Stoichiometry Stoichiometry Stoichiometry mass and quantity relationships among











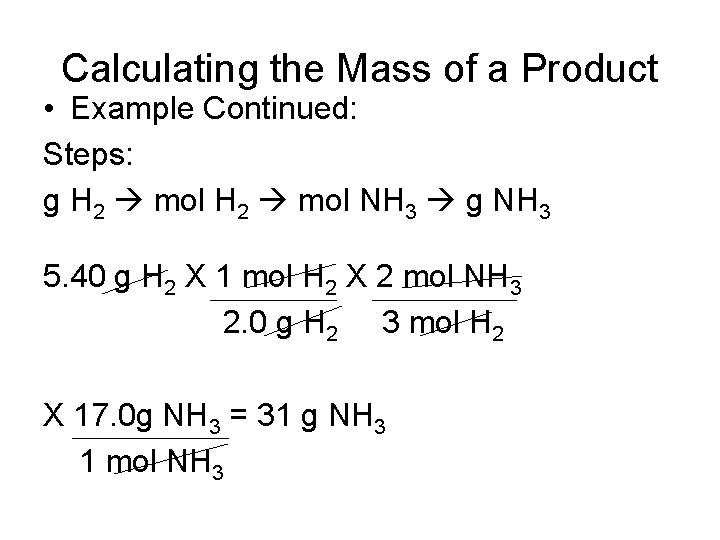










- Slides: 50

Stoichiometry

Stoichiometry • Stoichiometry- mass and quantity relationships among reactants and products in a chemical reaction • Chemists use balanced chemical equations as a basis to calculate how much reactant is needed or product is formed in a reaction

Stoichiometry • How is a balanced equation like a recipe? -A balanced chemical equation provides the same kind of quantitative information

Solving Stoichiometry Problems 1. List what you know 2. Set up the problem 3. Estimate and calculate

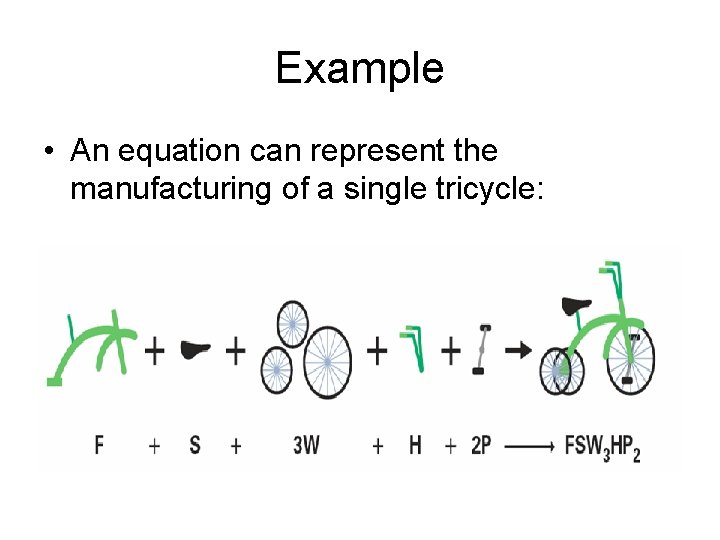
Example • An equation can represent the manufacturing of a single tricycle:

Example • In a five-day workweek, Tiny Tyke is scheduled to make 640 tricycles. How many wheels should be in the plant on Monday morning to make these tricycles? • Knowns: • Number of tricycles = 640 FSW 3 HP 2 • F + S + 3 W + H + 2 P = FSW 3 HP 2

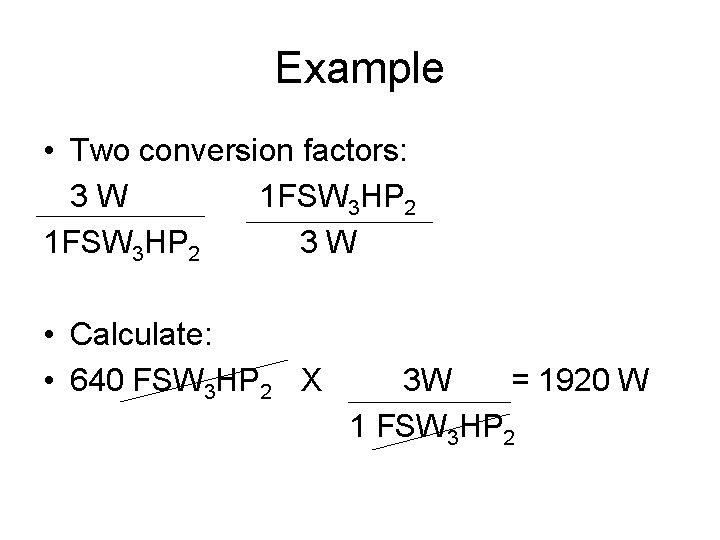
Example • Two conversion factors: 3 W 1 FSW 3 HP 2 3 W • Calculate: • 640 FSW 3 HP 2 X 3 W = 1920 W 1 FSW 3 HP 2

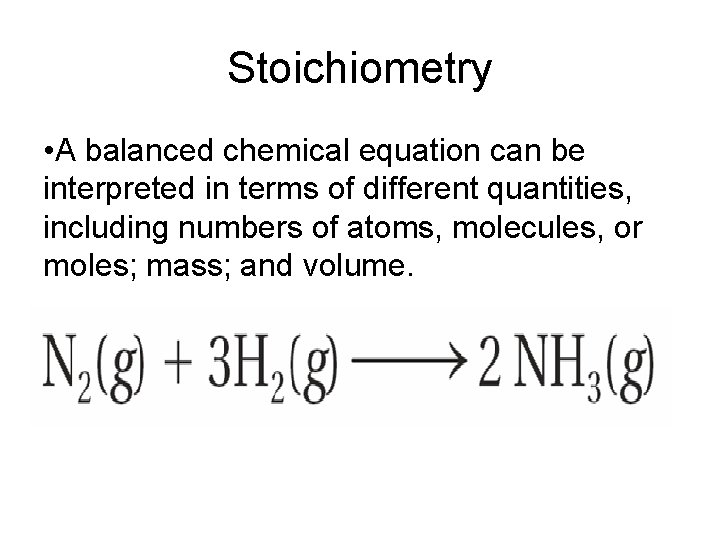
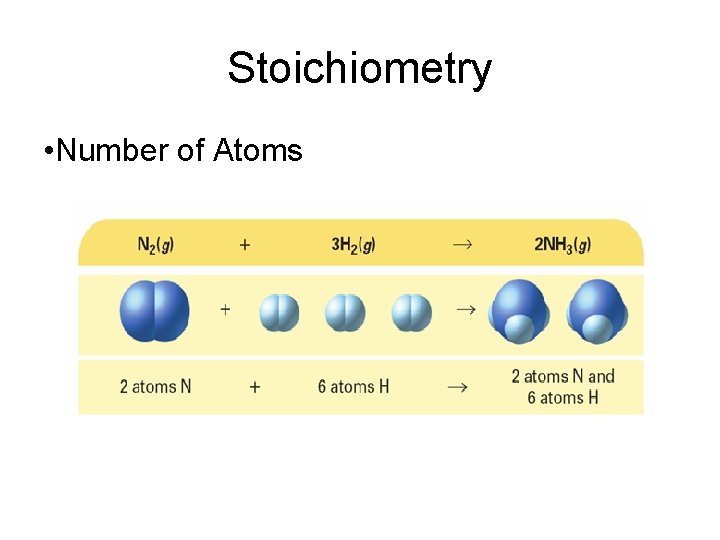
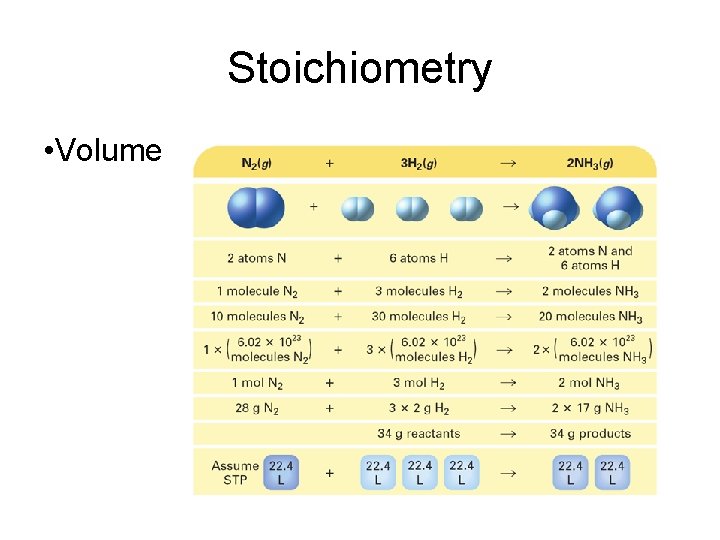
Stoichiometry • A balanced chemical equation can be interpreted in terms of different quantities, including numbers of atoms, molecules, or moles; mass; and volume.

Stoichiometry • Number of Atoms

Stoichiometry • Number of Molecules

Stoichiometry • Moles

Stoichiometry • Mass

Stoichiometry • Volume

Stoichiometry • Mass and atoms are conserved in every chemical reaction

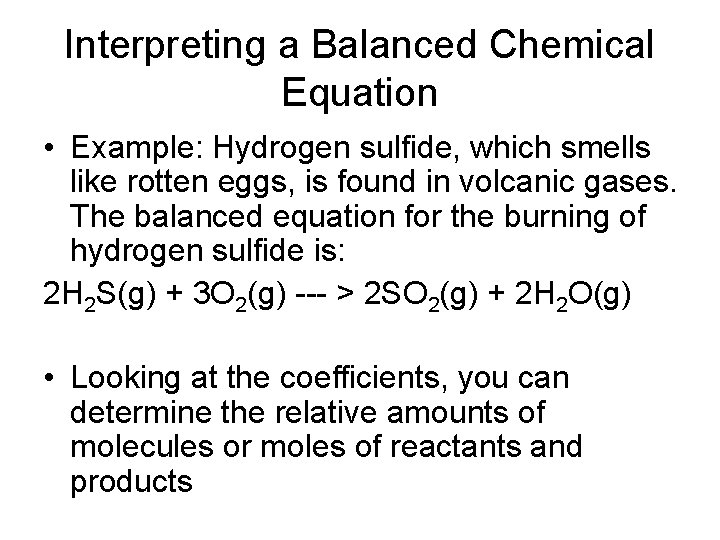
Interpreting a Balanced Chemical Equation • Example: Hydrogen sulfide, which smells like rotten eggs, is found in volcanic gases. The balanced equation for the burning of hydrogen sulfide is: 2 H 2 S(g) + 3 O 2(g) --- > 2 SO 2(g) + 2 H 2 O(g) • Looking at the coefficients, you can determine the relative amounts of molecules or moles of reactants and products

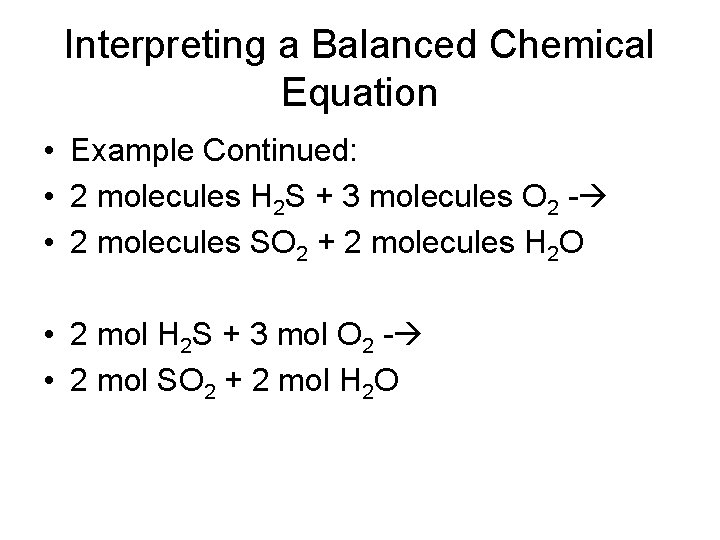
Interpreting a Balanced Chemical Equation • Example Continued: • 2 molecules H 2 S + 3 molecules O 2 - • 2 molecules SO 2 + 2 molecules H 2 O • 2 mol H 2 S + 3 mol O 2 - • 2 mol SO 2 + 2 mol H 2 O

Interpreting a Balanced Chemical Equation • Example Continued: 2 mol H 2 S + 3 mol O 2 2 mol SO 2 + 2 mol H 20 (2 mol X 34. 1 g/mol) + (3 mol X 32 g/mol) -> (2 mol X 64. 1 g/mol) + (2 mol X 18. 0 g/mol) 68. 2 g H 2 S + 96. 0 g O 2 -> 128. 2 g SO 2 + 36. 0 g H 2 O 164. 2 g = 164. 2 g

Writing and Using Mole Ratios • In chemical calculations, mole ratios are used to convert between: - moles of reactant and moles of product, between moles of reactants, or between moles of products

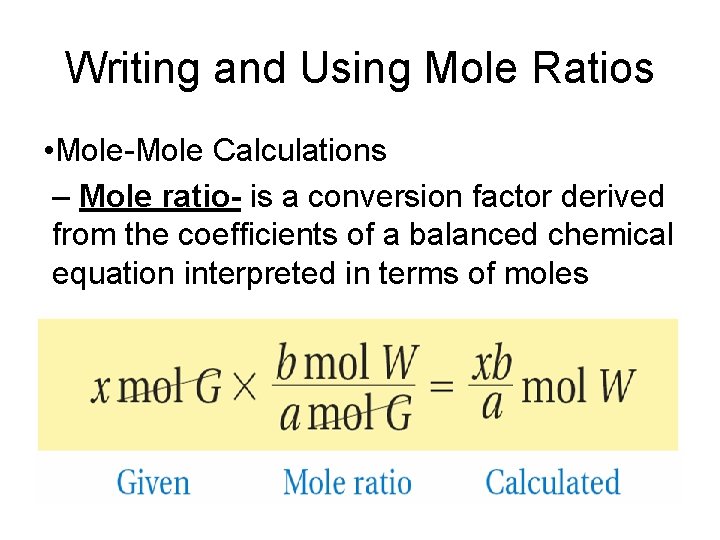
Writing and Using Mole Ratios • Mole-Mole Calculations – Mole ratio- is a conversion factor derived from the coefficients of a balanced chemical equation interpreted in terms of moles

Writing and Using Mole Ratios • To determine the number of moles in a sample of a compound, first measure the mass of the sample • Then use the molar mass to calculate the number of moles in that mass

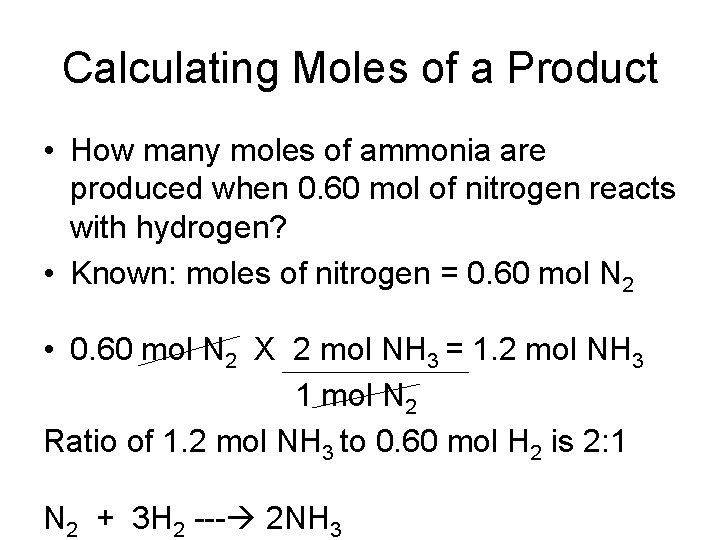
Calculating Moles of a Product • How many moles of ammonia are produced when 0. 60 mol of nitrogen reacts with hydrogen? • Known: moles of nitrogen = 0. 60 mol N 2 • 0. 60 mol N 2 X 2 mol NH 3 = 1. 2 mol NH 3 1 mol N 2 Ratio of 1. 2 mol NH 3 to 0. 60 mol H 2 is 2: 1 N 2 + 3 H 2 --- 2 NH 3

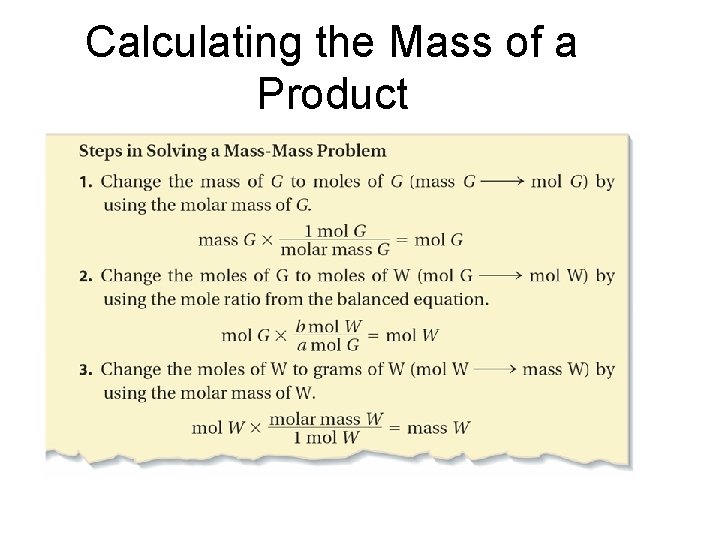
Calculating the Mass of a Product –Mass-Mass Calculations

Calculating the Mass of a Product • Calculate the number of grams of NH 3 produced by the reaction of 5. 40 g of hydrogen with an excess of nitrogen. The balanced equation is: N 2(g) + 3 H 2(g) - 2 NH 3(g) • Known: • Mass of hydrogen = 5. 40 g H 2 • 3 mol H 2 = 2 mol NH 3 (from equation) • 1 mol H 2 = 2. 0 g H 2 (molar mass) • 1 mol NH 3 = 17. 0 g NH 3 (molar mass)
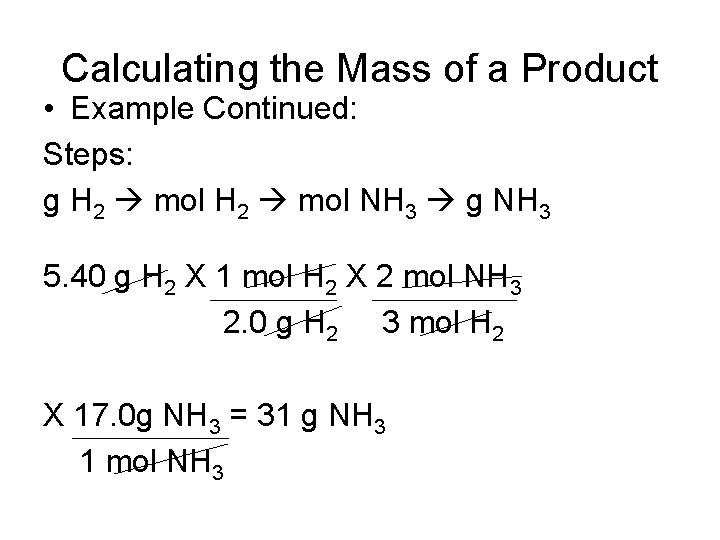
Calculating the Mass of a Product • Example Continued: Steps: g H 2 mol NH 3 g NH 3 5. 40 g H 2 X 1 mol H 2 X 2 mol NH 3 2. 0 g H 2 3 mol H 2 X 17. 0 g NH 3 = 31 g NH 3 1 mol NH 3

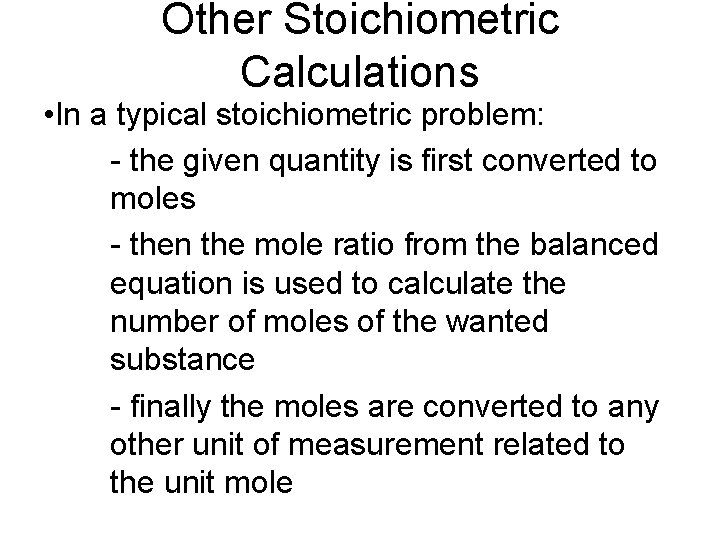
Other Stoichiometric Calculations • In a typical stoichiometric problem: - the given quantity is first converted to moles - then the mole ratio from the balanced equation is used to calculate the number of moles of the wanted substance - finally the moles are converted to any other unit of measurement related to the unit mole

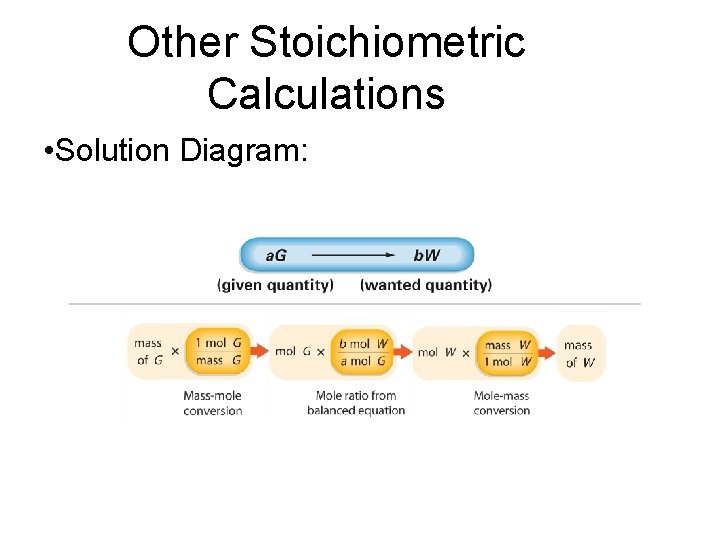
Other Stoichiometric Calculations • Solution Diagram:

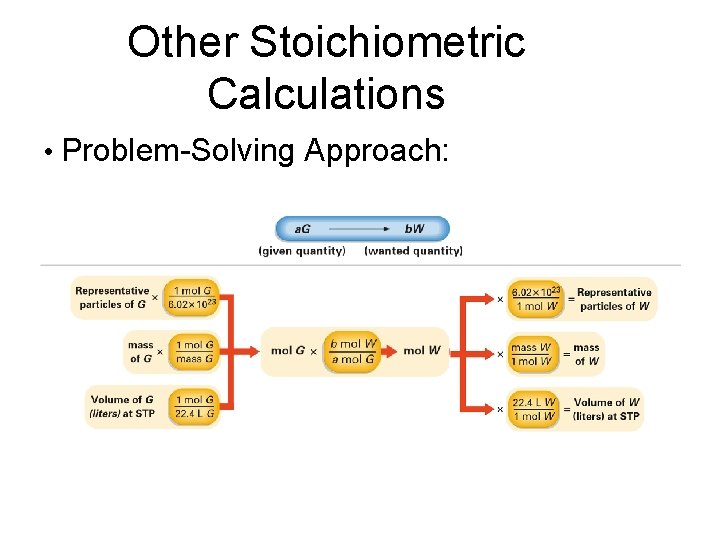
Other Stoichiometric Calculations • Problem-Solving Approach:

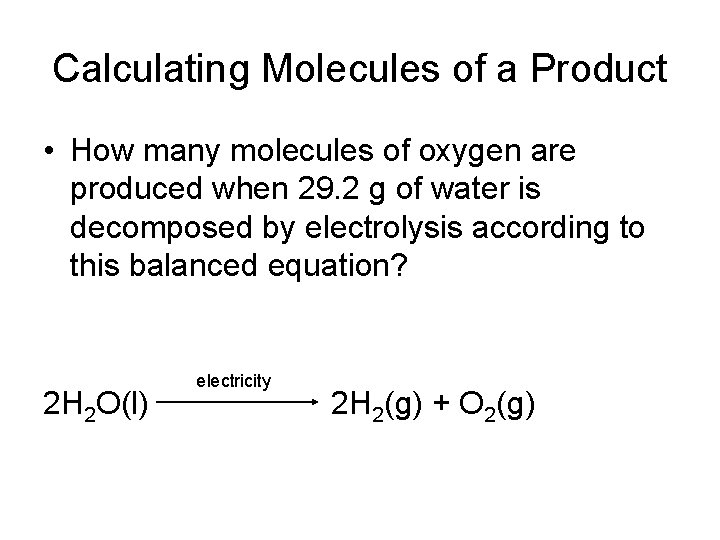
Calculating Molecules of a Product • How many molecules of oxygen are produced when 29. 2 g of water is decomposed by electrolysis according to this balanced equation? 2 H 2 O(l) electricity 2 H 2(g) + O 2(g)

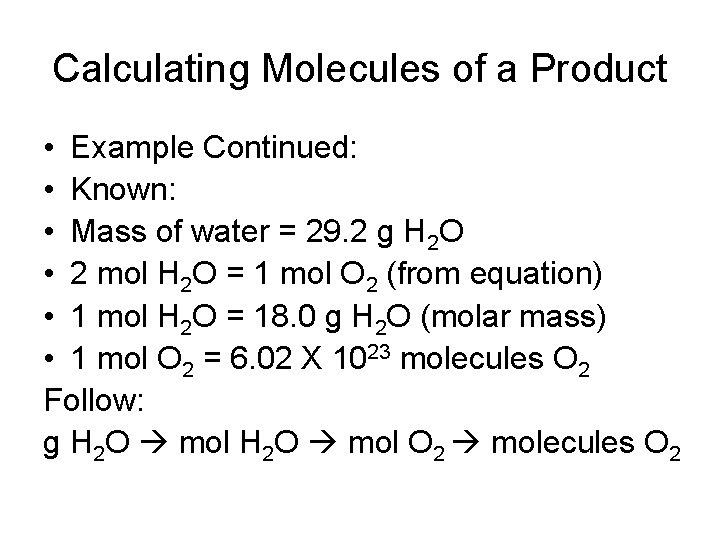
Calculating Molecules of a Product • Example Continued: • Known: • Mass of water = 29. 2 g H 2 O • 2 mol H 2 O = 1 mol O 2 (from equation) • 1 mol H 2 O = 18. 0 g H 2 O (molar mass) • 1 mol O 2 = 6. 02 X 1023 molecules O 2 Follow: g H 2 O mol O 2 molecules O 2

Calculating Molecules of a Product • Example Continued: 29. 2 g H 2 O X 1 mol O 2 18. 0 g H 2 O 2 mol H 2 O X 6. 02 X 1023 molecules O 2 1 mol O 2 = 4. 88 X 1023 molecules O 2

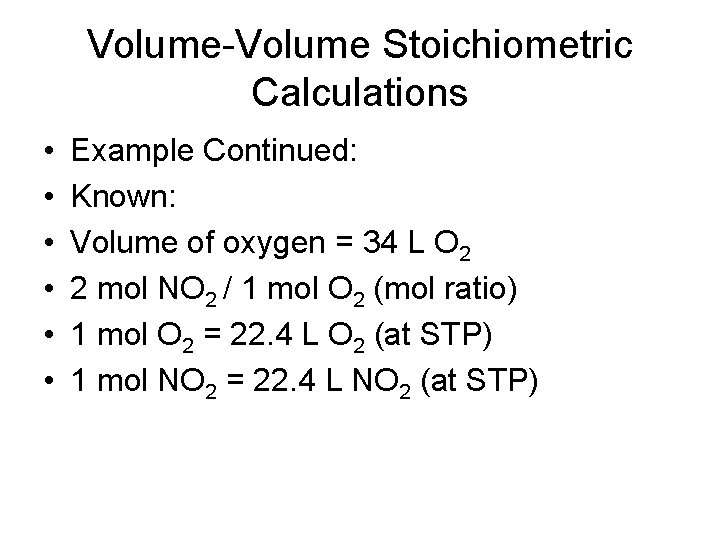
Volume-Volume Stoichiometric Calculations • Nitrogen monoxide and oxygen gas combine to form the brown gas nitrogen dioxide, which contributes to photochemical smog. How many liters of nitrogen dioxide are produced when 34 L of oxygen reacts with an excess of nitrogen monoxide? Assume conditions of Standard Temperature and Pressure. 2 NO(g) + O 2(g) ----- 2 NO 2(g)

Volume-Volume Stoichiometric Calculations • • • Example Continued: Known: Volume of oxygen = 34 L O 2 2 mol NO 2 / 1 mol O 2 (mol ratio) 1 mol O 2 = 22. 4 L O 2 (at STP) 1 mol NO 2 = 22. 4 L NO 2 (at STP)

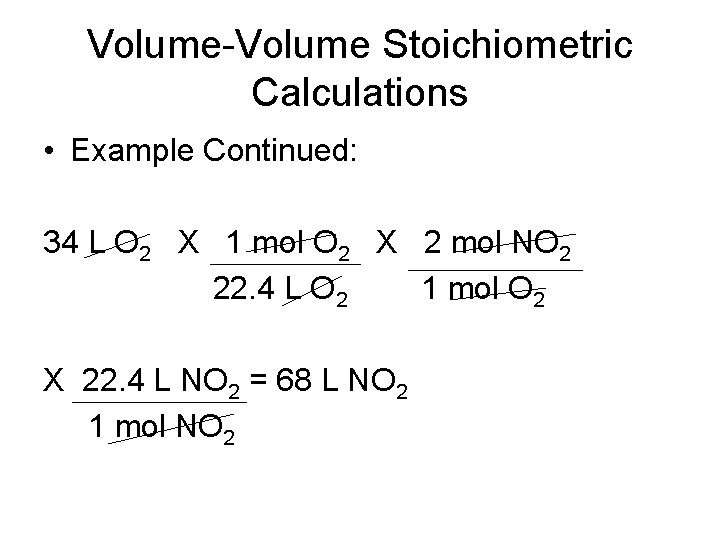
Volume-Volume Stoichiometric Calculations • Example Continued: 34 L O 2 X 1 mol O 2 X 2 mol NO 2 22. 4 L O 2 1 mol O 2 X 22. 4 L NO 2 = 68 L NO 2 1 mol NO 2

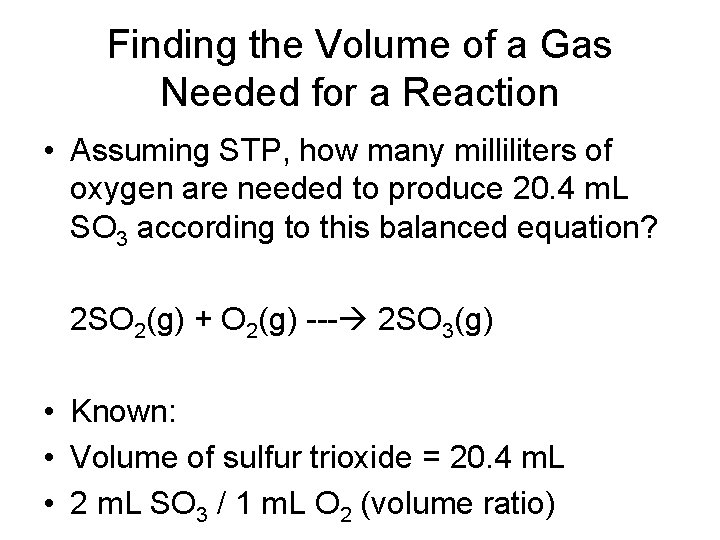
Finding the Volume of a Gas Needed for a Reaction • Assuming STP, how many milliliters of oxygen are needed to produce 20. 4 m. L SO 3 according to this balanced equation? 2 SO 2(g) + O 2(g) --- 2 SO 3(g) • Known: • Volume of sulfur trioxide = 20. 4 m. L • 2 m. L SO 3 / 1 m. L O 2 (volume ratio)

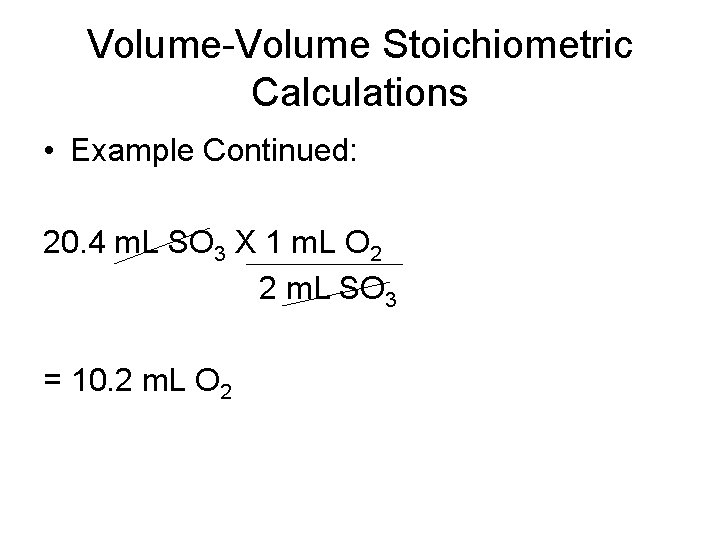
Volume-Volume Stoichiometric Calculations • Example Continued: 20. 4 m. L SO 3 X 1 m. L O 2 2 m. L SO 3 = 10. 2 m. L O 2

Limiting and Excess Reagents • In a chemical reaction, an insufficient quantity of any of the reactants will limit the amount of product that forms. – limiting reagent- is the reagent that determines the amount of product that can be formed by a reaction – Excess Reagent- the reagent that is not used up

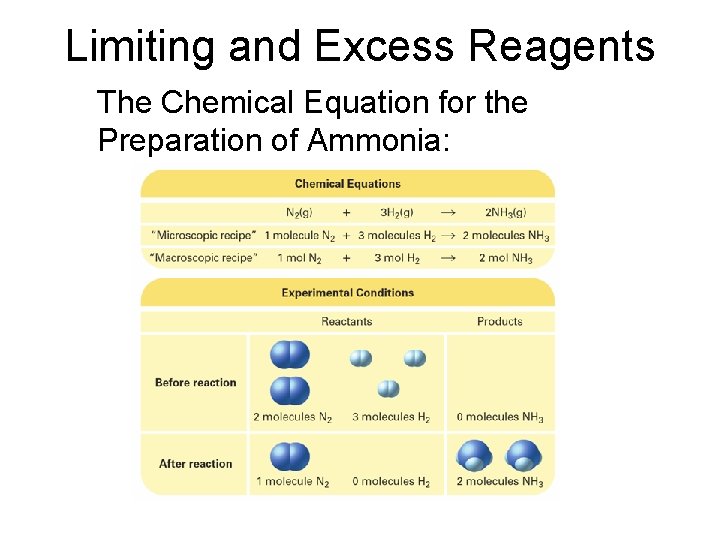
Limiting and Excess Reagents The Chemical Equation for the Preparation of Ammonia:

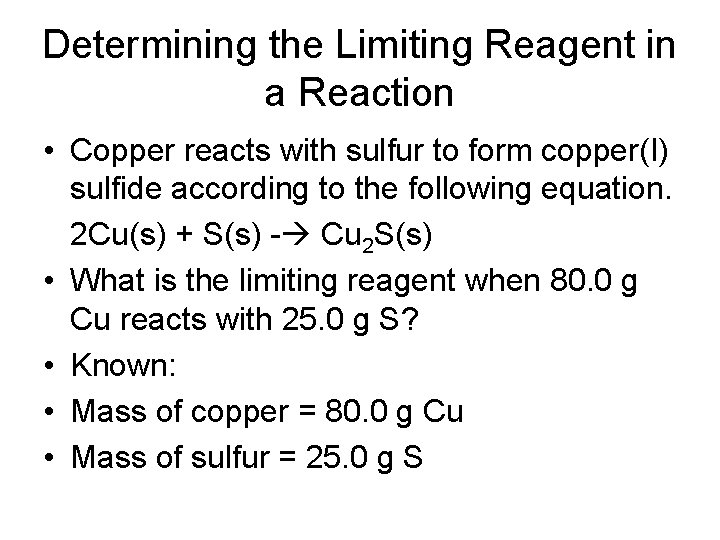
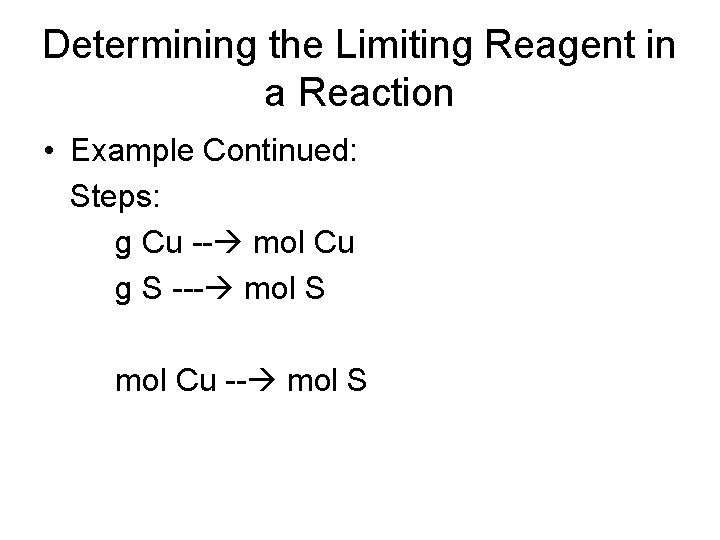
Determining the Limiting Reagent in a Reaction • Copper reacts with sulfur to form copper(I) sulfide according to the following equation. 2 Cu(s) + S(s) - Cu 2 S(s) • What is the limiting reagent when 80. 0 g Cu reacts with 25. 0 g S? • Known: • Mass of copper = 80. 0 g Cu • Mass of sulfur = 25. 0 g S

Determining the Limiting Reagent in a Reaction • Example Continued: Steps: g Cu -- mol Cu g S --- mol S mol Cu -- mol S

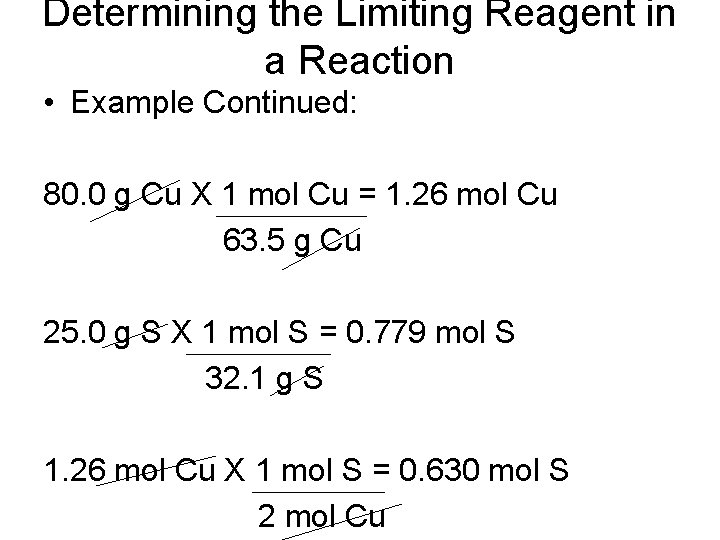
Determining the Limiting Reagent in a Reaction • Example Continued: 80. 0 g Cu X 1 mol Cu = 1. 26 mol Cu 63. 5 g Cu 25. 0 g S X 1 mol S = 0. 779 mol S 32. 1 g S 1. 26 mol Cu X 1 mol S = 0. 630 mol S 2 mol Cu

Determining the Limiting Reagent in a Reaction • Example Continued: • You can compare the amount of sulfur needed (0. 630 mol S) with the given amount (0. 779 mol S). - indicates sulfur is in excess - copper is the limiting reagent

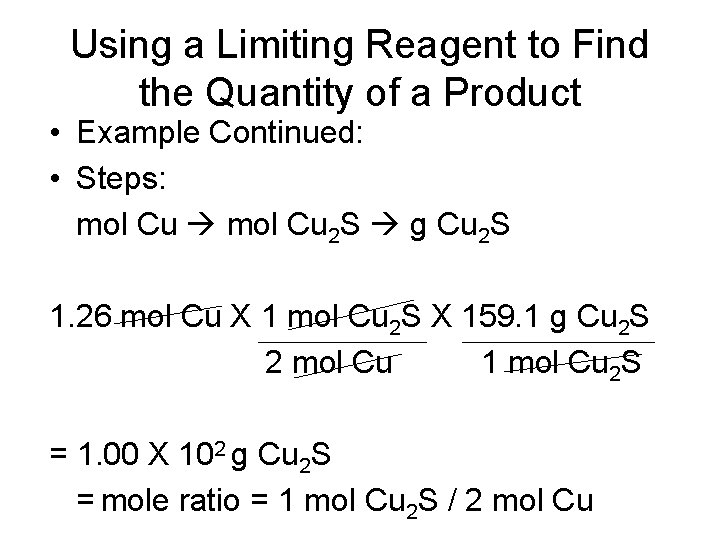
Using a Limiting Reagent to Find the Quantity of a Product • What is the maximum number of grams of Cu 2 S that can be formed when 80. 0 g Cu reacts with 25. 0 g S? 2 Cu(s) + S(s) --- Cu 2 S(s) • Known: • Limiting reagent = 1. 26 mol Cu • 1 mol Cu 2 S = 159. 1 g Cu 2 S (molar mass)

Using a Limiting Reagent to Find the Quantity of a Product • Example Continued: • Steps: mol Cu 2 S g Cu 2 S 1. 26 mol Cu X 1 mol Cu 2 S X 159. 1 g Cu 2 S 2 mol Cu 1 mol Cu 2 S = 1. 00 X 102 g Cu 2 S = mole ratio = 1 mol Cu 2 S / 2 mol Cu

Percent Yield • Percent yield is a measure of the efficiency of a reaction carried out in the laboratory – Example: A batting average is actually a percent yield.

Percent Yield • Theoretical yield- maximum amount of product that could be formed from given amounts of reactants • Actual yield- the amount of product that actually forms when the reaction is carried out in the laboratory

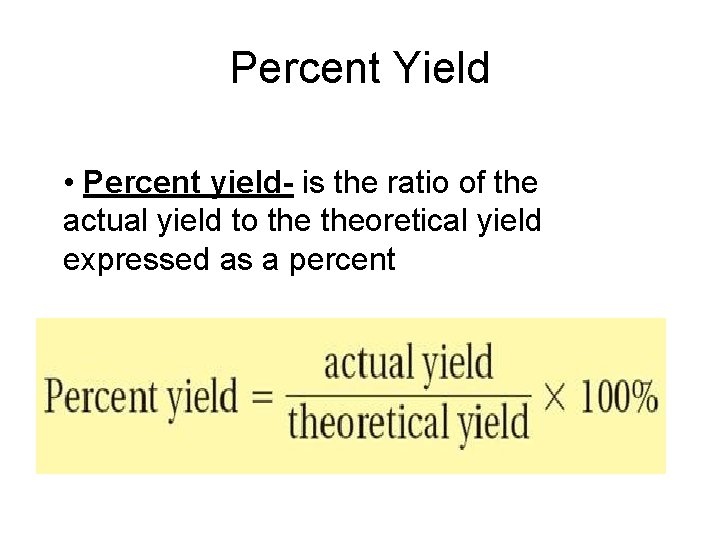
Percent Yield • Percent yield- is the ratio of the actual yield to theoretical yield expressed as a percent

Calculating the Theoretical Yield of a Reaction • Calcium carbonate, which is found in seashells, is decomposed by heating. The balanced equation for this reaction is: Δ • • Ca. CO 3(s) Ca. O(s) + CO 2(g) Known: Mass of calcium carbonate=24. 8 g Ca. CO 3 1 mol Ca. CO 3 = 100. 1 g Ca. CO 3 1 mol Ca. O = 56. 1 g Ca. O

Calculating the Theoretical Yield of a Reaction • Example Continued: Steps: g Ca. CO 3 mol Ca. O g Ca. O 24. 8 g Ca. CO 3 X 1 mol Ca. O 100. 1 g Ca. CO 3 1 mol Ca. CO 3 X 56. 1 g Ca. O 1 mol Ca. O = 13. 9 g Ca. O

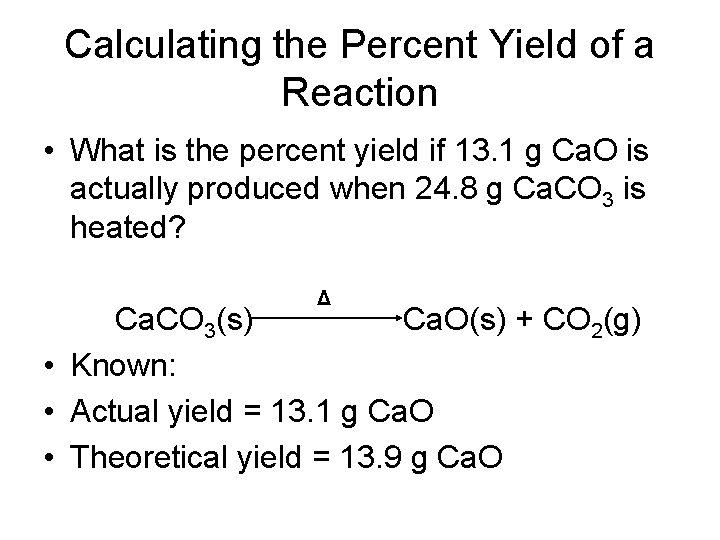
Calculating the Percent Yield of a Reaction • What is the percent yield if 13. 1 g Ca. O is actually produced when 24. 8 g Ca. CO 3 is heated? Δ Ca. CO 3(s) Ca. O(s) + CO 2(g) • Known: • Actual yield = 13. 1 g Ca. O • Theoretical yield = 13. 9 g Ca. O

Calculating the Percent Yield of a Reaction • Example Continued: percent yield = actual yield theoretical yield X 100% Percent yield = 13. 1 g Ca. O X 100% = 94. 2% 13. 9 g Ca. O