names of arylamines depend on the presence of aromatic radicals and their locations.

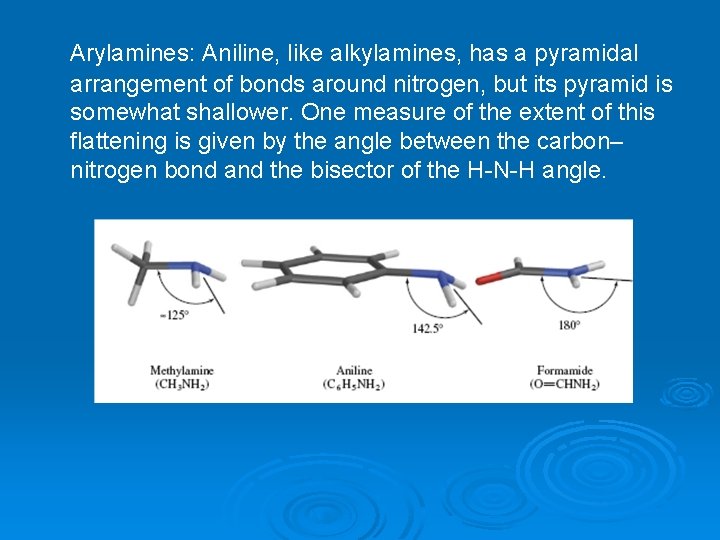
Arylamines: Aniline, like alkylamines, has a pyramidal arrangement of bonds around nitrogen, but its pyramid is somewhat shallower. One measure of the extent of this flattening is given by the angle between the carbon– nitrogen bond and the bisector of the H-N-H angle.

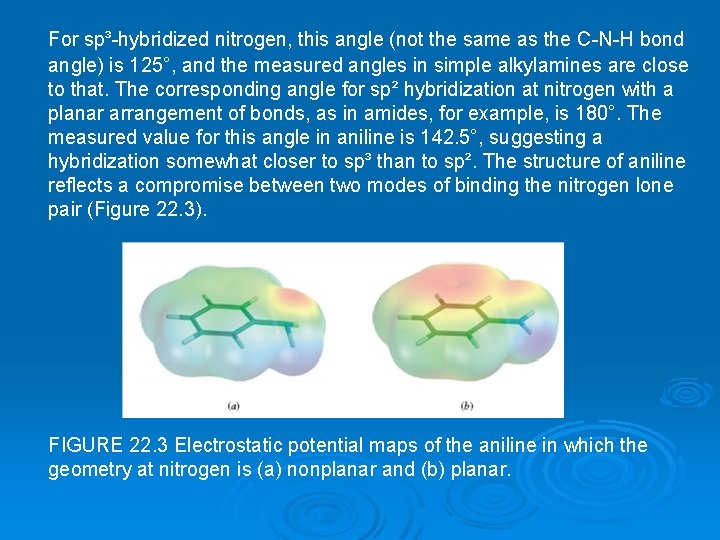
For sp³-hybridized nitrogen, this angle (not the same as the C-N-H bond angle) is 125°, and the measured angles in simple alkylamines are close to that. The corresponding angle for sp² hybridization at nitrogen with a planar arrangement of bonds, as in amides, for example, is 180°. The measured value for this angle in aniline is 142. 5°, suggesting a hybridization somewhat closer to sp³ than to sp². The structure of aniline reflects a compromise between two modes of binding the nitrogen lone pair (Figure 22. 3). FIGURE 22. 3 Electrostatic potential maps of the aniline in which the geometry at nitrogen is (a) nonplanar and (b) planar.

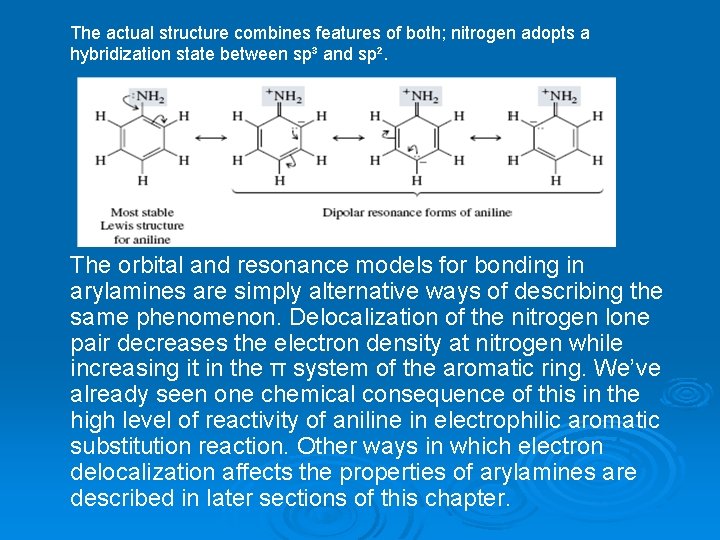
The electrons are more strongly attracted to nitrogen when they are in an orbital with some s character—an sp³hybridized orbital, for example— than when they are in a p orbital. On the other hand, delocalization of these electrons into the aromatic π system is better achieved if they occupy a p orbital. A p orbital of nitrogen is better aligned for overlap with the p orbitals of the benzene ring to forman extended π system than is an sp³-hybridized orbital. As a result of these two opposing forces, nitrogen adopts an orbital hybridization that is between sp³ and sp². The corresponding resonance description shows the delocalization of the nitrogen lone-pair electrons in terms of contributions from dipolar structures. In the nonplanar geometry, the unshared pair occupies an sp³ hybrid orbital of nitrogen. The region of highest electron density in (a) is associated with nitrogen. In the planar geometry, nitrogen is sp²-hybridized and the electron pair is delocalized between a p orbital of nitrogen and the π system of the ring. The region of highest electron density in (b) encompasses both the ring and nitrogen.

The actual structure combines features of both; nitrogen adopts a hybridization state between sp³ and sp². The orbital and resonance models for bonding in arylamines are simply alternative ways of describing the same phenomenon. Delocalization of the nitrogen lone pair decreases the electron density at nitrogen while increasing it in the π system of the aromatic ring. We’ve already seen one chemical consequence of this in the high level of reactivity of aniline in electrophilic aromatic substitution reaction. Other ways in which electron delocalization affects the properties of arylamines are described in later sections of this chapter.

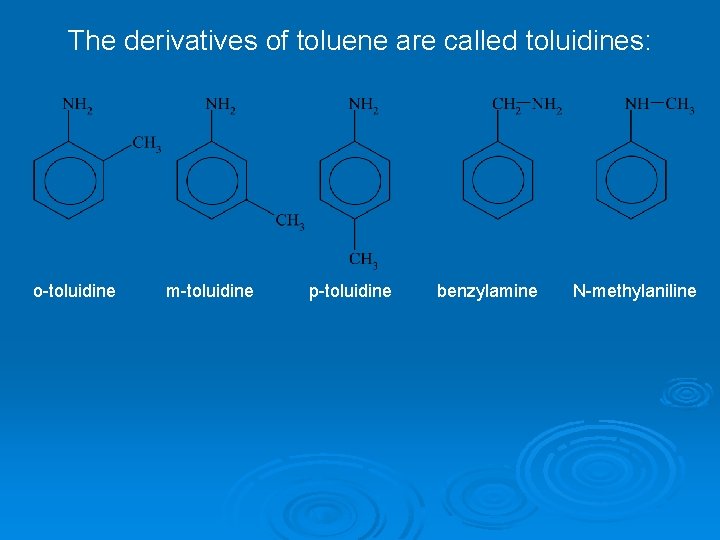
The derivatives of toluene are called toluidines: o-toluidine m-toluidine p-toluidine benzylamine N-methylaniline

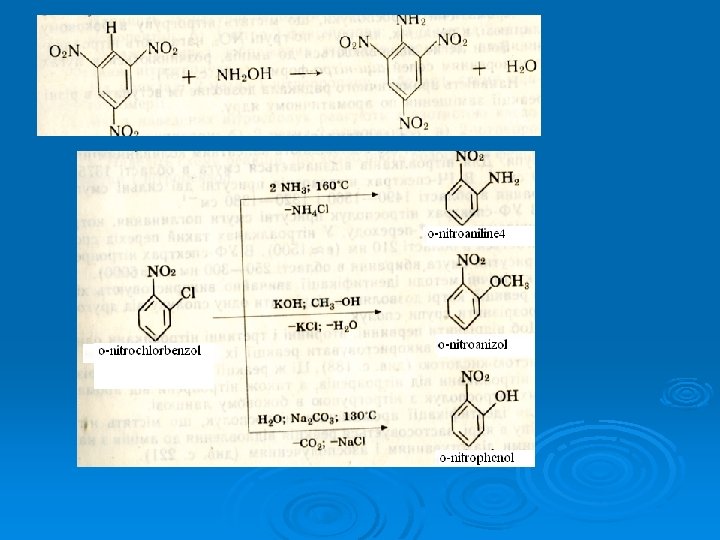
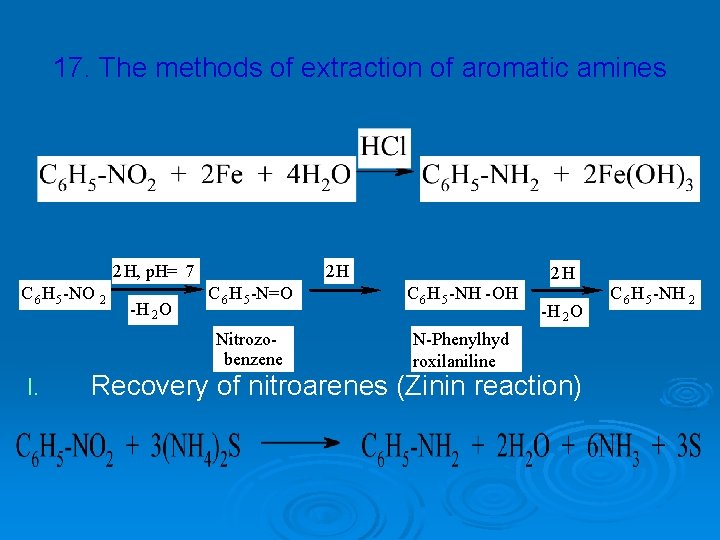
17. The methods of extraction of aromatic amines 2 H, p. H= 7 C 6 H 5 -NO 2 I. -H 2 O 2 H C 6 H 5 -N=O C 6 H 5 -NH -OH Nitrozobenzene N-Phenylhyd roxilaniline 2 H -H 2 O Recovery of nitroarenes (Zinin reaction) C 6 H 5 -NH 2

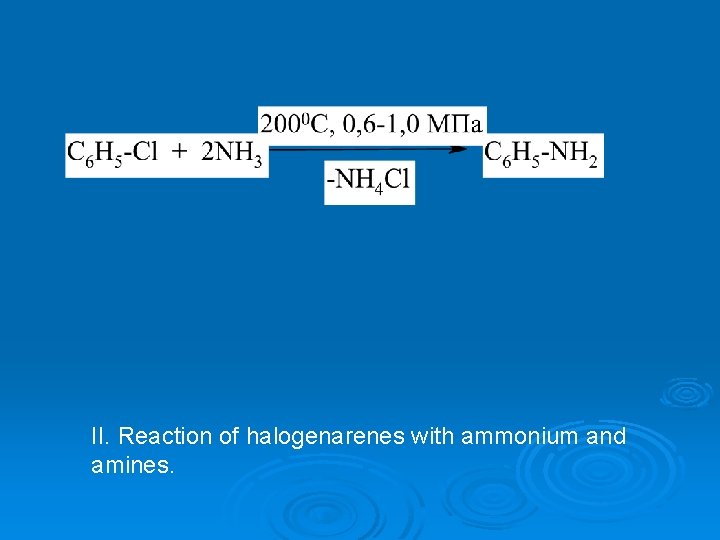
II. Reaction of halogenarenes with ammonium and amines.

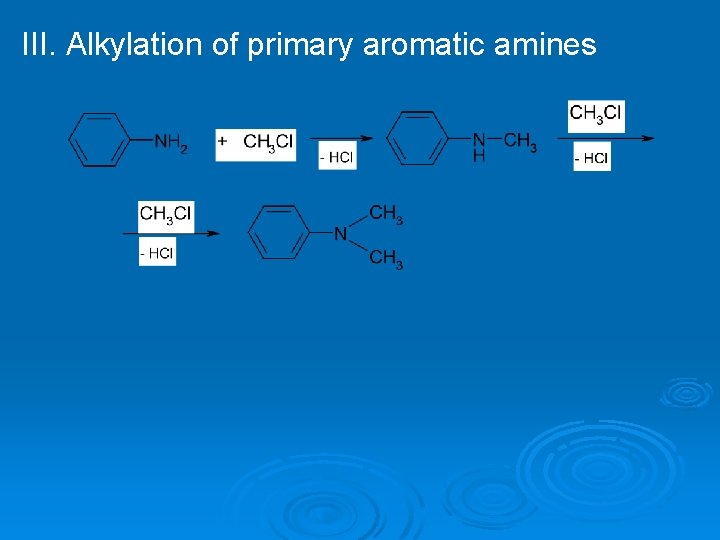
III. Alkylation of primary aromatic amines

18. Physical properties of aromatic amines Aromatic amines are colourless liquids or solids with peculiar smell. They can be oxidized by open air very easily. Aromatic amines are very toxic compounds. Hydrogen bonding significantly influences the properties of primary and secondary amines. Thus the boiling point of amines is higher than those of the corresponding phosphines, but generally lower than those of the corresponding alcohols. Thus methylamine and ethylamine are gases under standard conditions, whereas the corresponding methyl alcohol and ethyl alcohols are liquids. Gaseous amines possess a characteristic ammonia smell, liquid amines have a distinctive "fishy" smell. Also reflecting their ability to form hydrogen bonds, most aliphatic amines display some solubility in water. Solubility decreases with the increase in the number of carbon atoms. Aliphatic amines display significant solubility in organic solvents, especially polar organic solvents. Primary amines react with ketones such as acetone, and most amines are incompatible with chloroform and carbon tetrachloride. The aromatic amines, such as aniline, have their lone pair electrons conjugated into the benzene ring, thus their tendency to engage in hydrogen bonding is diminished. Their boiling points are high and their solubility in water low

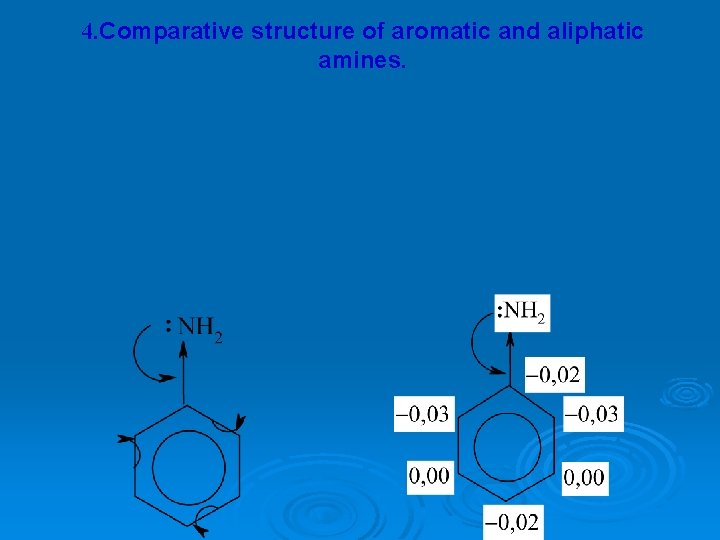
4. Comparative structure of aromatic and aliphatic amines.

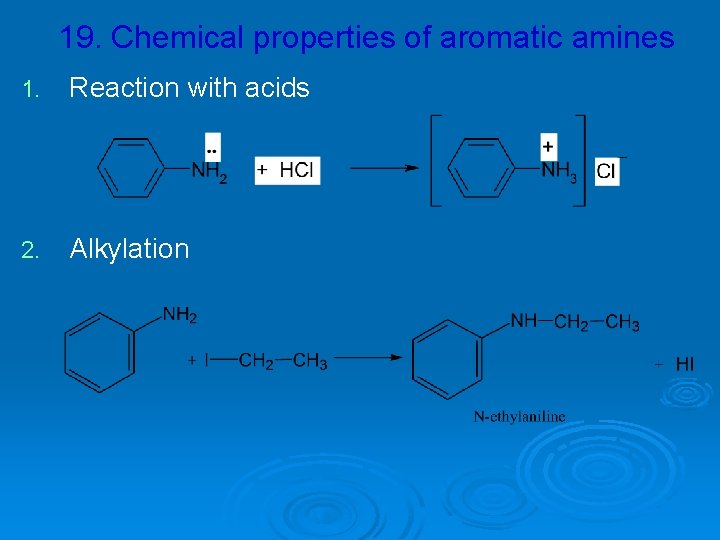
19. Chemical properties of aromatic amines 1. Reaction with acids 2. Alkylation

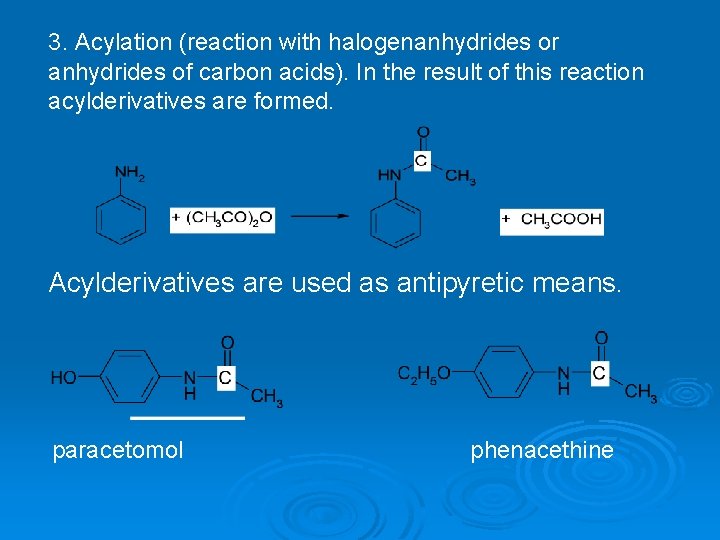
3. Acylation (reaction with halogenanhydrides or anhydrides of carbon acids). In the result of this reaction acylderivatives are formed. Acylderivatives are used as antipyretic means. paracetomol phenacethine

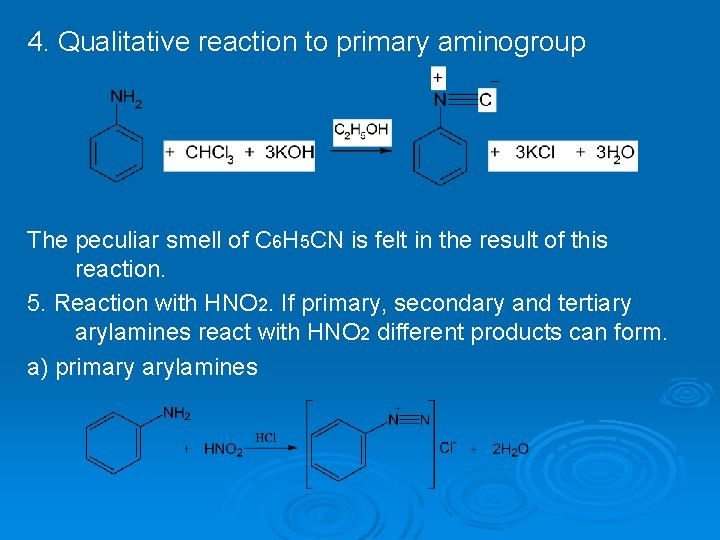
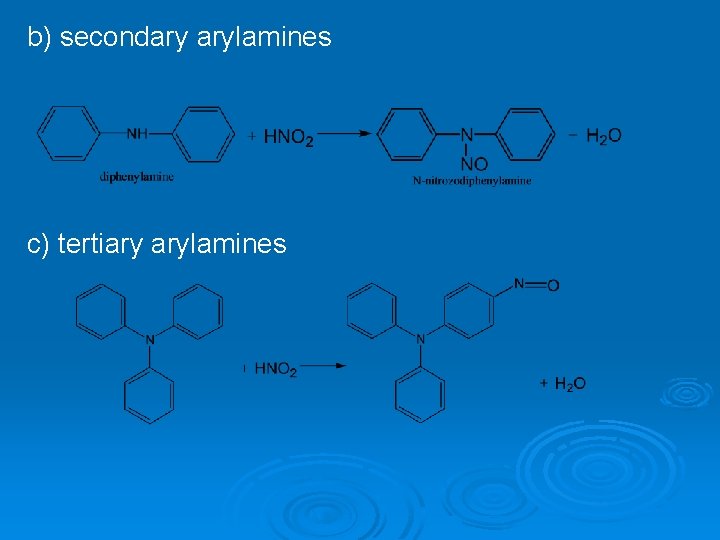
4. Qualitative reaction to primary aminogroup The peculiar smell of C 6 H 5 CN is felt in the result of this reaction. 5. Reaction with HNO 2. If primary, secondary and tertiary arylamines react with HNO 2 different products can form. a) primary arylamines

b) secondary arylamines c) tertiary arylamines

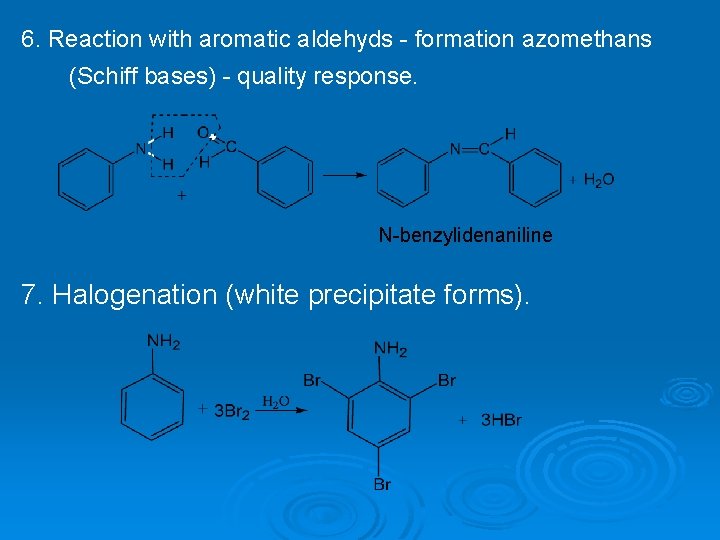
6. Reaction with aromatic aldehyds - formation azomethans (Schiff bases) - quality response. N-benzylidenaniline 7. Halogenation (white precipitate forms).

8. Nitration reaction - reaction of transmitting, making protection of amino groups.

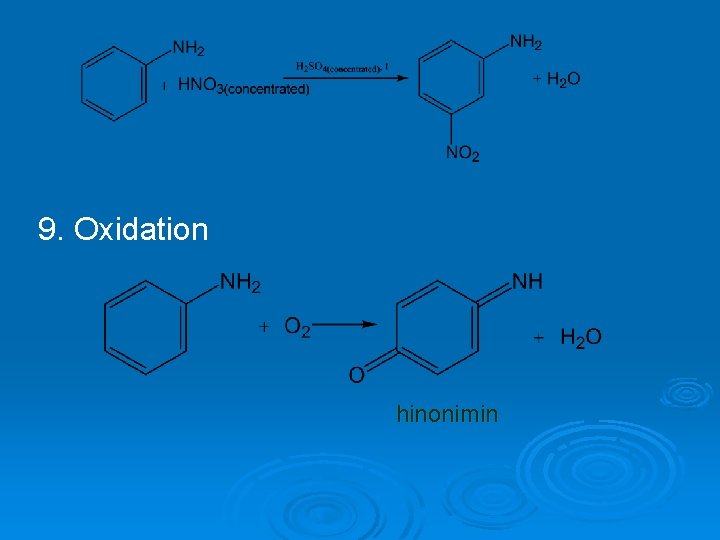
9. Oxidation hinonimin
![NH 2 NO O H 2 SO 5 nitrozobenzene NH 2 NO 2 NH 2 NO + [O] H 2 SO 5 nitrozobenzene NH 2 NO 2](https://slidetodoc.com/presentation_image/8014489fe77ea2802fa35d39dee1779a/image-59.jpg)
NH 2 NO + [O] H 2 SO 5 nitrozobenzene NH 2 NO 2 + [O] CF 3 COOOH nitrobenzene

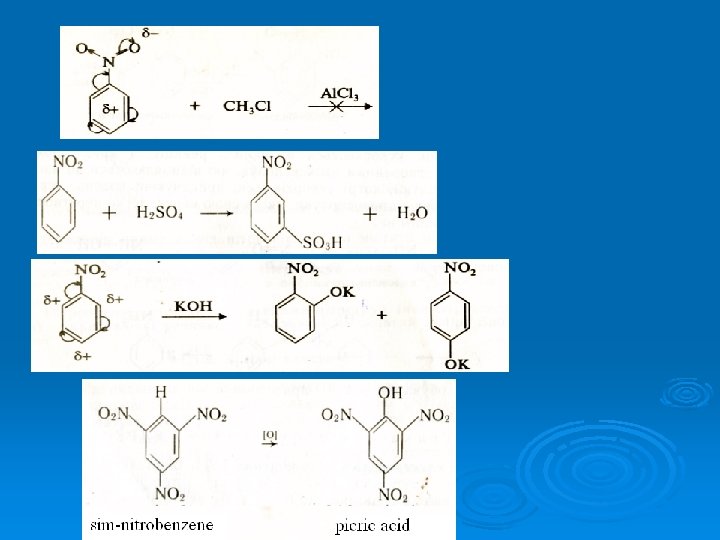
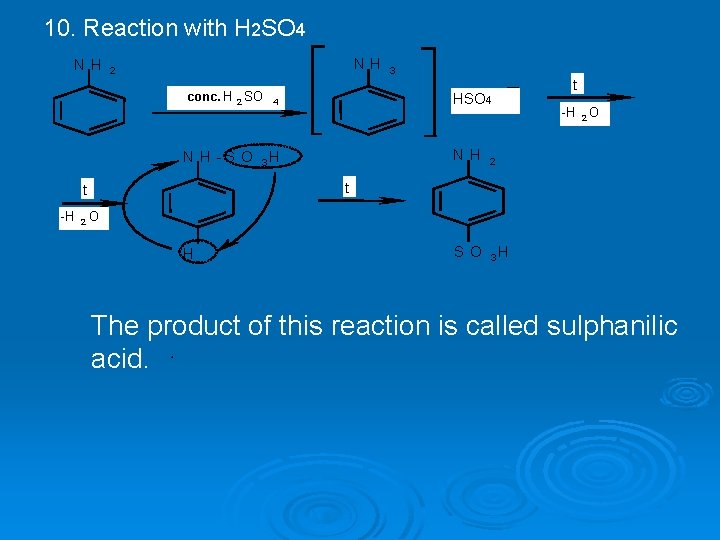
10. Reaction with H 2 SO 4 N H 2 conc. H 2 SO N H -S O HSO 4 4 3 H N H 2 S O 3 H t -H 2 O t t -H 3 2 O H The product of this reaction is called sulphanilic acid.

20. Sulphanilic acid has acidic (-SO 3 H) and alkaline (NH 2) centers in its molecule. Sulphanilic acid is quite active acid. It easily forms salts with alkalis. But it does not react with mineral acids. Although it has alkaline (NH 2) group it does not have alkaline properties. Sulphanilic acid is widely used in production of some medicines and dyes. It is the structural part of a large group of medicines which have antibacterial action. They are called sulphanylamides. The basic compound of all sulphanylamides is streptocide. It is amide of sulphanilic acid:

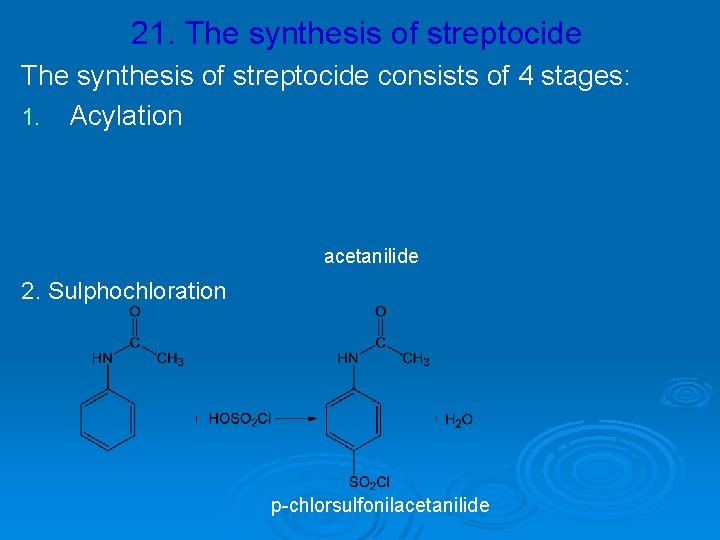
21. The synthesis of streptocide consists of 4 stages: 1. Acylation acetanilide 2. Sulphochloration p-chlorsulfonilacetanilide

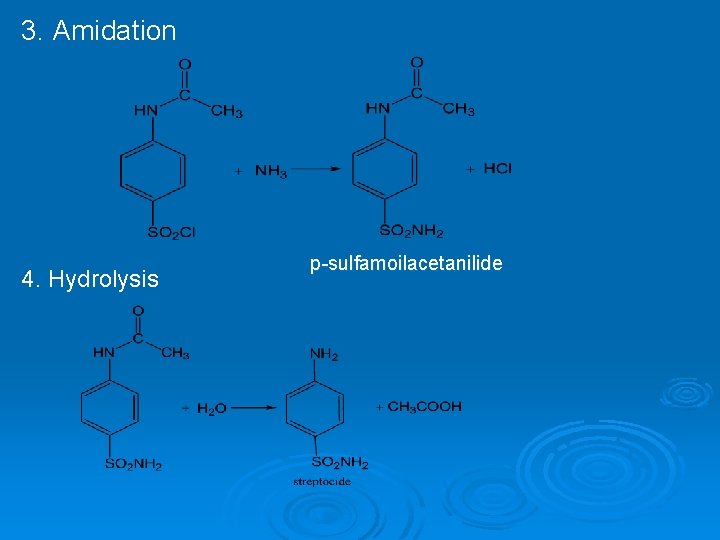
3. Amidation 4. Hydrolysis p-sulfamoilacetanilide

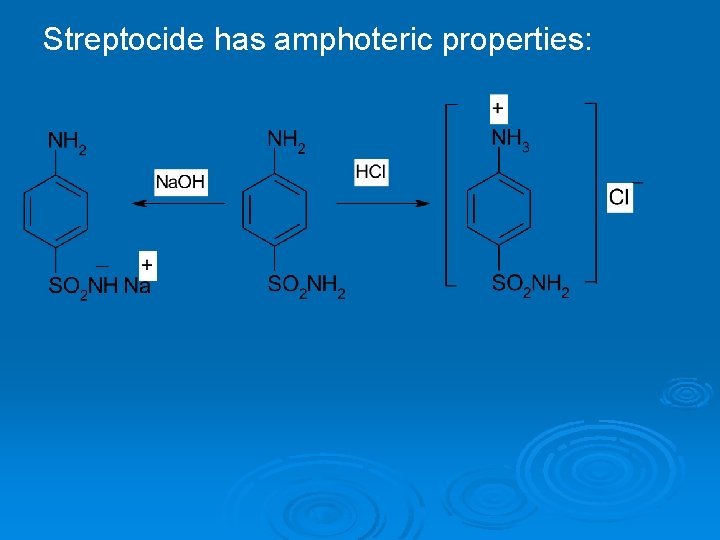
Streptocide has amphoteric properties:

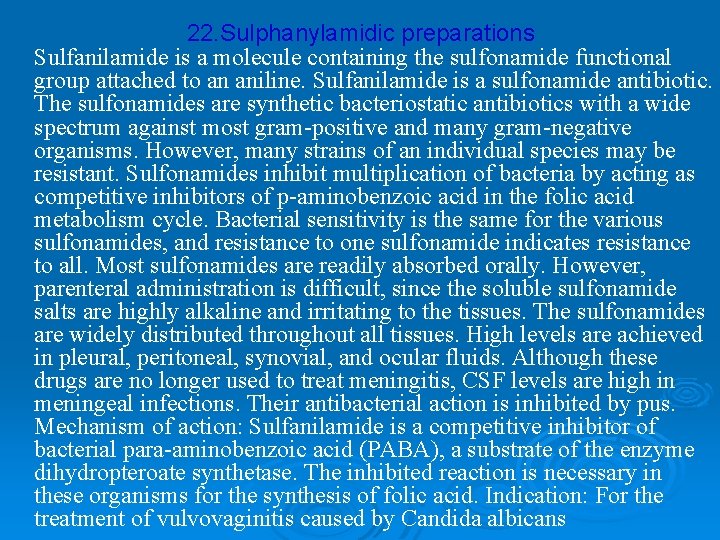
22. Sulphanylamidic preparations Sulfanilamide is a molecule containing the sulfonamide functional group attached to an aniline. Sulfanilamide is a sulfonamide antibiotic. The sulfonamides are synthetic bacteriostatic antibiotics with a wide spectrum against most gram-positive and many gram-negative organisms. However, many strains of an individual species may be resistant. Sulfonamides inhibit multiplication of bacteria by acting as competitive inhibitors of p-aminobenzoic acid in the folic acid metabolism cycle. Bacterial sensitivity is the same for the various sulfonamides, and resistance to one sulfonamide indicates resistance to all. Most sulfonamides are readily absorbed orally. However, parenteral administration is difficult, since the soluble sulfonamide salts are highly alkaline and irritating to the tissues. The sulfonamides are widely distributed throughout all tissues. High levels are achieved in pleural, peritoneal, synovial, and ocular fluids. Although these drugs are no longer used to treat meningitis, CSF levels are high in meningeal infections. Their antibacterial action is inhibited by pus. Mechanism of action: Sulfanilamide is a competitive inhibitor of bacterial para-aminobenzoic acid (PABA), a substrate of the enzyme dihydropteroate synthetase. The inhibited reaction is necessary in these organisms for the synthesis of folic acid. Indication: For the treatment of vulvovaginitis caused by Candida albicans

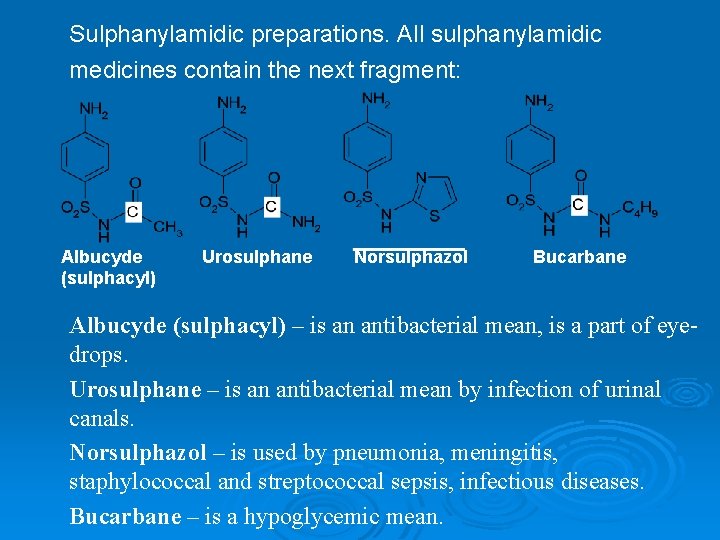
Sulphanylamidic preparations. All sulphanylamidic medicines contain the next fragment: Albucyde (sulphacyl) Urosulphane Norsulphazol Bucarbane Albucyde (sulphacyl) – is an antibacterial mean, is a part of eyedrops. Urosulphane – is an antibacterial mean by infection of urinal canals. Norsulphazol – is used by pneumonia, meningitis, staphylococcal and streptococcal sepsis, infectious diseases. Bucarbane – is a hypoglycemic mean.

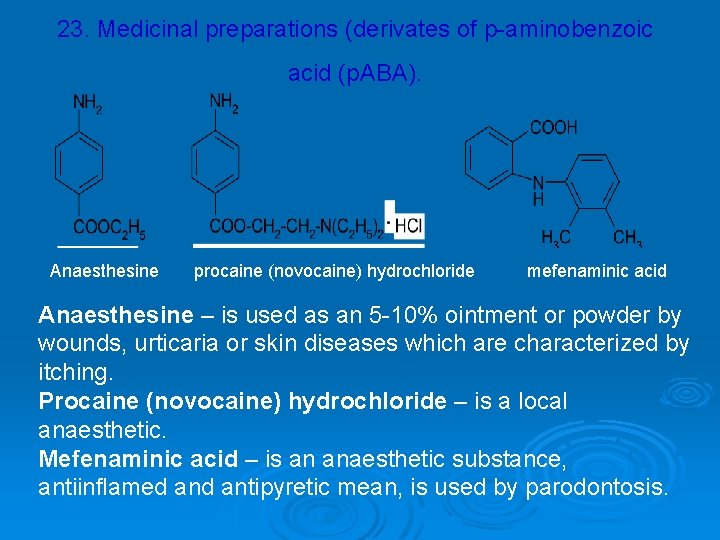
23. Medicinal preparations (derivates of p-aminobenzoic acid (p. ABA). Anaesthesine procaine (novocaine) hydrochloride mefenaminic acid Anaesthesine – is used as an 5 -10% ointment or powder by wounds, urticaria or skin diseases which are characterized by itching. Procaine (novocaine) hydrochloride – is a local anaesthetic. Mefenaminic acid – is an anaesthetic substance, antiinflamed antipyretic mean, is used by parodontosis.

4 -Aminobenzoic acid (also known as paraaminobenzoic acid or PABA) is an organic compound with the molecular formula C 7 H 7 NO 2. PABA is a white crystalline substance that is only slightly soluble in water. It consists of a benzene ring substituted with an amino group and a carboxyl group. PABA is an essential nutrient for some bacteria and is sometimes called Vitamin Bx. In humans, PABA is normally made by E. coli in the colon and therefore PABA from food is not normally essential to human health. PABA is therefore not officially classified as a vitamin. PABA is an intermediate in bacterial synthesis of folate. Although humans lack the ability to synthesize folate from PABA, that is also normally done by E. coli. PABA is sometimes marketed as an essential nutrient for use whenever normal PABA synthesis by intestinal bacteria is insufficient.

Medical use of 4 -Aminobenzoic acid (also known as para-aminobenzoic acid or PABA) The potassium salt is used as a drug against fibrotic skin disorders, such as Peyronie's disease, under the trade name Potaba. PABA is also occasionally used in pill form by sufferers of Irritable bowel syndrome to treat its associated gastrointestinal symptoms, and in nutritional epidemiological studies to assess the completeness of 24 -hour urine collection for the determination of urinary sodium, potassium, or nitrogen levels.

24. Diazocompounds are organic compounds that contain NNgroup which is connected with hydrocarbon radical and radical of mineral acid. The general formula of diazocompounds is: RN 2 X, where R – is a hydrocarbon radical X – is a radical of mineral acid (Cl−, Br−, NO 3−, SO 4 H−, OH−, CN−, SO 3 H−, SH−. There aliphatic and aromatic diazocompounds. But aromatic diazocompounds are more important for production of dyes and medicines, in pharmaceutical analysis. The general formula of aromatic diazocompounds is Ar. N 2 X, where Ar – is an aromatic radical X – is a radical of mineral acid (Cl−, Br−, NO 3−, SO 4 H−, OH−, CN−, SO 3 H−, SH−.

In acid medium aromatic diazocompounds have ionic structure and they are called salts of diazonium (Ar– N+≡NX−). In neutral medium aromatic diazocompounds have covalent structure (Ar–N=N–X). In alkaline medium aromatic diazocompounds are diazotates. acid medium neutral medium alkaline medium

The systematic (IUPAC) name of aromatic diazocompounds is obtained by adding the suffix “-diazo”or “-dizonium-” (in the case of salts of diazonium). For example: benzenediazohydroxide sodium benzenediazotate 4 -chlorobenzenedizocyanide 4 -methylbenzenediazonium chloride Physical properties salts of diazonium Salts of diazonium are colorless crystalline substance, easily soluble in water. They are unstable on heating and mechanical actions of explosion. That why decomposed in reactions usually use them freshly prepared aqueous solutions

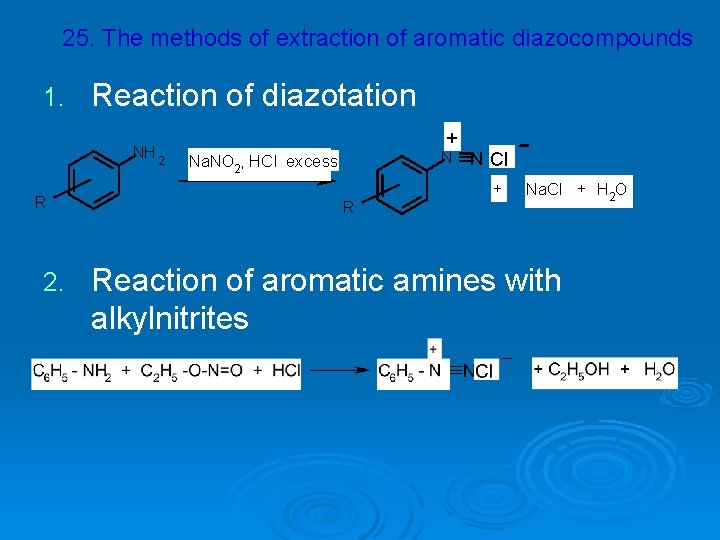
25. The methods of extraction of aromatic diazocompounds 1. Reaction of diazotation NH 2 R 2. + N N Cl Nа. NO 2, HCl excess R + Nа. Cl + H 2 O Reaction of aromatic amines with alkylnitrites

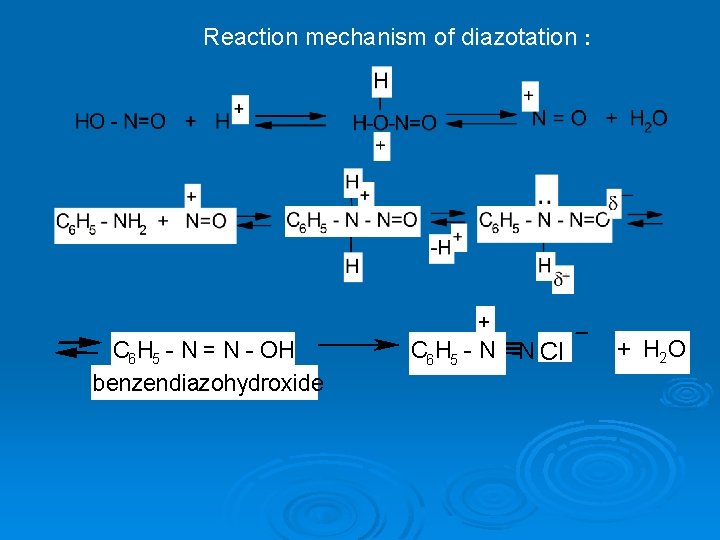
Reaction mechanism of diazotation : + С 6 H 5 - N = N - OH benzendiazohydroxide С 6 H 5 - N N Cl + H 2 O

26. Chemical properties of aromatic diazocompounds I. Reaction with extraction of N 2 +KBr

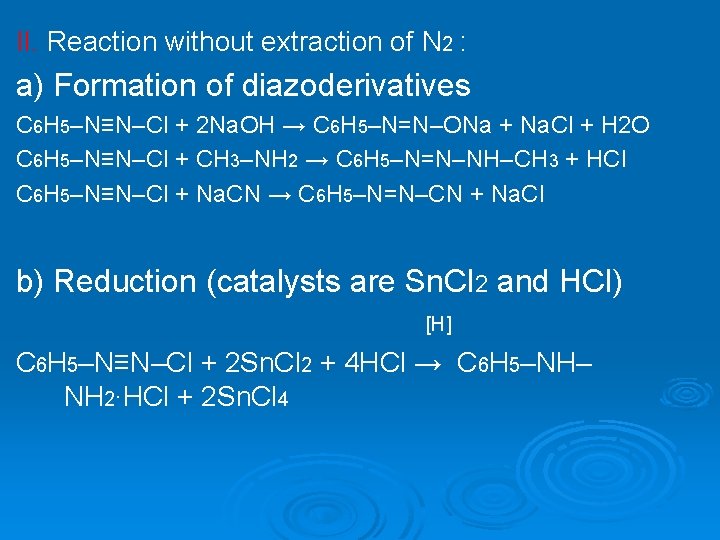
II. Reaction without extraction of N 2 : a) Formation of diazoderivatives C 6 H 5–N≡N–Cl + 2 Na. OH → C 6 H 5–N=N–ONa + Na. Cl + H 2 O C 6 H 5–N≡N–Cl + CH 3–NH 2 → C 6 H 5–N=N–NH–CH 3 + HCl C 6 H 5–N≡N–Cl + Na. CN → C 6 H 5–N=N–CN + Na. Cl b) Reduction (catalysts are Sn. Cl 2 and HCl) [H] C 6 H 5–N≡N–Cl + 2 Sn. Cl 2 + 4 HCl → C 6 H 5–NH– NH 2∙HCl + 2 Sn. Cl 4

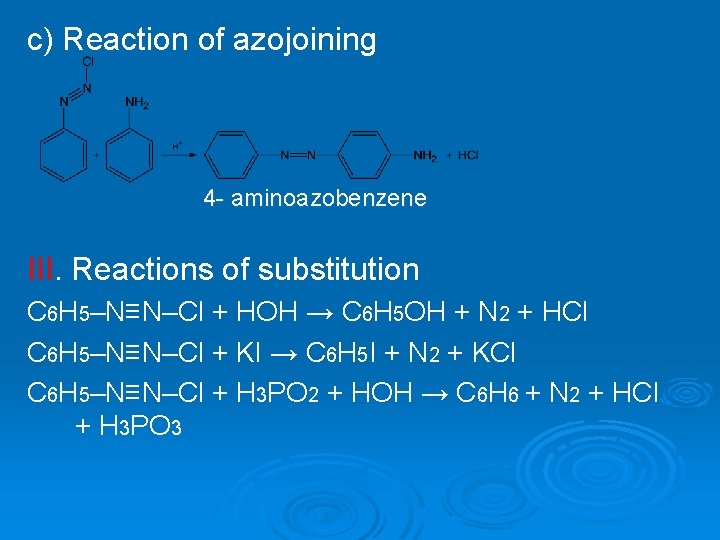
c) Reaction of azojoining 4 - aminoazobenzene III. Reactions of substitution C 6 H 5–N≡N–Cl + HOH → C 6 H 5 OH + N 2 + HCl C 6 H 5–N≡N–Cl + KI → C 6 H 5 I + N 2 + KCl C 6 H 5–N≡N–Cl + H 3 PO 2 + HOH → C 6 H 6 + N 2 + HCl + H 3 PO 3

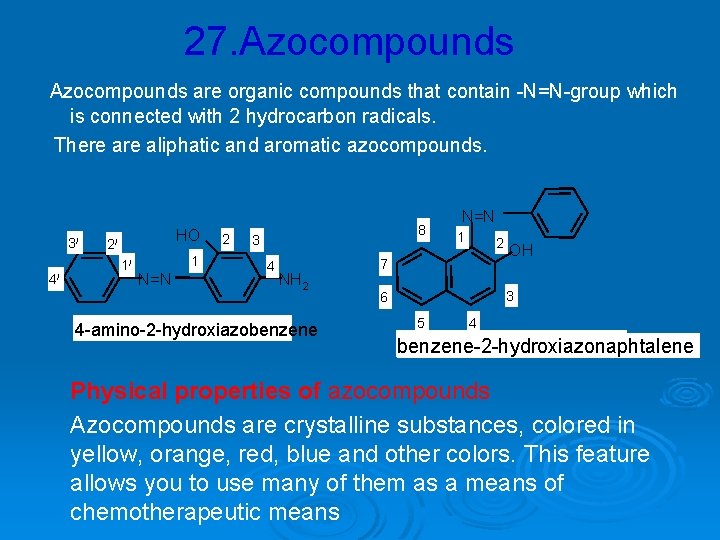
27. Azocompounds are organic compounds that contain -N=N-group which is connected with 2 hydrocarbon radicals. There aliphatic and aromatic azocompounds. 3/ 4/ HO 2/ 1/ 1 N=N 2 8 3 4 NH 2 4 -amino-2 -hydroxiazobenzene N=N 1 2 7 OH 3 6 5 4 benzene-2 -hydroxiazonaphtalene Physical properties of azocompounds Azocompounds are crystalline substances, colored in yellow, orange, red, blue and other colors. This feature allows you to use many of them as a means of chemotherapeutic means

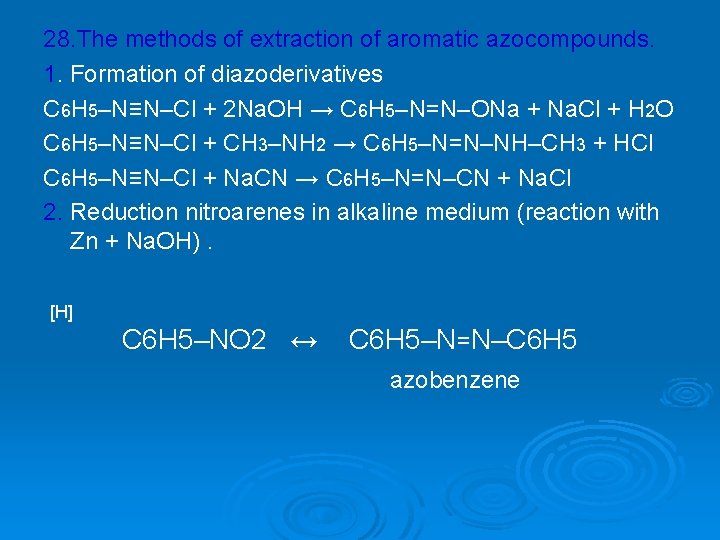
28. The methods of extraction of aromatic azocompounds. 1. Formation of diazoderivatives C 6 H 5–N≡N–Cl + 2 Na. OH → C 6 H 5–N=N–ONa + Na. Cl + H 2 O C 6 H 5–N≡N–Cl + CH 3–NH 2 → C 6 H 5–N=N–NH–CH 3 + HCl C 6 H 5–N≡N–Cl + Na. CN → C 6 H 5–N=N–CN + Na. Cl 2. Reduction nitroarenes in alkaline medium (reaction with Zn + Na. OH). [H] C 6 H 5–NO 2 ↔ C 6 H 5–N=N–C 6 H 5 azobenzene

29. Chemical properties of aromatic azocompounds: Chemical properties are specified by group –N=N–: 1. Reaction with mineral acids: C 6 H 5–N=N–C 6 H 5 + HCl ↔ C 6 H 5–N+H=N–C 6 H 5 + Cl¯ 2. Oxidation (reaction with peroxiacids) [O] C 6 H 5–N=N–C 6 H 5 ↔ C 6 H 5–N+O ¯ =N–C 6 H 5 3. Reduction (reaction with Zn + Na. OH) [2 H] C 6 H 5–N=N–C 6 H 5 ↔ C 6 H 5–NH–NH–C 6 H 5

30. Physical bases of theory of colouration. The theory of colouration studies the dependence of colour of organic compounds on the structure of molecules. The colour of any compound is specified its ability to absorb electromagnetic radiation. Color or colour (see spelling differences) is the visual perceptual property corresponding in humans to the categories called red, yellow, blue and others. Color derives from the spectrum of light (distribution of light energy versus wavelength) interacting in the eye with the spectral sensitivities of the light receptors. Color categories and physical specifications of color are also associated with objects, materials, light sources, etc. , based on their physical properties such as light absorption, reflection, or emission spectra.

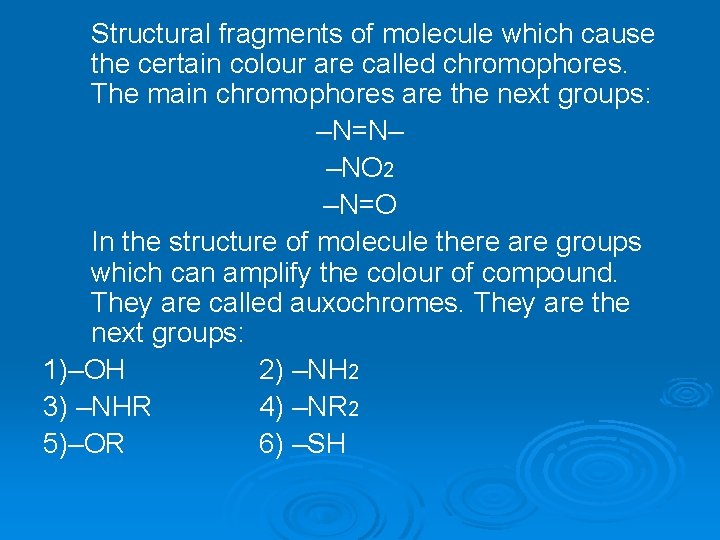
Structural fragments of molecule which cause the certain colour are called chromophores. The main chromophores are the next groups: –N=N– –NO 2 –N=O In the structure of molecule there are groups which can amplify the colour of compound. They are called auxochromes. They are the next groups: 1)–OH 2) –NH 2 3) –NHR 4) –NR 2 5)–OR 6) –SH

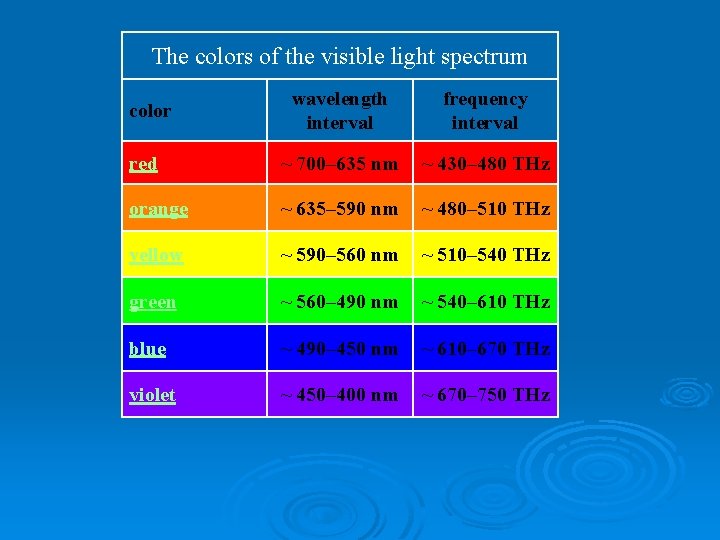
The colors of the visible light spectrum wavelength interval frequency interval red ~ 700– 635 nm ~ 430– 480 THz orange ~ 635– 590 nm ~ 480– 510 THz yellow ~ 590– 560 nm ~ 510– 540 THz green ~ 560– 490 nm ~ 540– 610 THz blue ~ 490– 450 nm ~ 610– 670 THz violet ~ 450– 400 nm ~ 670– 750 THz color

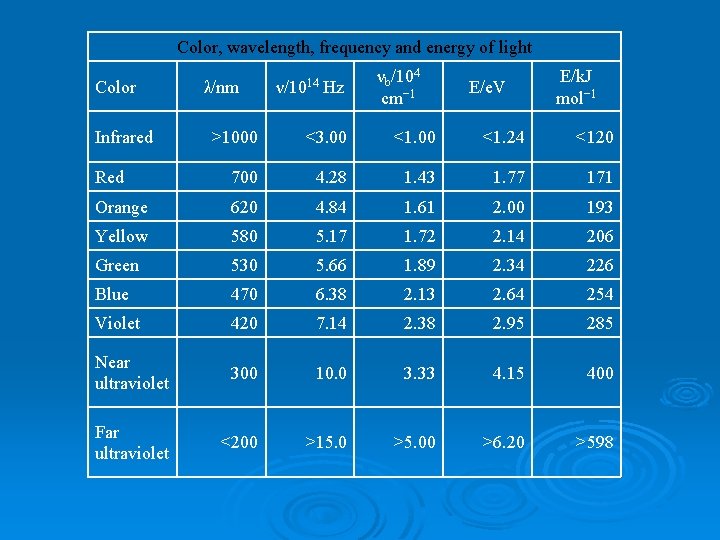
Color, wavelength, frequency and energy of light Color Infrared λ/nm ν/1014 Hz νb/104 cm− 1 E/e. V E/k. J mol− 1 >1000 <3. 00 <1. 24 <120 Red 700 4. 28 1. 43 1. 77 171 Orange 620 4. 84 1. 61 2. 00 193 Yellow 580 5. 17 1. 72 2. 14 206 Green 530 5. 66 1. 89 2. 34 226 Blue 470 6. 38 2. 13 2. 64 254 Violet 420 7. 14 2. 38 2. 95 285 Near ultraviolet 300 10. 0 3. 33 4. 15 400 Far ultraviolet <200 >15. 0 >5. 00 >6. 20 >598

In the 1969 study Basic Color Terms: Their Universality and Evolution, Brent Berlin and Paul Kay describe a pattern in naming "basic" colors (like "red" but not "red-orange" or "dark red" or "blood red", which are "shades" of red). All languages that have two "basic" color names distinguish dark/cool colors from bright/warm colors. The next colors to be distinguished are usually red and then yellow or green. All languages with six "basic" colors include black, white, red, green, blue and yellow. The pattern holds up to a set of twelve: black, grey, white, pink, red, orange, yellow, green, blue, purple, brown, and azure (distinct from blue in Russian and Italian but not English).

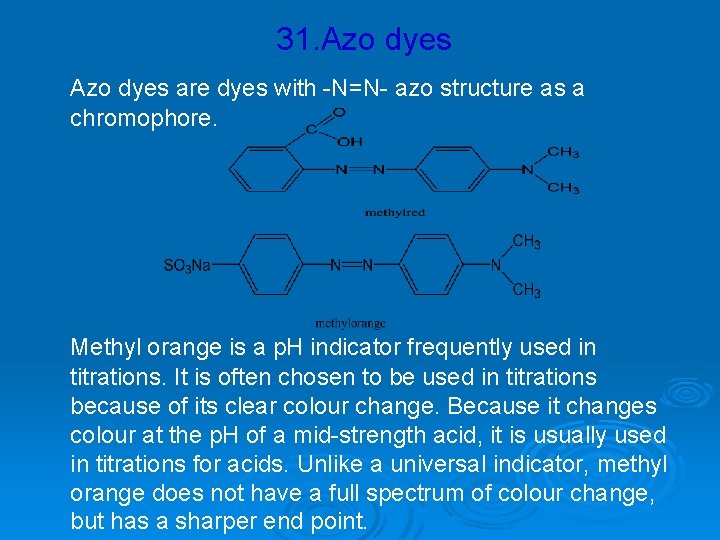
31. Azo dyes are dyes with -N=N- azo structure as a chromophore. Methyl orange is a p. H indicator frequently used in titrations. It is often chosen to be used in titrations because of its clear colour change. Because it changes colour at the p. H of a mid-strength acid, it is usually used in titrations for acids. Unlike a universal indicator, methyl orange does not have a full spectrum of colour change, but has a sharper end point.

In a solution becoming less acidic, methyl orange moves from red to orange and finally to yellow with the reverse occurring for a solution increasing in acidity. It should be noted that the entire colour change occurs in acidic conditions. In an acid it is reddish and in alkali it is yellow.

Methyl red, also called C. I. Acid Red 2, is an indicator dye that turns red in acidic solutions. It is an azo-dye, and is a dark red crystalline powder. Methyl red is a p. H indicator; it is red in p. H under 4. 4, yellow in p. H over 6. 2, and orange in between, with a p. Ka of approximately 5. Preparation of methyl red As an azo dye, methyl red may be prepared by diazotization of anthranilic acid, followed by reaction with dimethylaniline:

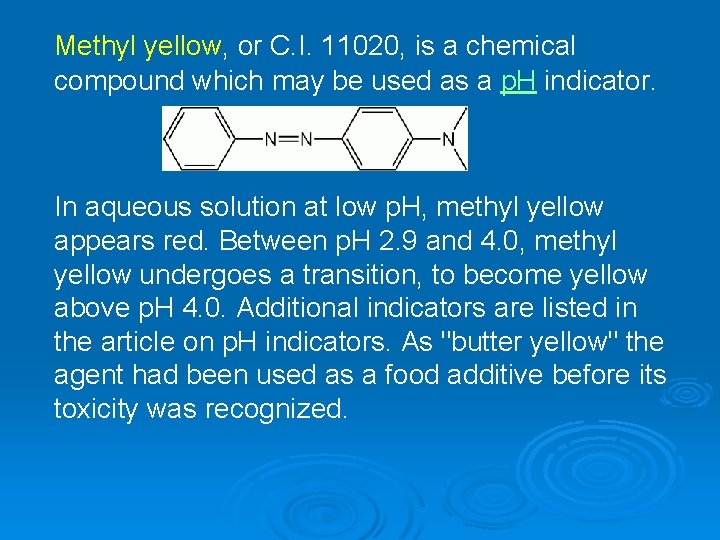
Methyl yellow, or C. I. 11020, is a chemical compound which may be used as a p. H indicator. In aqueous solution at low p. H, methyl yellow appears red. Between p. H 2. 9 and 4. 0, methyl yellow undergoes a transition, to become yellow above p. H 4. 0. Additional indicators are listed in the article on p. H indicators. As "butter yellow" the agent had been used as a food additive before its toxicity was recognized.

Thank you for attention!