The Nitrogen Cycle Nitrogen Nitrogen N is an












- Slides: 23

The Nitrogen Cycle

Nitrogen • Nitrogen (N) is an essential component of DNA, RNA, and proteins, the building blocks of life. • All organisms require nitrogen to live and grow. • The majority (78%) of the Earth’s atmosphere is N 2.

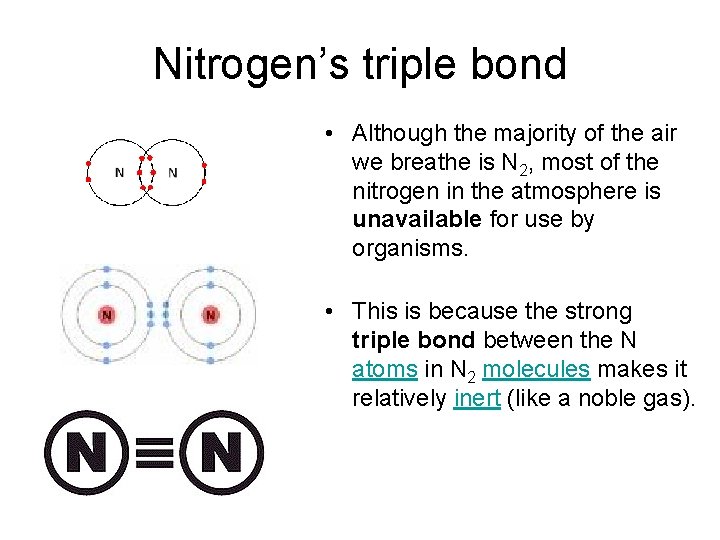
Nitrogen’s triple bond • Although the majority of the air we breathe is N 2, most of the nitrogen in the atmosphere is unavailable for use by organisms. • This is because the strong triple bond between the N atoms in N 2 molecules makes it relatively inert (like a noble gas).

How can we use N 2? WE CAN’T! But BACTERIA CAN! • In order for plants and animals to be able to use nitrogen, N 2 gas must first be converted to more a chemically available form such as ammonium (NH 4+) or nitrate (NO 3 -). https: //www. youtube. com/watch? v=K 5 EOZen. SSB 8

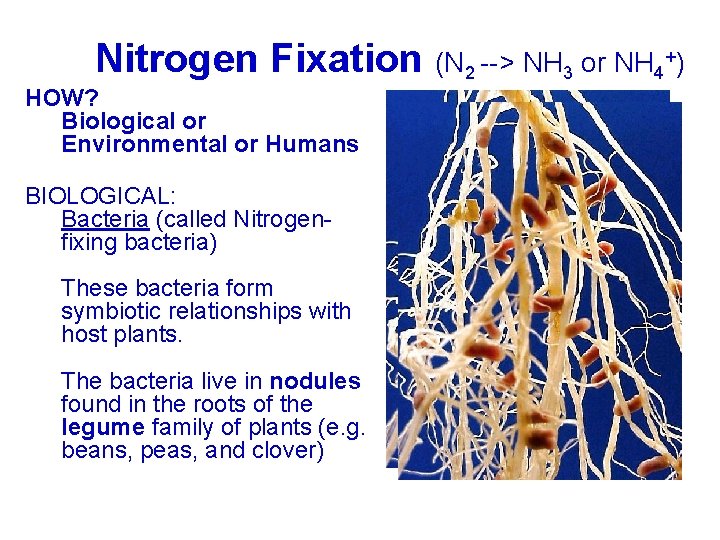
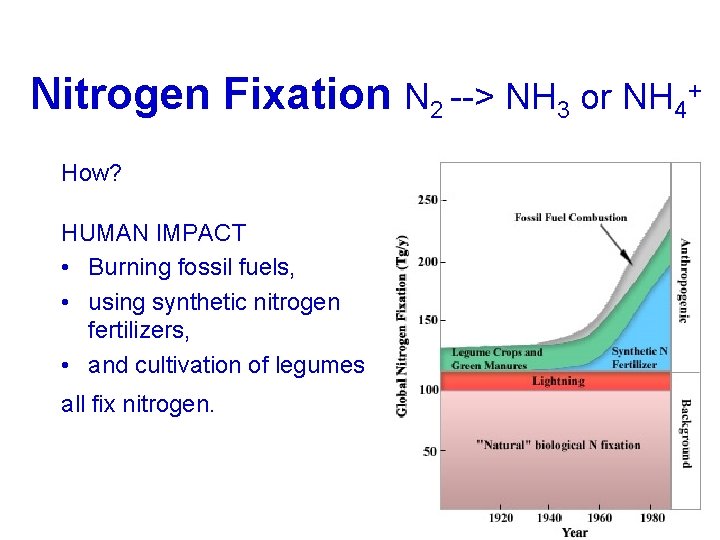
+) Nitrogen Fixation (N --> NH or NH Nitrogen Fixation (N 2 2 --> NH 3 3 or NH 4 4+) HOW? Biologicaloror Biological Environmentaloror. Humans Environmental BIOLOGICAL: Bacteria(called. Nitrogen. Bacteria fixingbacteria) fixing Thesebacteriaform These symbioticrelationshipswith symbiotic hostplants. host Thebacterialiveininnodules The foundininthe therootsofofthe found legumefamilyofofplants(e. g. legume beans, peas, andclover) beans,

Nitrogen Fixation (N 2 --> NH 3 or NH 4+) How? BIOLOGICAL • In aquatic environments (like fishtanks), blue-green algae (cyanobacteria) is an important free-living nitrogen fixer.

Nitrogen Fixation (N 2 --> NH 3 or NH 4+) ENVIRONMENTAL High-energy natural events which break the bond N 2 Examples: lightning forest fires hot lava flows

Nitrogen Fixation N 2 --> NH 3 or NH 4+ How? HUMAN IMPACT • Burning fossil fuels, • using synthetic nitrogen fertilizers, • and cultivation of legumes all fix nitrogen.

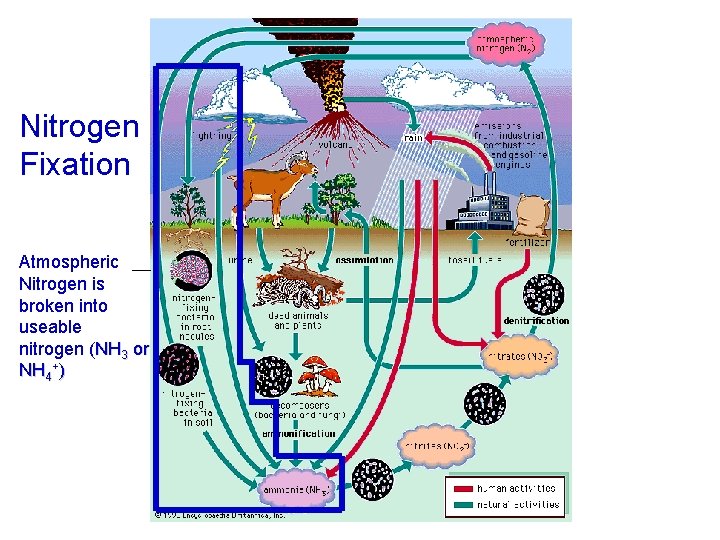
Nitrogen Fixation Atmospheric Nitrogen is broken into useable nitrogen (NH 3 or NH 4+)

Nitrogen Mineralization also called Ammonification Organic N --> NH 4+ • Decay of dead things, manure, etc. puts N in the soil • Done by decomposers (bacteria, fungi, etc. ) • During this process, a significant amount of the nitrogen contained within the dead organism is converted to ammonium (NH 4+).

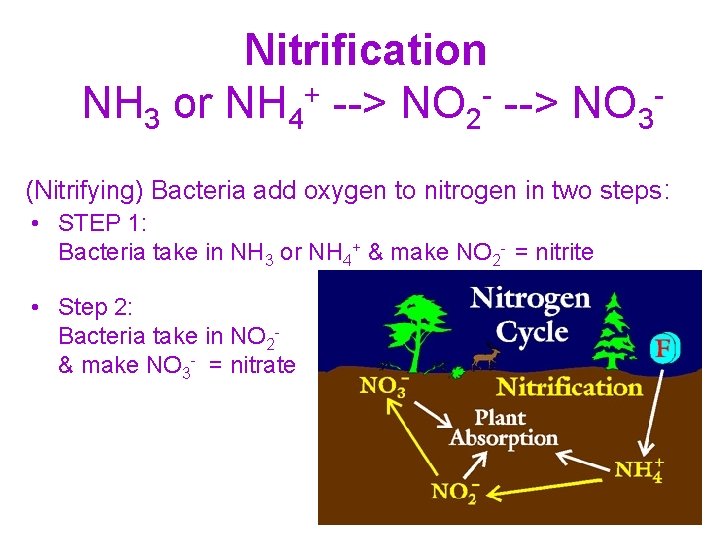
Nitrification NH 3 or NH 4+ --> NO 2 - --> NO 3(Nitrifying) Bacteria add oxygen to nitrogen in two steps: • STEP 1: Bacteria take in NH 3 or NH 4+ & make NO 2 - = nitrite • Step 2: Bacteria take in NO 2& make NO 3 - = nitrate

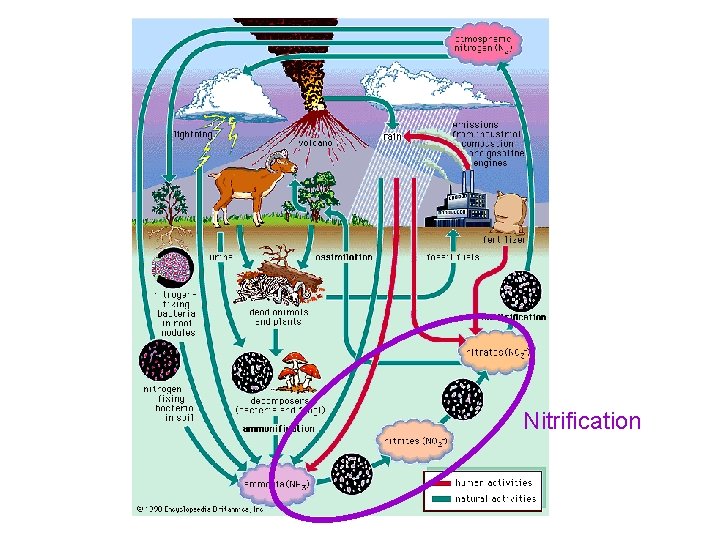
Nitrification

Nitrogen Uptake • The ammonia (NH 3) produced by nitrogen-fixing bacteria is usually quickly incorporated into protein and other organic nitrogen compounds (organisms!). • Nitrogen is changed to either be absorbed by a plant, by the bacteria itself, or by another soil organism. • Organisms at the top of the food chain (like us!) eat and grow, uptaking nitrogen (that has already been fixed).

Denitrification NO 3 - --> N 2 (Denitrifying) Bacteria do it. Denitrification removes nitrogen from ecosystems, and converts it back to atmospheric N 2.

Human Impact • FERTILIZERS! • Extra nitrogen fertilizer can runoff, where it contaminates surface water or infiltrates into ground water. • In drinking water, excess nitrogen can lead to cancer in humans and respiratory distress in infants.

Human Impact • In surface waters, extra nitrogen can lead to nutrient over-enrichment. • This leads to – fish-kills, – harmful algal blooms, – and species shifts in aquatic and land ecosystems.

Human Impact Some forms of nitrogen (like NO 3 - and NH 4+) can also enter the atmosphere to become: 1. smog- nitric oxide (NO) 2. Greenhouse gasnitrous oxide (N 2 O) 3. Acid Rain(nitrogen oxides)

Nitrogen Cycle Pictures Good pictures of the nitrogen cycle have these processes: – Nitrogen fixation (N 2 bonds are broken) – Nitrification (oxygen is added to form nitrogen oxides) – Denitrification (N 2 is put back into the air) It is also helpful to have – Ammonification (mineralization/waste conversion by decomposers) – Assimilation (intake by producers)




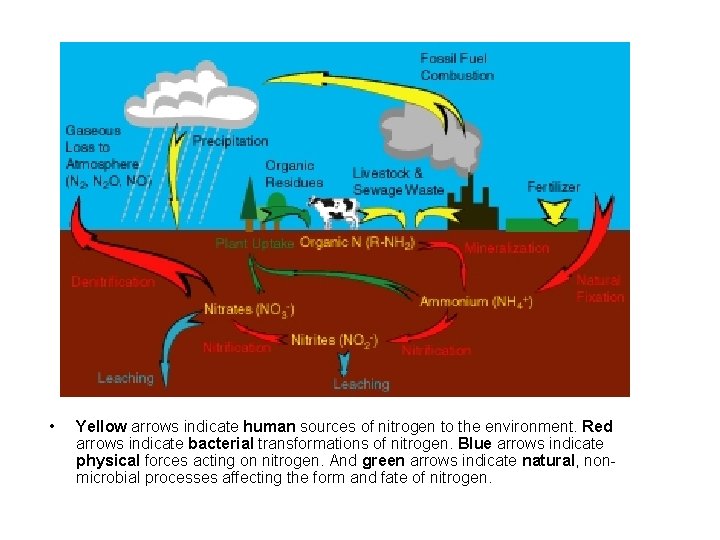
• Yellow arrows indicate human sources of nitrogen to the environment. Red arrows indicate bacterial transformations of nitrogen. Blue arrows indicate physical forces acting on nitrogen. And green arrows indicate natural, nonmicrobial processes affecting the form and fate of nitrogen.
