Chapter 7 7 3 Percentage Yield Theoretical Yield


- Slides: 8

Chapter 7 7. 3: Percentage Yield

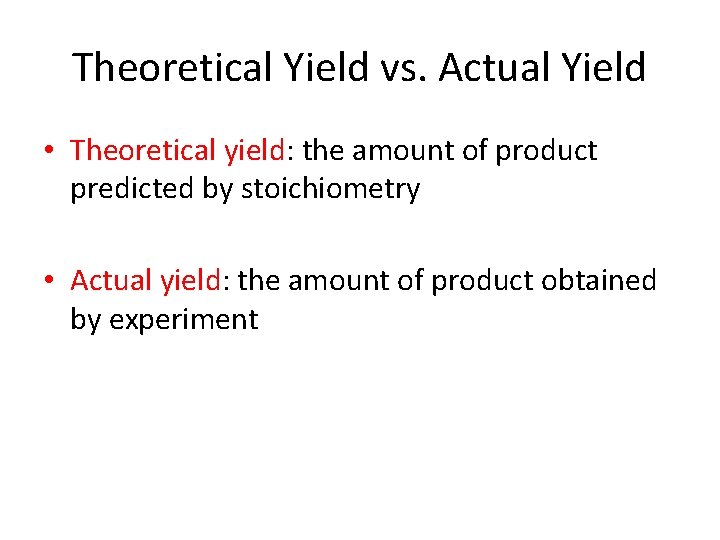
Theoretical Yield vs. Actual Yield • Theoretical yield: the amount of product predicted by stoichiometry • Actual yield: the amount of product obtained by experiment

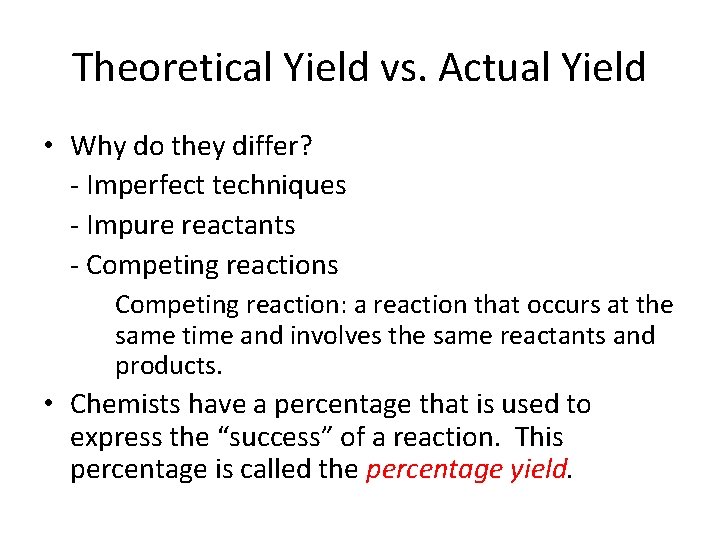
Theoretical Yield vs. Actual Yield • Why do they differ? - Imperfect techniques - Impure reactants - Competing reactions Competing reaction: a reaction that occurs at the same time and involves the same reactants and products. • Chemists have a percentage that is used to express the “success” of a reaction. This percentage is called the percentage yield.

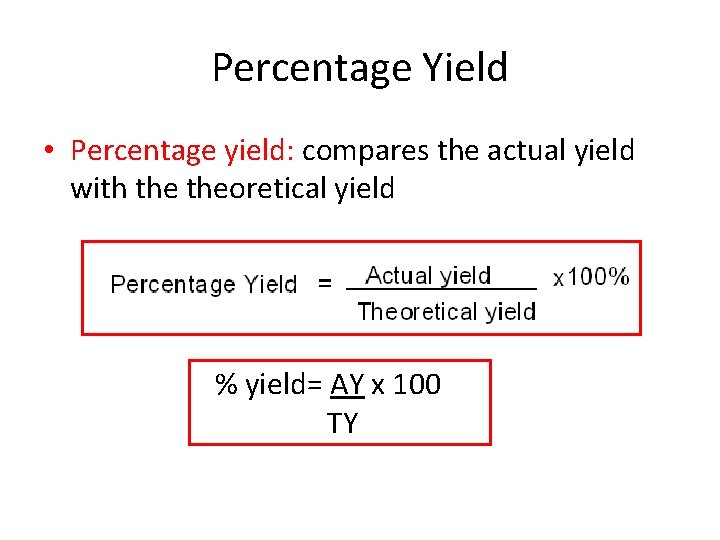
Percentage Yield • Percentage yield: compares the actual yield with theoretical yield % yield= AY x 100 TY

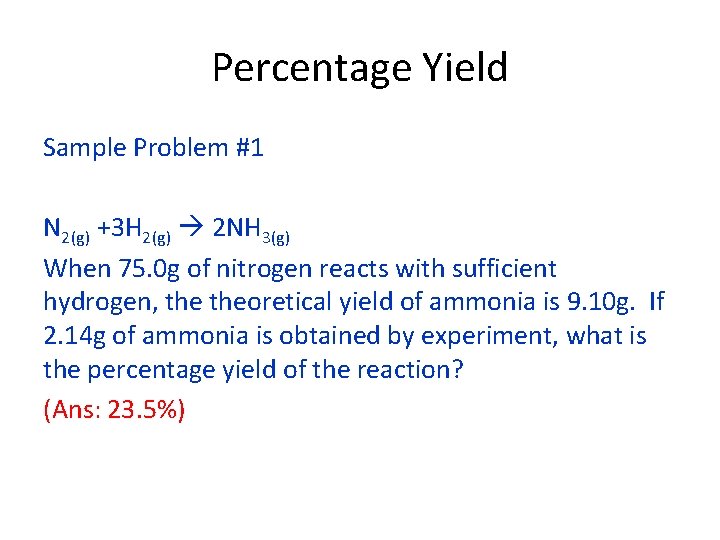
Percentage Yield Sample Problem #1 N 2(g) +3 H 2(g) 2 NH 3(g) When 75. 0 g of nitrogen reacts with sufficient hydrogen, theoretical yield of ammonia is 9. 10 g. If 2. 14 g of ammonia is obtained by experiment, what is the percentage yield of the reaction? (Ans: 23. 5%)

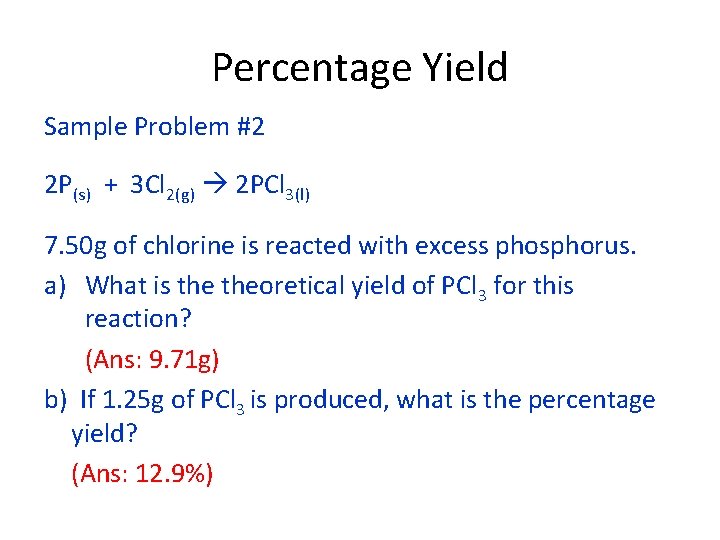
Percentage Yield Sample Problem #2 2 P(s) + 3 Cl 2(g) 2 PCl 3(l) 7. 50 g of chlorine is reacted with excess phosphorus. a) What is theoretical yield of PCl 3 for this reaction? (Ans: 9. 71 g) b) If 1. 25 g of PCl 3 is produced, what is the percentage yield? (Ans: 12. 9%)

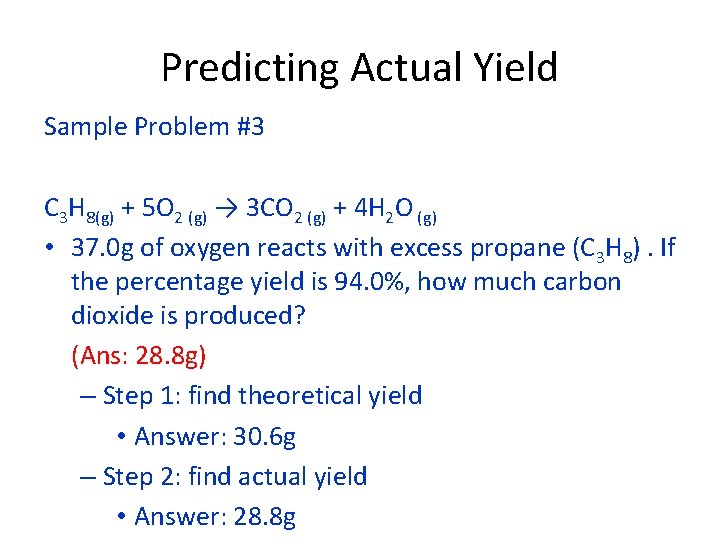
Predicting Actual Yield Sample Problem #3 C 3 H 8(g) + 5 O 2 (g) → 3 CO 2 (g) + 4 H 2 O (g) • 37. 0 g of oxygen reacts with excess propane (C 3 H 8). If the percentage yield is 94. 0%, how much carbon dioxide is produced? (Ans: 28. 8 g) – Step 1: find theoretical yield • Answer: 30. 6 g – Step 2: find actual yield • Answer: 28. 8 g

k o o b t x Te -33 1 3 # 2 26 n p o B i t 7 c i v -37 d In 4 e r 3 p # e 6 t le 26 p p m o C e l ab t s b o - Copy - Read l report) (forma rs: e uiz d q n i b a m l Re s… s a l c t ex * n * s e y u a d d – 3 n i ) 7 Inv 7 A 5 h C ( 3 nit U T S **TE