Percent Yield Theoretical Yield The theoretical yield is







- Slides: 13

Percent Yield

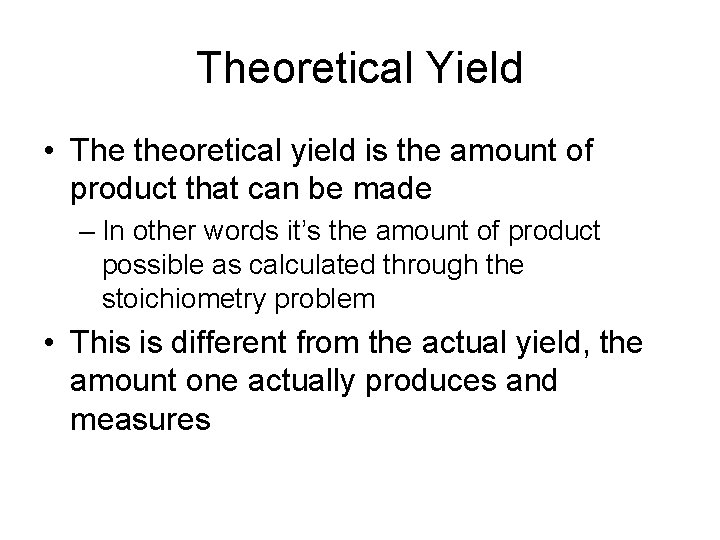
Theoretical Yield • The theoretical yield is the amount of product that can be made – In other words it’s the amount of product possible as calculated through the stoichiometry problem • This is different from the actual yield, the amount one actually produces and measures

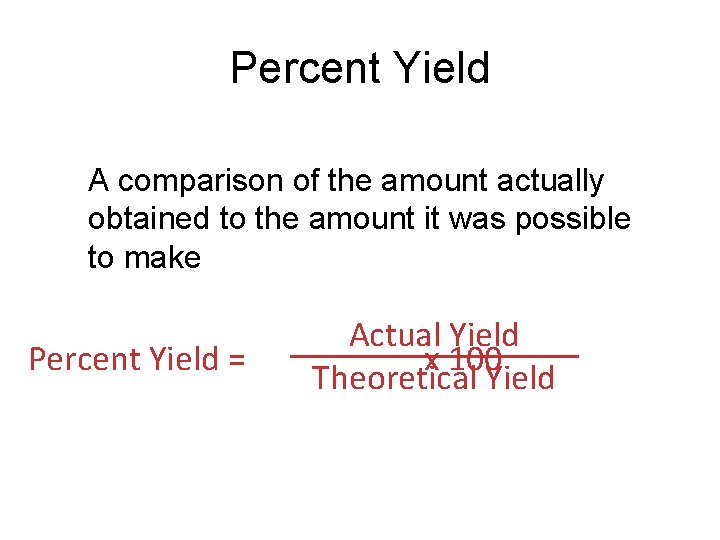
Percent Yield A comparison of the amount actually obtained to the amount it was possible to make Percent Yield = Actual Yield x 100 Theoretical Yield

• When you write an exam, the highest grade that you can earn is usually 100%. • Most people do not regularly earn a grade of 100%. • A percentage on a exam is calculated using the following equation: • Percentage grade = marks earnedx 100% maximum possible marks

In this Section… • You will learn about a percentage that chemists use to predict and express the success of reaction. • Chemists use stoichiometry to predict the amount of product that can be expected from a chemical reaction. • The amount of of product that is predicted by stoichiometry is called theoretical yield.

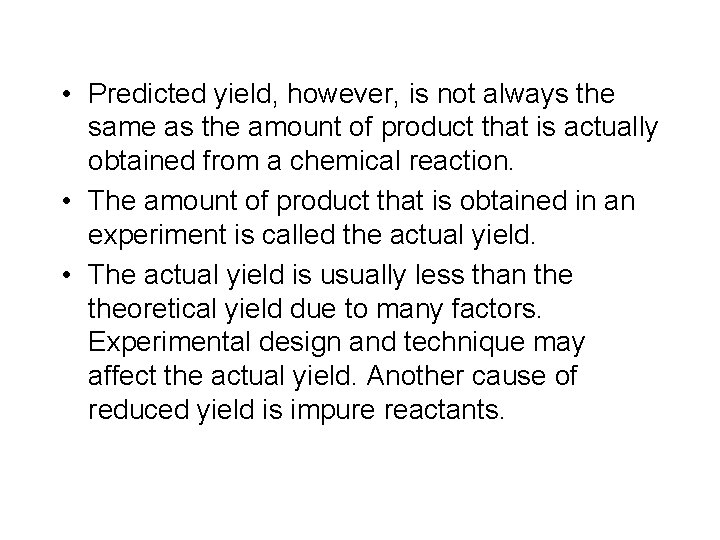
• Predicted yield, however, is not always the same as the amount of product that is actually obtained from a chemical reaction. • The amount of product that is obtained in an experiment is called the actual yield. • The actual yield is usually less than theoretical yield due to many factors. Experimental design and technique may affect the actual yield. Another cause of reduced yield is impure reactants.

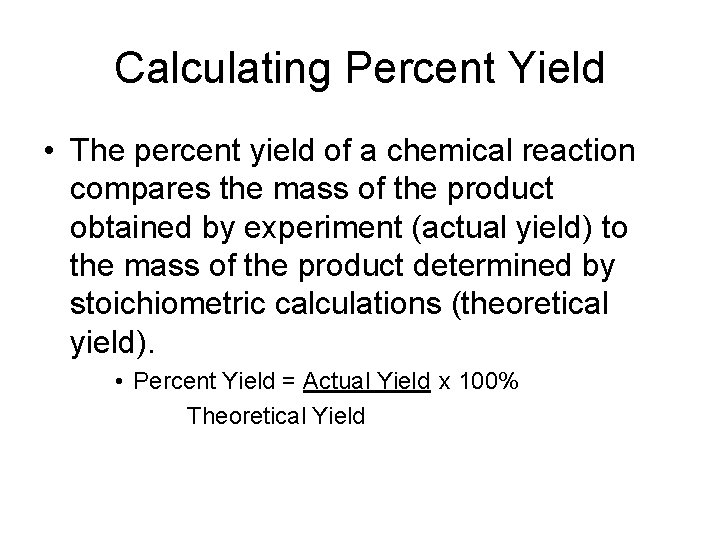
Calculating Percent Yield • The percent yield of a chemical reaction compares the mass of the product obtained by experiment (actual yield) to the mass of the product determined by stoichiometric calculations (theoretical yield). • Percent Yield = Actual Yield x 100% Theoretical Yield

Example • Ammonia can be prepared by reacting nitrogen gas (from the atmosphere) with hydrogen gas. • When 75 g of nitrogen react with sufficient hydrogen, theoretical yield of ammonia is 9. 10 g. • If 1. 72 g of ammonia is obtained, what is the percent yield of the reaction?

• You know: • Actual yield is 1. 71 g • Theoretical yield is 9. 10 g • Calculate: • 1. 72 g / 9. 10 g • Answer: • 18. 9% x 100%

• Sometimes chemists know what percent yield to expect from a chemical reaction. • The next example shows how to predict the actual yield of a reaction from a known percent yield.

Example 2 • Calcium Carbonate can be thermally decomposed to calcium oxide and carbon dioxide. • Under certain conditions, this reaction proceeds with 92. 4% yield of calcium oxide. • How many grams of calcium oxide can a chemist expect to obtain if 12. 4 g of calcium carbonate is heated?

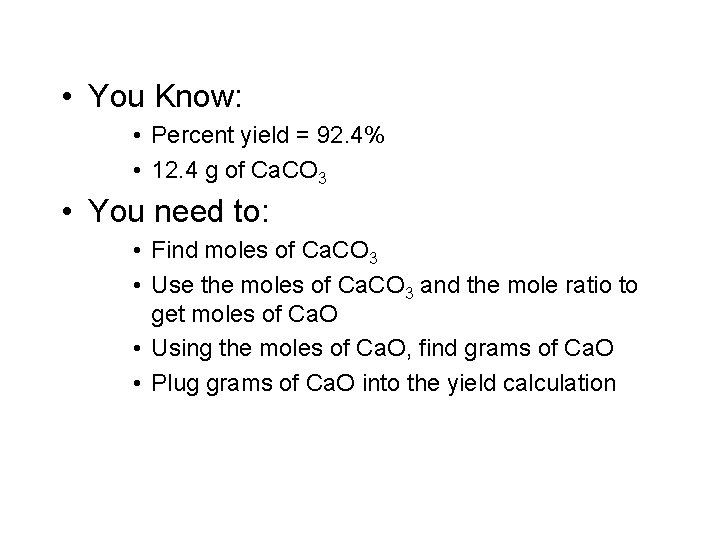
• You Know: • Percent yield = 92. 4% • 12. 4 g of Ca. CO 3 • You need to: • Find moles of Ca. CO 3 • Use the moles of Ca. CO 3 and the mole ratio to get moles of Ca. O • Using the moles of Ca. O, find grams of Ca. O • Plug grams of Ca. O into the yield calculation

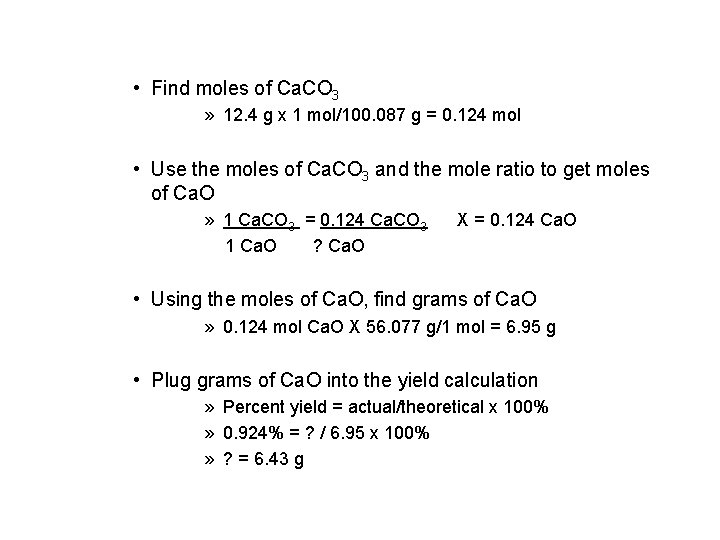
• Find moles of Ca. CO 3 » 12. 4 g x 1 mol/100. 087 g = 0. 124 mol • Use the moles of Ca. CO 3 and the mole ratio to get moles of Ca. O » 1 Ca. CO 3 = 0. 124 Ca. CO 3 1 Ca. O ? Ca. O X = 0. 124 Ca. O • Using the moles of Ca. O, find grams of Ca. O » 0. 124 mol Ca. O X 56. 077 g/1 mol = 6. 95 g • Plug grams of Ca. O into the yield calculation » Percent yield = actual/theoretical x 100% » 0. 924% = ? / 6. 95 x 100% » ? = 6. 43 g