Monitoring Air Quality Air 78 nitrogen 21 oxygen










- Slides: 10

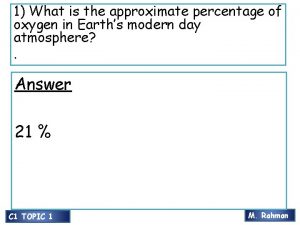
Monitoring Air Quality Air: 78% nitrogen 21% oxygen <1% argon 0. 03% carbon dioxide traces of hydrogen and neon Air quality can be determined in 2 ways: -by measuring the levels of pollutants in the air. -by estimating the amount of emissions from pollution sources

Sulfur Dioxide The major source of sulfur dioxide is industrial processes. In Alberta it is caused mainly by the oil and gas industry. Sulfur dioxide (SO 2) is a major pollutant that forms both smog and acid rain. To reduce sulfur dioxide emissions, plants use “scrubbers”. Scrubbers use limestone (calcium carbonate) to convert the pollutant sulfur dioxide to a useful product, gypsum. 2 SO 2(g) + 4 H 2 O(l) + 2 Ca. CO 3(s) + O 2(g) sulfur dioxide + water + limestone + oxygen 2 Ca. SO 4· 2 H 2 O(s) + 2 CO 2(g) gypsum + carbon dioxide

Nitrogen Oxides Nitrogen oxides are a major pollutant that creates both smog and acid rain from mainly the combustion in vehicles. They are also formed by combustion in generating plants and some industrial processes, such as oil refining. Nitrogen forms by the burning of fuels, combines with oxygen to produce nitrogen monoxide. The nitrogen monoxide then combines with the oxygen in the air to produce nitrogen dioxide, the brown smog.

Carbon Monoxide The main source of carbon monoxide occurs from motor vehicles. Other sources are wood, natural gas, airplanes, industrial processes And even cigarette smoking. Carbon monoxide is known as the silent killer. It forms when there is not enough oxygen in the air to complete combustion. If there is enough oxygen, nitrogen dioxide is formed.

Ground – Level Ozone is an odourless, colourless gas composed of three oxygen atoms. Ozone in the atmosphere protects Earth from harmful ultraviolet radiation. Ozone near the ground is a harmful pollutant. The major source of ground-level ozone is fuel combustion in vehicle engines and industry. As a result, ozone pollution is a problem mainly in larger cities.

2. 3 Monitoring the Atmosphere Life on Earth thrives because we live in a natural greenhouse. Some gases in the atmosphere act like the glass in a greenhouse. They trap heat from the Sun’s energy. This heat keeps Earth at temperatures that allow living things to live, grow, and reproduce. The atmospheric gases that trap heat are called greenhouse gases. Water vapour Carbon dioxide Methane Nitrogen oxides are all greenhouse gases.


"Al Gore’s movie about global warming is called 'An Inconvenient Truth. ' It's described as a detailed scientific view of global warming. President Bush said he just saw a film about global warming, 'Ice Age 2; The Meltdown. ' He said, 'It's so much better than that boring Al Gore movie. '" --Jay Leno "President Bush said global warming is happening much quicker than he thought, and then his staff pulled him aside and said 'It's just springtime. '" --Jay Leno

Enhanced Greenhouse Effect: greenhouse effect made greater by human activities, such as burning fossil fuels and clearing land, that add greenhouse gases to the atmosphere. Global Warming: increased average temperatures worldwide caused by the enhanced greenhouse effect. To slow down global warming countries have started to reduce carbon dioxide emissions through various means. They have also started to plant trees to offset the company’s carbon dioxide production.

The ozone layer provides Earth with protection from the Sun’s damaging ultraviolet rays. Exposure to greater UV rays could cause cancer, cataracts and plankton deaths. Chlorofluorocarbons have caused the thinning of our ozone layer. They are used in refrigerators, aerosol cans and fire extinguishers.