1 What is the approximate percentage of oxygen














- Slides: 25

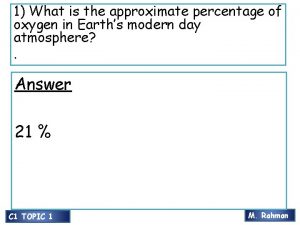
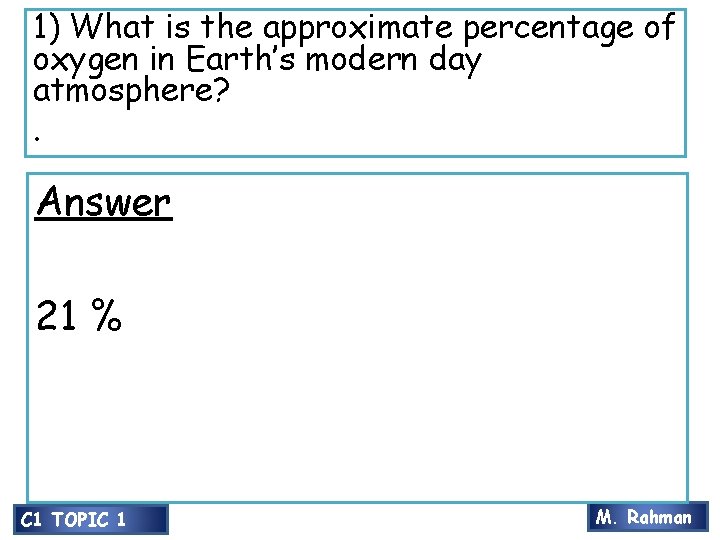
1) What is the approximate percentage of oxygen in Earth’s modern day atmosphere? . Answer 21 % C 1 TOPIC 1 M. Rahman

2) Which gas has the largest percentage in Earth’s modern day atmosphere? Answer Nitrogen C 1 TOPIC 1 M. Rahman

3) Which gas made up the majority of the Earth’s early atmosphere? Answer Carbon dioxide C 1 TOPIC 1 M. Rahman

4) Where is much of the gas that once made up the Earth’s early atmosphere now located? Answer. It dissolved in oceans and was then taken up by marine organisms. C 1 TOPIC 1 M. Rahman

5) What is the approximate percentage of carbon dioxide currently found in the atmosphere? Answer. 0. 04 % C 1 TOPIC 1 M. Rahman

6) Give three ways humans have contributed to the amount of CO 2 in the atmosphere Answer a) deforestation b) burning fossil fuels c) farming C 1 TOPIC 1 M. Rahman

7) Which of the following activities is not a human activity that causes small changes to the atmosphere over time? a) b) c) d) deforestation burning fossil fuels volcanoes erupting farming Answer C) volcanoes erupting C 1 TOPIC 1 M. Rahman

8) Which process was responsible for increasing the amount of oxygen in the atmosphere over time? Answer photosynthesis C 1 TOPIC 1 M. Rahman

9) Which gas do plants take out of the atmosphere? carbon dioxide C 1 TOPIC 1 M. Rahman

10) Which of the following gases are released by volcanoes? a) b) c) d) oxygen and nitrogen carbon dioxide and hydrogen oxygen only carbon dioxide and steam Answer d) carbon dioxide and steam C 1 TOPIC 1 M. Rahman

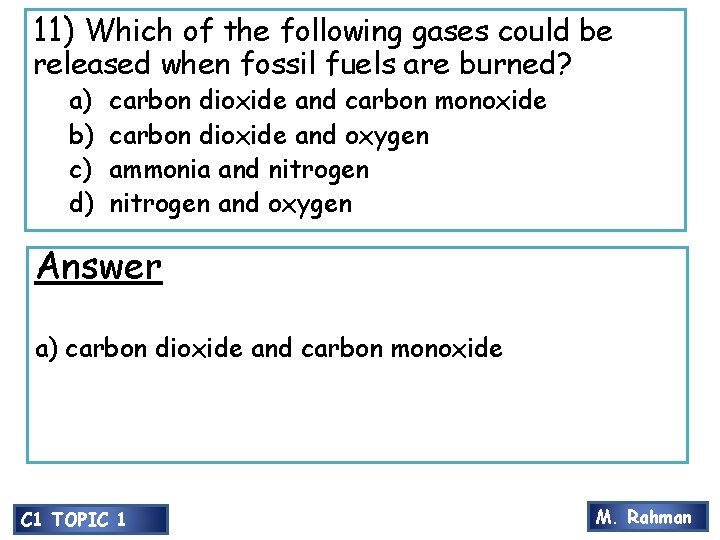
11) Which of the following gases could be released when fossil fuels are burned? a) b) c) d) carbon dioxide and carbon monoxide carbon dioxide and oxygen ammonia and nitrogen and oxygen Answer a) carbon dioxide and carbon monoxide C 1 TOPIC 1 M. Rahman

12) How were oceans formed? (3) Answer 1) Water vapour released from volcanoes 2)Water vapour condensed as the earth cooled. 3) Rain falls to fill up the hollows in the crust. C 1 TOPIC 1 M. Rahman

13) Describe why burning fossil is harmful to the earth and its environment (2). Answer • Increases amount of CO 2 in the atmosphere, ; • carbon dioxide is a green house gas, which causes global warming; • greenhouse gases prevent heat from leaving the earths atmosphere; • Trapped heat causes the earth temperature to rise. . C 1 TOPIC 1 M. Rahman

14) Billions of years ago, which gas was in the greatest amount? Answer Carbon dioxide C 1 TOPIC 1 M. Rahman

15) Give the origins of one piece of evidence that scientist have collected to explain the composition of the earths atmosphere Answer • Antarctic ice cores-difference layers contain different amount of each gas. Each layer represents a different time. or • Oxygen locked in rocks. C 1 TOPIC 1 M. Rahman

16) In todays atmosphere, which gas is present that was either in very small amounts or not at all? Answer Oxygen C 1 TOPIC 1 M. Rahman

17) Explain how the levels of oxygen has changed over billions of years (2). Answer Primitive green plants evolved using carbon dioxide (1) and released oxygen by photosynthesis (1). C 1 TOPIC 1 M. Rahman

18) Describe how carbon gets locked into sedimentary rocks (2). Answer Carbon dioxide dissolved in sea water reacts with other chemicals to form calcium carbonate and calcium hydrogen carbonate (1). The shells of dead sea creatures accumulate and are transformed into sedimentary rocks (1). C 1 TOPIC 1 M. Rahman

19) Describe how the levels of carbon dioxide in the atmosphere in the earths early atmosphere was reduced(2). Answer By photosynthesis (1) and by dissolving in the oceans (1). C 1 TOPIC 1 M. Rahman

20) Name three ways humans are changing the atmosphere. Answer 1) Burning of fossil fuels 2) deforestation 3) livestock farming C 1 TOPIC 1 M. Rahman

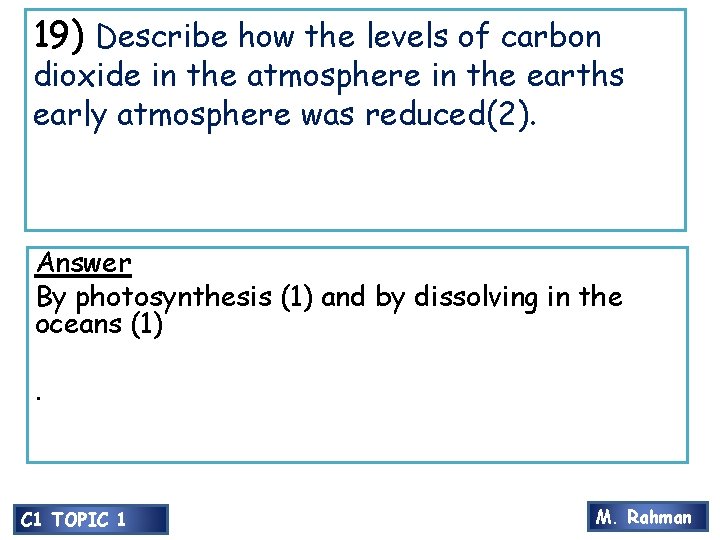
21) iron 100 mm 79 mm 1) Explain how the above experiment could be used to determine the amount of oxygen in the atmosphere (4 marks) Answer • Iron reacts with oxygen to from iron oxide. • Reaction uses up oxygen and total amount of air reduces. • The water rises inside the tube to • fill up the gap C 1 TOPIC 1 M. Rahman

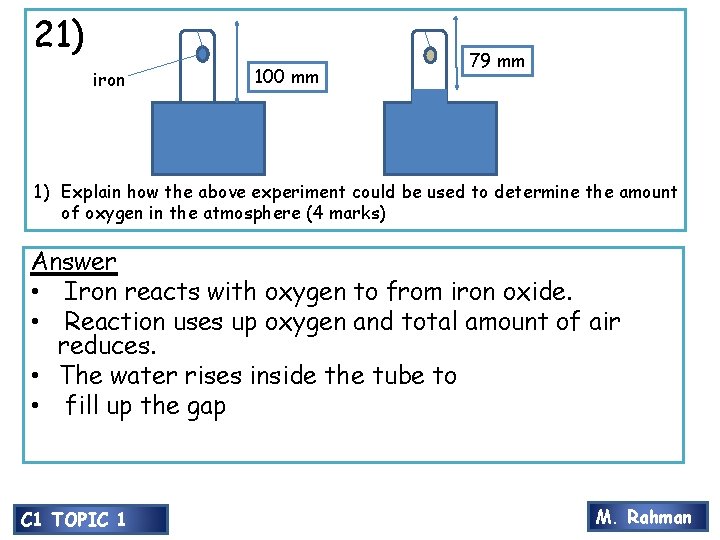
20) 22) iron 300 mm ? mm 1) If the atmosphere contains 21 % oxygen, what will the final reading be. Show your working out. (3 marks) Answer 300 x 0. 21=63 mm (1) 300 -63= 237 mm (2) C 1 TOPIC 1 M. Rahman

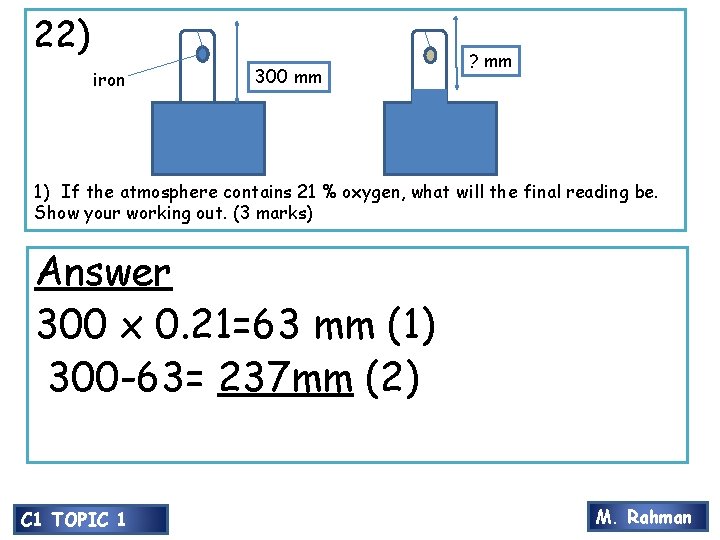
20) 23) iron 750 mm 592 mm 1) Show that the percentage of oxygen in the atmosphere is 21 %. Answer 750 -592 =158 (1) 158/750= 0. 21 (1) 0. 21 x 100 = 21 % C 1 TOPIC 1 M. Rahman

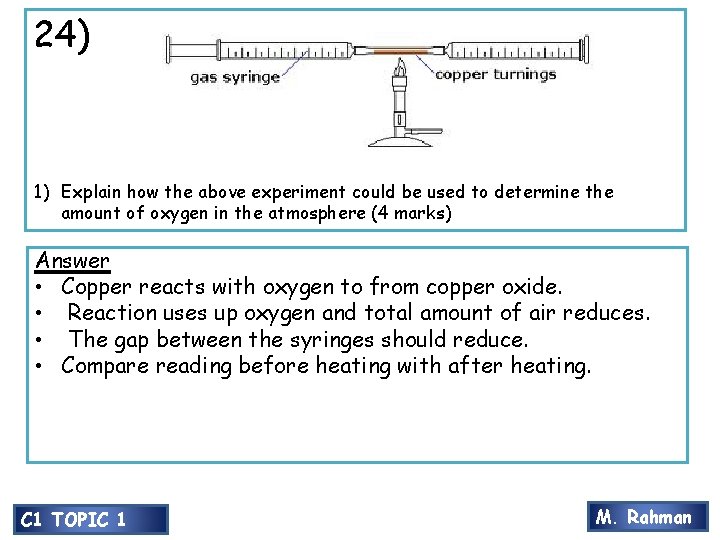
24) 1) Explain how the above experiment could be used to determine the amount of oxygen in the atmosphere (4 marks) Answer • Copper reacts with oxygen to from copper oxide. • Reaction uses up oxygen and total amount of air reduces. • The gap between the syringes should reduce. • Compare reading before heating with after heating. C 1 TOPIC 1 M. Rahman

25) Iron reacts with oxygen to produce iron oxide. Write the word equations. Answer Iron + Oxygen ---- Iron Oxide C 1 TOPIC 1 M. Rahman
 What is the approximate percentage of oxygen in the air?
What is the approximate percentage of oxygen in the air? What is the percentage composition of oxygen
What is the percentage composition of oxygen Pasteur point oxygen cascade
Pasteur point oxygen cascade Fourteen billion years represents the approximate age of
Fourteen billion years represents the approximate age of What are the approximate dates of the baroque period
What are the approximate dates of the baroque period Sketch techniques for approximate query processing
Sketch techniques for approximate query processing A guided tour to approximate string matching
A guided tour to approximate string matching Collector emitter loop
Collector emitter loop Approximate computing
Approximate computing Lshzoo.cc
Lshzoo.cc Musical devices in poetry
Musical devices in poetry Approximate cell decomposition
Approximate cell decomposition Approximate computing
Approximate computing Times are approximate
Times are approximate Board ga,e
Board ga,e Approximate the best fitting line for the data
Approximate the best fitting line for the data Fast exact and approximate geodesics on meshes
Fast exact and approximate geodesics on meshes What is the approximate
What is the approximate Approximate counting algorithm
Approximate counting algorithm Sonnet example
Sonnet example A guided tour to approximate string matching
A guided tour to approximate string matching Vancouver art gallery
Vancouver art gallery A building bent deflects in the way same as a:-
A building bent deflects in the way same as a:- Grade 10 literary devices
Grade 10 literary devices Draw a table for the different units of storage of data
Draw a table for the different units of storage of data It is the inner terminus of the fingerprints pattern.
It is the inner terminus of the fingerprints pattern.