Ch 7 5 STOICHIOMETRY Percentage Yield and Percentage








- Slides: 15

Ch 7. 5: STOICHIOMETRY: Percentage Yield and Percentage Purity

A+B AB 1/10/2022

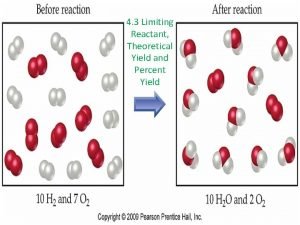
Various factors can affect the actual amount of products obtained, such as: 1) The reactants may not all react. This can occur either because: a)The lab conditions are not ideal (temperature, humidity, not enough time, etc. ) b)The reactants are not 100 % pure. 2) Some of the products are lost during procedures such as solvent extraction, filtration and crystallization, which are needed to physically separate and purify the products.

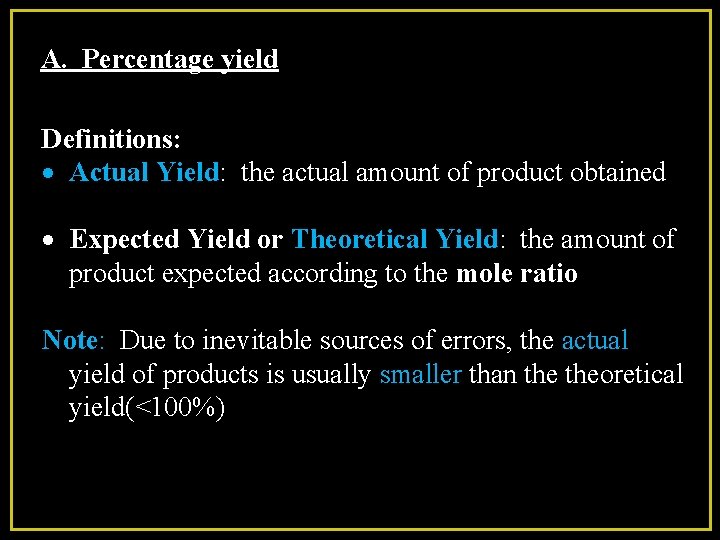
A. Percentage yield Definitions: Actual Yield: the actual amount of product obtained Expected Yield or Theoretical Yield: the amount of product expected according to the mole ratio Note: Due to inevitable sources of errors, the actual yield of products is usually smaller than theoretical yield(<100%)

Percentage yield = _Actual Yield (mass of product obtained) x 100% Expected Yield (mass of product expected) Approach: (i) Calculate the “amount of product expected” according to mole ratio and amount of limiting reactant. Ignore any percentage yield or purities and (ii) Actual Yield is obtained from experimental data

Example 1: CH 4 + Cl 2 CH 3 Cl + HCl Production of chloromethane, which is used as local anesthetic, intermediate in organic synthesis, solvent, herbicide If 15. 0 g CH 4 is reacted with an excess of Cl 2 to produce 29. 7 g of CH 3 Cl, what is the percentage yield? (i) Expected yield of CH 3 Cl: # moles of CH 4 n=15. 0 g/16. 0 g/mol = 0. 9375 mol Mass of CH 3 Cl =0. 9375 mol x 50. 5 g/mol= 47. 34 g

(ii) Percentage yield: Percentage yield = _Actual Yield (mass of product obtained) x 100% Expected Yield (mass of product expected) % yield = (29. 7 g/47. 34 g) x 100% = 62. 7 % (iii) Analyse: What may have happened during this reaction to cause the difference between the actual yield and theoretical yield? • Reactants did not react completely • The lab conditions are not ideal • Reactants were not 100% pure 1/10/2022

Example 2: Consider the reaction: 4 KO 2(s) + 2 CO 2(g) ® 2 K 2 CO 3(s) + 3 O 2(g) The Russian Space Agency has had success using potassium superoxide in chemical oxygen generators for its spacesuits and Soyuz spacecraft. KO 2 has also been utilized in canisters for rebreathers for fire fighting and mine rescue work What mass of K 2 CO 3 is produced when 2. 50 g of KO 2 is reacted with an excess of CO 2, if the reaction has a 23. 0 % yield?

(i) Expected yield of K 2 CO 3(s): # moles of KO 2 =2. 50 g/71. 1 g/mol = 0. 03516 mol Mass of K 2 CO 3 = (0. 03516 mol : 2) x (138. 2 g/mol)= 2. 430 g (ii) Actual Yield Percentage yield =Actual Yield x 100% Expected Yield Actual yield = (Percentage yield) (Expected Yield)/100 % = =23. 0 % x 2. 430 g /100% =0. 559 g

Abbreviations • n= # of moles; n(X) # moles of x • M(X)= molar mass of X • C(X)= molarity or concentration of X [sometimes we use [X] or M(X)] • m (X)= mass of X • V= volume • d=density • % yield = percentage yield • % purity= percentage purity 1/10/2022

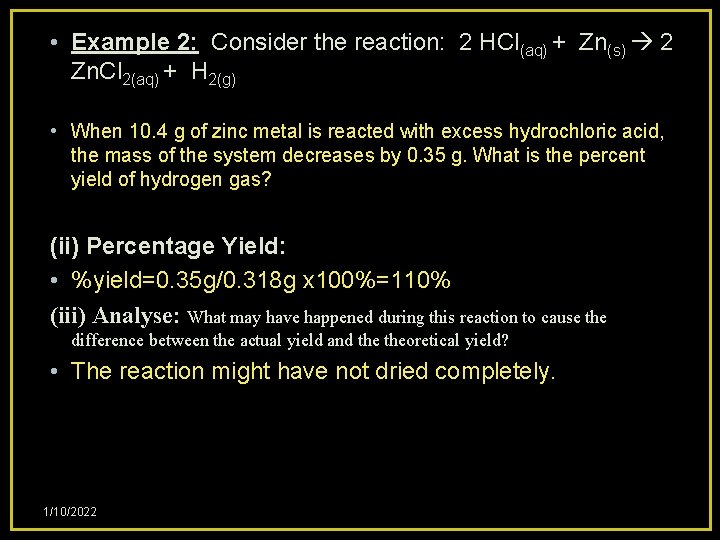
• Example 2: Consider the reaction: 2 HCl(aq) + Zn(s) 2 Zn. Cl 2(aq) + H 2(g) • When 10. 4 g of zinc metal is reacted with excess hydrochloric acid, the mass of the system decreases by 0. 35 g. What is the percent yield of hydrogen gas? (i) Expected yield of H 2(g): • 2 HCl+ Zn 2 Zn. Cl + H 2 • n(Zn)=10. 4 g/65. 4 g/mol=0. 159 mol Zn • n(Zn)=n(H 2)=0. 159 mol H 2 • m(H 2)expected=0. 159 mol H 2 x 2. 0 g/mol=0. 318 g H 2 1/10/2022

• Example 2: Consider the reaction: 2 HCl(aq) + Zn(s) 2 Zn. Cl 2(aq) + H 2(g) • When 10. 4 g of zinc metal is reacted with excess hydrochloric acid, the mass of the system decreases by 0. 35 g. What is the percent yield of hydrogen gas? (ii) Percentage Yield: • %yield=0. 35 g/0. 318 g x 100%=110% (iii) Analyse: What may have happened during this reaction to cause the difference between the actual yield and theoretical yield? • The reaction might have not dried completely. 1/10/2022

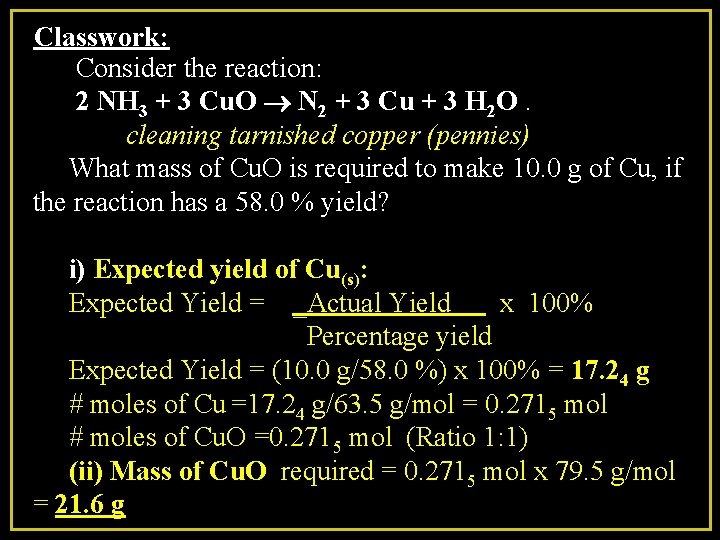
Classwork: Consider the reaction: 2 NH 3 + 3 Cu. O ® N 2 + 3 Cu + 3 H 2 O. cleaning tarnished copper (pennies) What mass of Cu. O is required to make 10. 0 g of Cu, if the reaction has a 58. 0 % yield? i) Expected yield of Cu(s): Expected Yield = _Actual Yield x 100% Percentage yield Expected Yield = (10. 0 g/58. 0 %) x 100% = 17. 24 g # moles of Cu =17. 24 g/63. 5 g/mol = 0. 2715 mol # moles of Cu. O =0. 2715 mol (Ratio 1: 1) (ii) Mass of Cu. O required = 0. 2715 mol x 79. 5 g/mol = 21. 6 g

Summary • Calculation of % yield based on actual yield and expected yield

Assignment: Hebden p. 137 #33 c, 35, 36
 Theoretical yield
Theoretical yield Dividend yield and capital gains yield
Dividend yield and capital gains yield Definition of percent yield
Definition of percent yield Actual yield
Actual yield Difference between actual yield and theoretical yield
Difference between actual yield and theoretical yield Dividend yield and capital gains yield
Dividend yield and capital gains yield Dividend yield and capital gains yield
Dividend yield and capital gains yield Calculating percentage yield
Calculating percentage yield Theoretical yield stoichiometry
Theoretical yield stoichiometry Current yield vs yield to maturity
Current yield vs yield to maturity Pv of coupon payments
Pv of coupon payments Junk bond rating
Junk bond rating Rolled throughput yield vs first pass yield
Rolled throughput yield vs first pass yield Calculate percentage yield
Calculate percentage yield How to find percentage yield
How to find percentage yield Calculate percentage yield
Calculate percentage yield