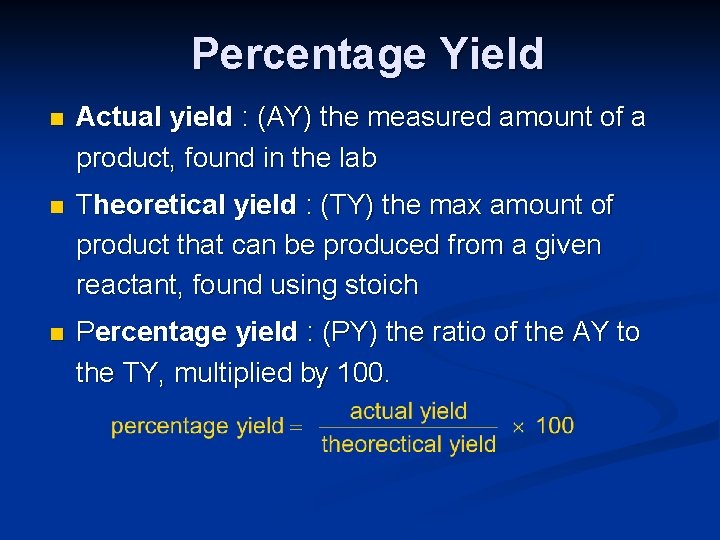
PERCENT YIELD Percentage Yield n Actual yield AY


- Slides: 9

PERCENT YIELD

Percentage Yield n Actual yield : (AY) the measured amount of a product, found in the lab n Theoretical yield : (TY) the max amount of product that can be produced from a given reactant, found using stoich n Percentage yield : (PY) the ratio of the AY to the TY, multiplied by 100.

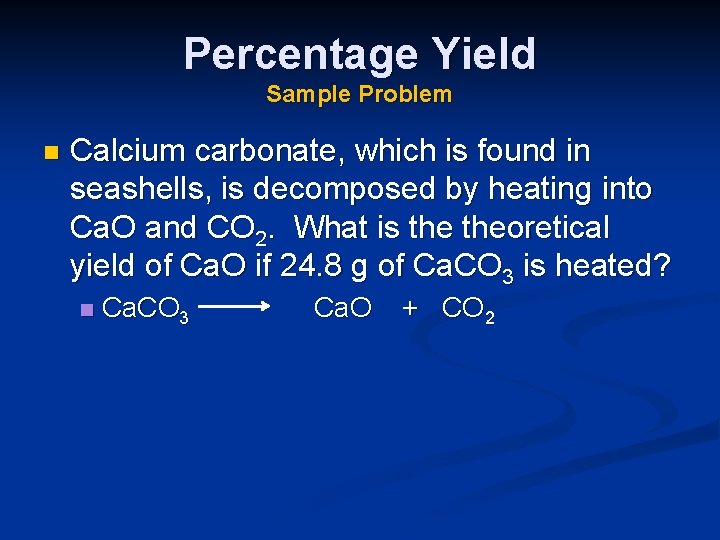
Percentage Yield Sample Problem n Calcium carbonate, which is found in seashells, is decomposed by heating into Ca. O and CO 2. What is theoretical yield of Ca. O if 24. 8 g of Ca. CO 3 is heated? n Ca. CO 3 Ca. O + CO 2

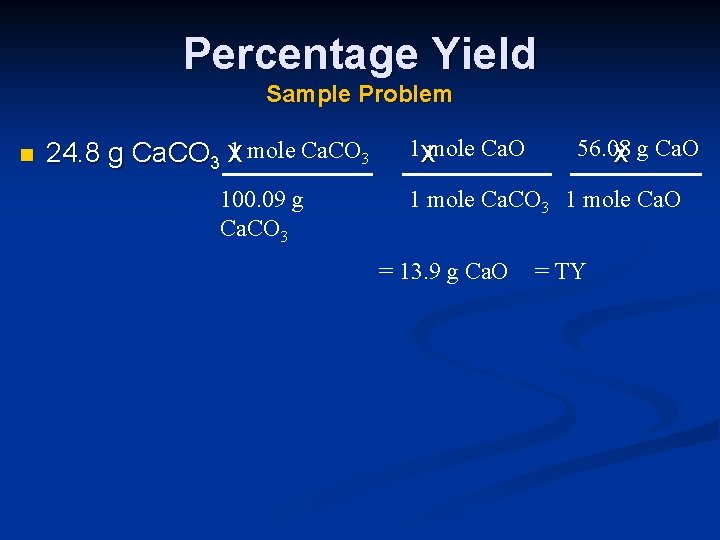
Percentage Yield Sample Problem n 1 mole Ca. CO 3 24. 8 g Ca. CO 3 x 100. 09 g Ca. CO 3 1 xmole Ca. O 56. 08 x g Ca. O 1 mole Ca. CO 3 1 mole Ca. O = 13. 9 g Ca. O = TY

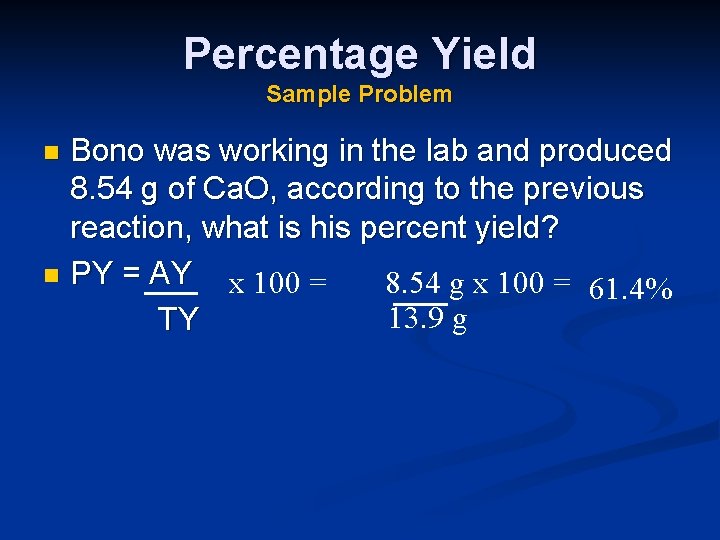
Percentage Yield Sample Problem Bono was working in the lab and produced 8. 54 g of Ca. O, according to the previous reaction, what is his percent yield? n PY = AY x 100 = 8. 54 g x 100 = 61. 4% 13. 9 g TY n

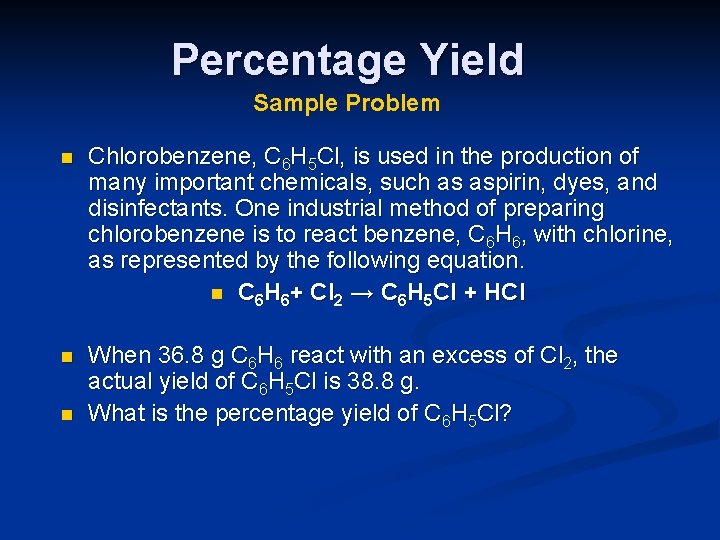
Percentage Yield Sample Problem n Chlorobenzene, C 6 H 5 Cl, is used in the production of many important chemicals, such as aspirin, dyes, and disinfectants. One industrial method of preparing chlorobenzene is to react benzene, C 6 H 6, with chlorine, as represented by the following equation. n C 6 H 6+ Cl 2 → C 6 H 5 Cl + HCl n When 36. 8 g C 6 H 6 react with an excess of Cl 2, the actual yield of C 6 H 5 Cl is 38. 8 g. What is the percentage yield of C 6 H 5 Cl? n

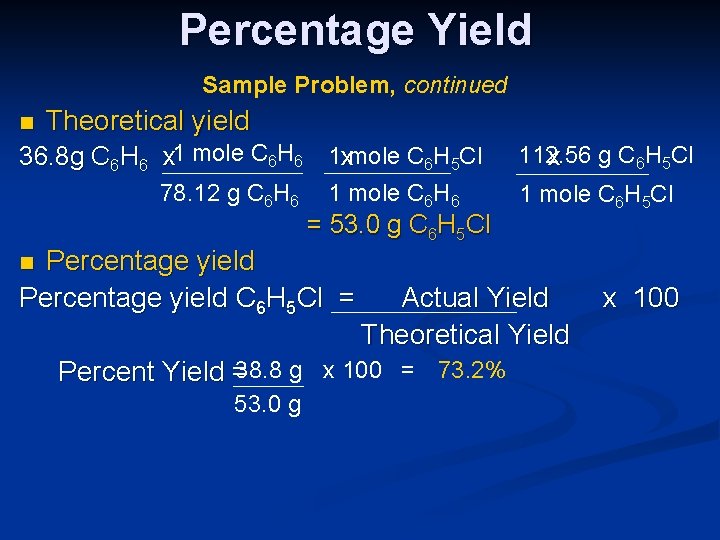
Percentage Yield Sample Problem, continued n Theoretical yield 36. 8 g C 6 H 6 x 1 mole C 6 H 6 1 xmole C 6 H 5 Cl 78. 12 g C 6 H 6 1 mole C 6 H 6 = 53. 0 g C 6 H 5 Cl Percentage yield C 6 H 5 Cl = 112. 56 x g C 6 H 5 Cl 1 mole C 6 H 5 Cl n Actual Yield Theoretical Yield Percent Yield =38. 8 g x 100 = 73. 2% 53. 0 g x 100

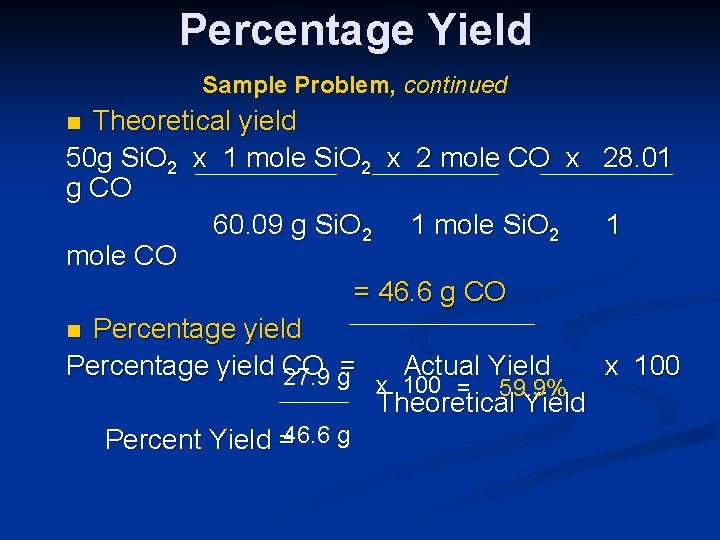
Percent Yield Example Problem n If 50. 0 g of silicon dioxide is heated with an excess of carbon, 27. 9 g of carbon monoxide is produced based on the following reaction. What is the percent yield of this reaction? Si. O 2 + 3 C Si. C + 2 CO n Actual Yield = 27. 9 g n

Percentage Yield Sample Problem, continued Theoretical yield 50 g Si. O 2 x 1 mole Si. O 2 x 2 mole CO x g CO 60. 09 g Si. O 2 1 mole Si. O 2 mole CO = 46. 6 g CO n Percentage yield CO Actual Yield 27. 9 g= x 100 = 59. 9% Theoretical Yield Percent Yield =46. 6 g n 28. 01 1 x 100