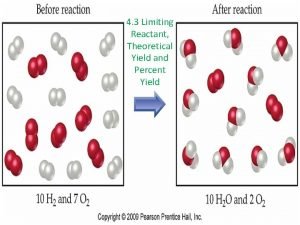
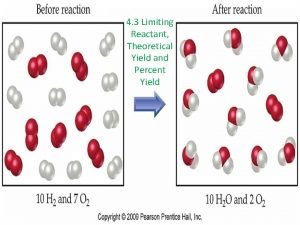
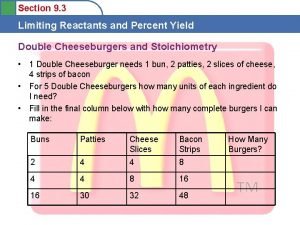
Yield the amount of product Theoretical yield the









- Slides: 12


Yield: the amount of product Theoretical yield: the amount of product we expect, based on stoichiometric calculations Actual yield: amount of product from a procedure or experiment (this is given in the question) Percent yield: actual yield x 100% theoretical yield

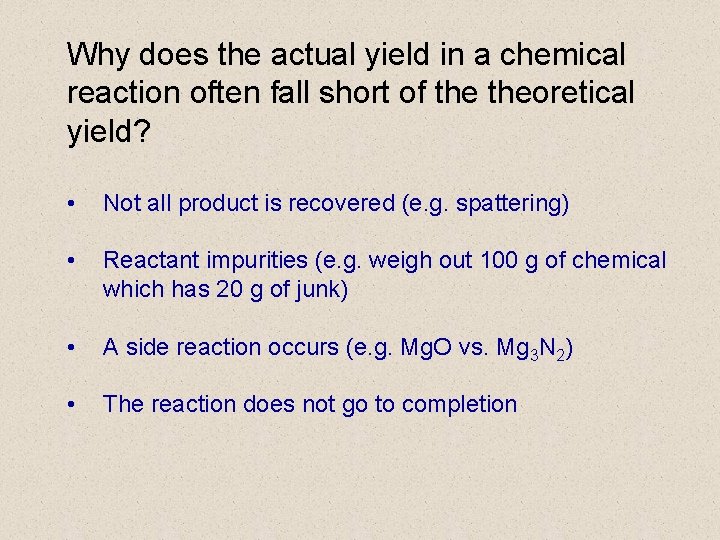
Why does the actual yield in a chemical reaction often fall short of theoretical yield? • Not all product is recovered (e. g. spattering) • Reactant impurities (e. g. weigh out 100 g of chemical which has 20 g of junk) • A side reaction occurs (e. g. Mg. O vs. Mg 3 N 2) • The reaction does not go to completion

Procedure Step 1: write the balanced chemical equation Step 2: determine actual and theoretical yield. Actual is given, theoretical is calculated: Step 3: Calculate % yield

Q 1 - What is the % yield of H 2 O if 138 g H 2 O is produced from 16 g H 2 and excess O 2?

Q 2 - What is the % yield of NH 3 if 40. 5 g NH 3 is produced from 20. 0 mol H 2 and excess N 2?

Q 3 When 5. 00 g of KCl. O 3 is heated it decomposes according to the equation: 2 KCl. O 3 2 KCl + 3 O 2 a) Calculate theoretical yield of oxygen.

b) Give the % yield if 1. 78 g of O 2 is produced. c) How much O 2 would be produced if the percentage yield was 78. 5%?

Q 4 What is the % yield of H 2 O if 58 g H 2 O are produced by combining 60 g O 2 and 7. 0 g H 2?


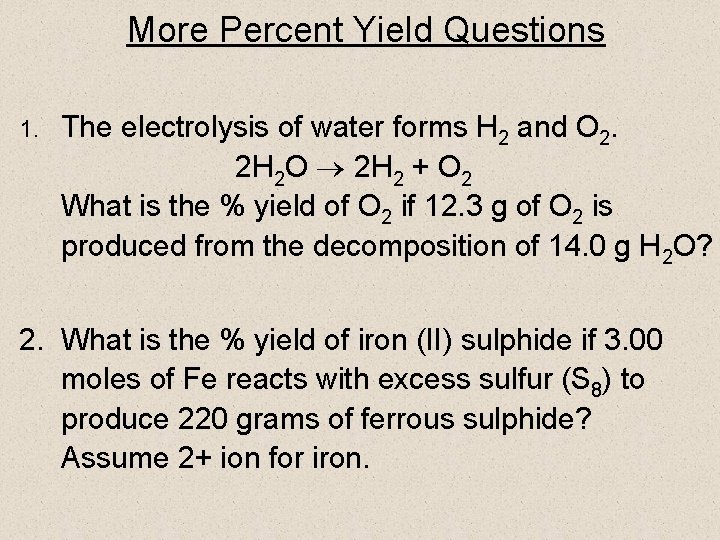
More Percent Yield Questions 1. The electrolysis of water forms H 2 and O 2. 2 H 2 O 2 H 2 + O 2 What is the % yield of O 2 if 12. 3 g of O 2 is produced from the decomposition of 14. 0 g H 2 O? 2. What is the % yield of iron (II) sulphide if 3. 00 moles of Fe reacts with excess sulfur (S 8) to produce 220 grams of ferrous sulphide? Assume 2+ ion for iron.

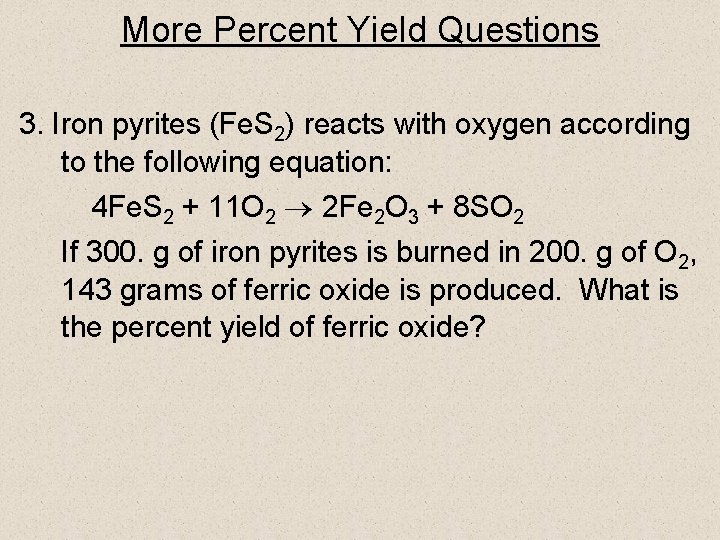
More Percent Yield Questions 3. Iron pyrites (Fe. S 2) reacts with oxygen according to the following equation: 4 Fe. S 2 + 11 O 2 2 Fe 2 O 3 + 8 SO 2 If 300. g of iron pyrites is burned in 200. g of O 2, 143 grams of ferric oxide is produced. What is the percent yield of ferric oxide?
 How do you get the theoretical yield
How do you get the theoretical yield Limiting reactant
Limiting reactant How do u find percent yield
How do u find percent yield Actual-theoretical/actual
Actual-theoretical/actual Difference between actual yield and theoretical yield
Difference between actual yield and theoretical yield Actual yield def
Actual yield def How to work out theoretical yield
How to work out theoretical yield Theoretical yield def
Theoretical yield def Theoretical yield
Theoretical yield Lab: limiting reactant and percent yield
Lab: limiting reactant and percent yield Theoretical yield stoichiometry
Theoretical yield stoichiometry Dividend yield and capital gains yield
Dividend yield and capital gains yield Dividend yield and capital gains yield
Dividend yield and capital gains yield