Percentage Yield What is Yield Its the amount


- Slides: 10

Percentage Yield

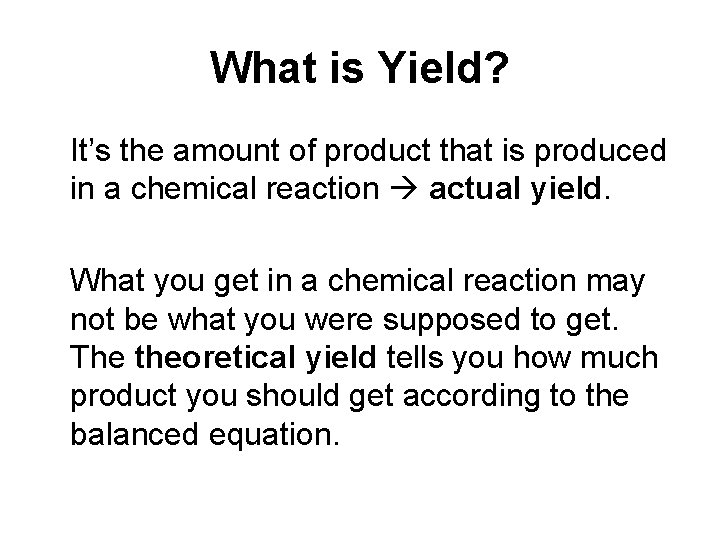
What is Yield? It’s the amount of product that is produced in a chemical reaction actual yield. What you get in a chemical reaction may not be what you were supposed to get. The theoretical yield tells you how much product you should get according to the balanced equation.

Factors affecting Yield • • • Splattering when heating a solution Filtering may not get all of the product Using impure/contaminated reactants Not letting wet solids dry properly Use of equipment (old or faulty) Side chemical reactions (eg. When you burn Mg in the air. It reacts with O 2. However, air also has nitrogen, so Mg may react with N 2 too!)

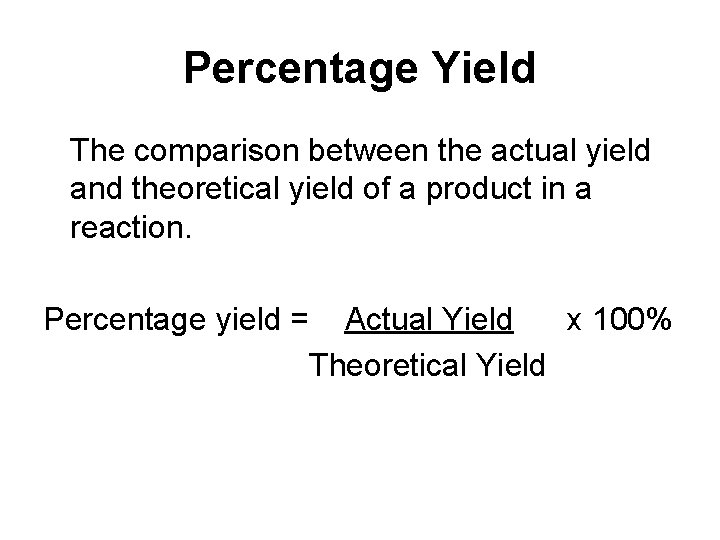
Percentage Yield The comparison between the actual yield and theoretical yield of a product in a reaction. Percentage yield = Actual Yield x 100% Theoretical Yield

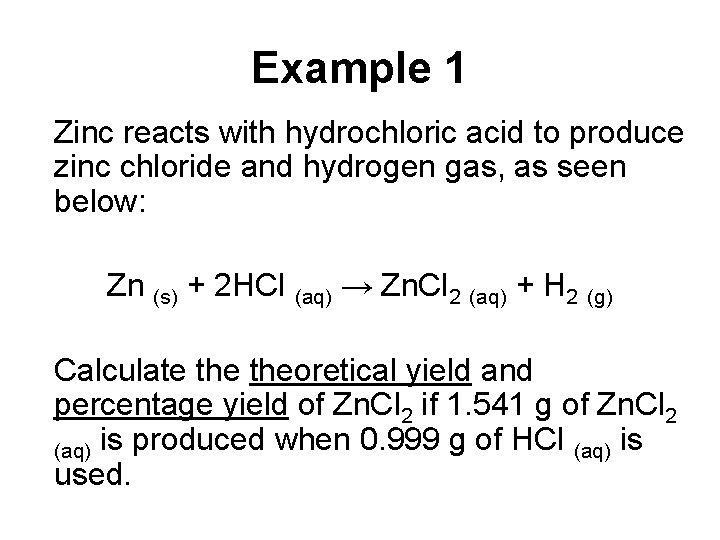
Example 1 Zinc reacts with hydrochloric acid to produce zinc chloride and hydrogen gas, as seen below: Zn (s) + 2 HCl (aq) → Zn. Cl 2 (aq) + H 2 (g) Calculate theoretical yield and percentage yield of Zn. Cl 2 if 1. 541 g of Zn. Cl 2 (aq) is produced when 0. 999 g of HCl (aq) is used.

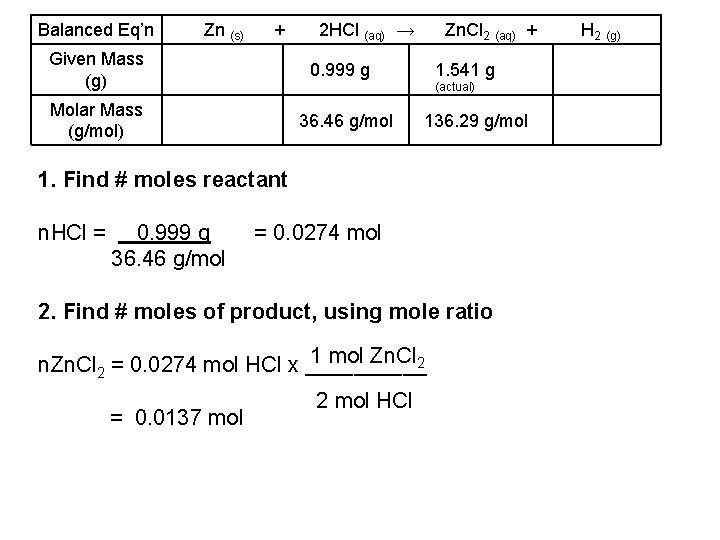
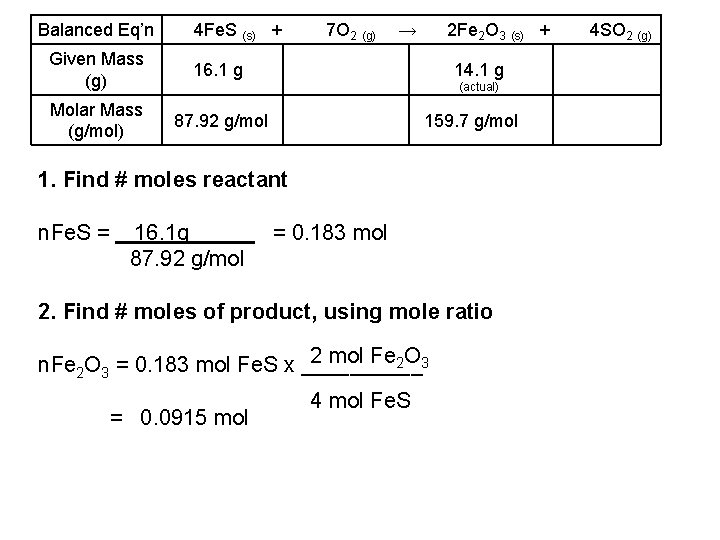
Balanced Eq’n Zn (s) + Given Mass (g) 2 HCl (aq) → Zn. Cl 2 (aq) + 0. 999 g 1. 541 g (actual) Molar Mass (g/mol) 36. 46 g/mol 136. 29 g/mol 1. Find # moles reactant n. HCl = 0. 999 g 36. 46 g/mol = 0. 0274 mol 2. Find # moles of product, using mole ratio 1 mol Zn. Cl 2 n. Zn. Cl 2 = 0. 0274 mol HCl x _____ = 0. 0137 mol 2 mol HCl H 2 (g)

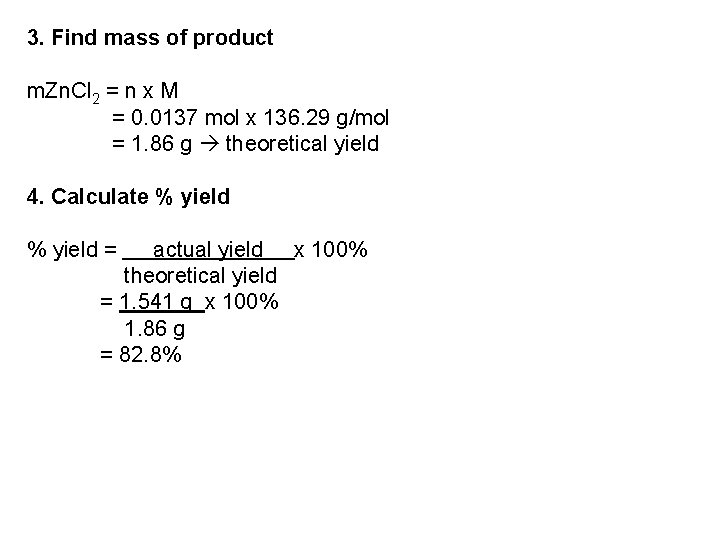
3. Find mass of product m. Zn. Cl 2 = n x M = 0. 0137 mol x 136. 29 g/mol = 1. 86 g theoretical yield 4. Calculate % yield = actual yield x 100% theoretical yield = 1. 541 g x 100% 1. 86 g = 82. 8%

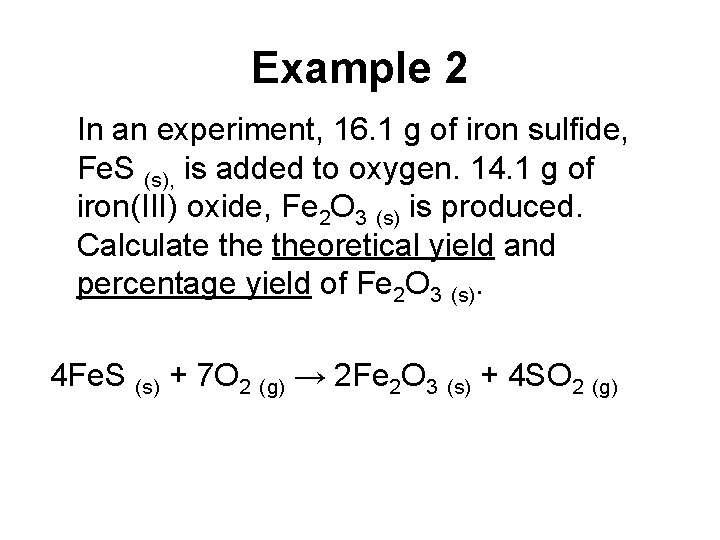
Example 2 In an experiment, 16. 1 g of iron sulfide, Fe. S (s), is added to oxygen. 14. 1 g of iron(III) oxide, Fe 2 O 3 (s) is produced. Calculate theoretical yield and percentage yield of Fe 2 O 3 (s). 4 Fe. S (s) + 7 O 2 (g) → 2 Fe 2 O 3 (s) + 4 SO 2 (g)

Balanced Eq’n 4 Fe. S (s) + Given Mass (g) 16. 1 g Molar Mass (g/mol) 87. 92 g/mol 7 O 2 (g) 2 Fe 2 O 3 (s) + → 14. 1 g (actual) 159. 7 g/mol 1. Find # moles reactant n. Fe. S = 16. 1 g 87. 92 g/mol = 0. 183 mol 2. Find # moles of product, using mole ratio 2 mol Fe 2 O 3 n. Fe 2 O 3 = 0. 183 mol Fe. S x _____ = 0. 0915 mol 4 mol Fe. S 4 SO 2 (g)

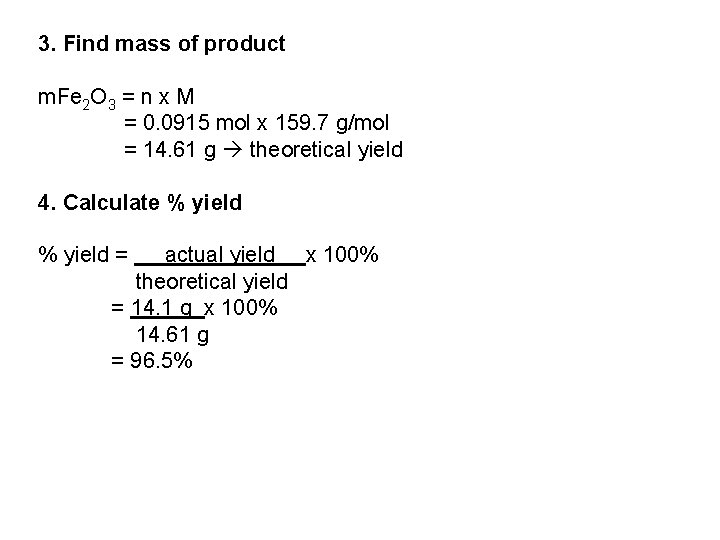
3. Find mass of product m. Fe 2 O 3 = n x M = 0. 0915 mol x 159. 7 g/mol = 14. 61 g theoretical yield 4. Calculate % yield = actual yield x 100% theoretical yield = 14. 1 g x 100% 14. 61 g = 96. 5%