Mrs Coyle Introduction to Chemistry Part I Chemistry






























- Slides: 39

Mrs. Coyle Introduction to Chemistry

Part I Chemistry and Technology

Chemistry The study of: n the composition (make-up) of matter n the changes that matter undergoes

What is matter? n Anything that: has mass and n occupies space (volume). n

Mass vs Weight n n Mass: a measure of the amount of matter that an object contains. (SI unit kilogram, kg) Weight: The force with which the earth pulls on an object. (SI unit Newton, N)

The 5 Branches of Chemistry Inorganic n Organic n Analytical n Physical n Biochemistry n

Inorganic Chemistry n The study of chemicals that do not contain carbon.

Organic Chemistry The study of chemicals that contain carbon. n Origin: study of chemicals in living organisms. n

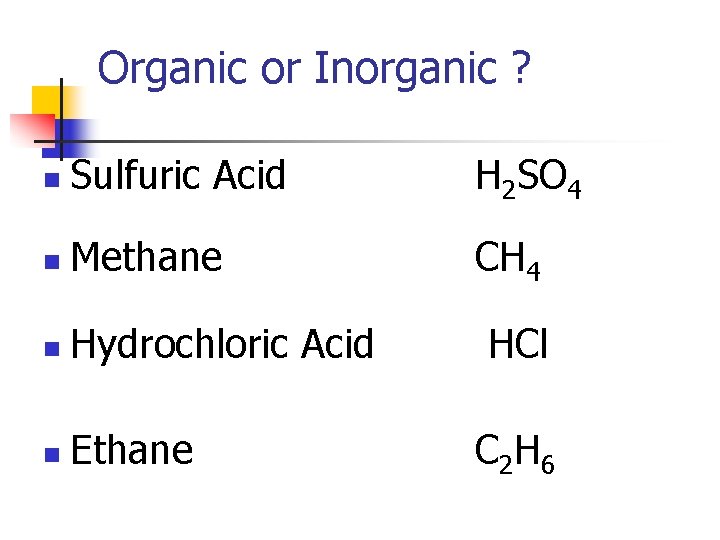
Organic or Inorganic ? n Sulfuric Acid H 2 SO 4 n Methane CH 4 n Hydrochloric Acid n Ethane HCl C 2 H 6

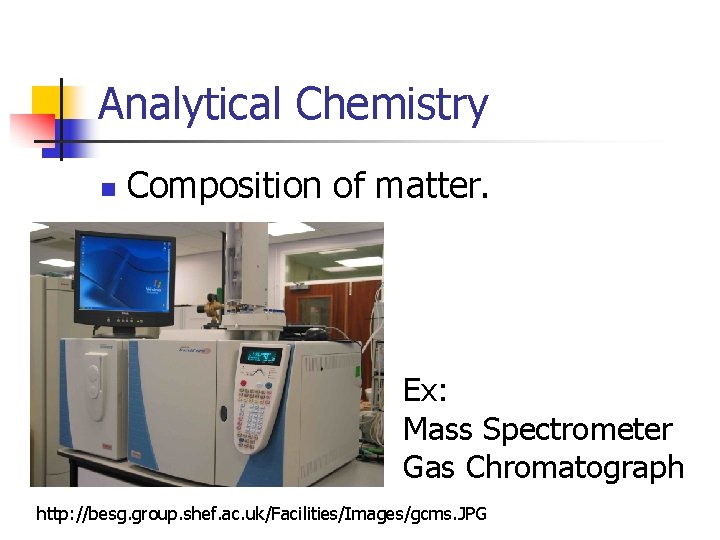
Analytical Chemistry n Composition of matter. Ex: Mass Spectrometer Gas Chromatograph http: //besg. group. shef. ac. uk/Facilities/Images/gcms. JPG

Physical Chemistry n The study of : The mechanism n The rate n The energy transfer n that happens when matter undergoes change.

Biochemistry n Study of processes that take place in organisms.

Science What? n Why? n How? n When? n

Science and Technology n Science Pure n n Does not necessarily have an application. Technology Applied Has practical applications in society. n Engineering. n

Question: Science or Technology? Studying or forming aspirin in a lab in small scale (small amounts).

Question: Science or Technology? n Producing aspirin tablets so that consumers can use them.

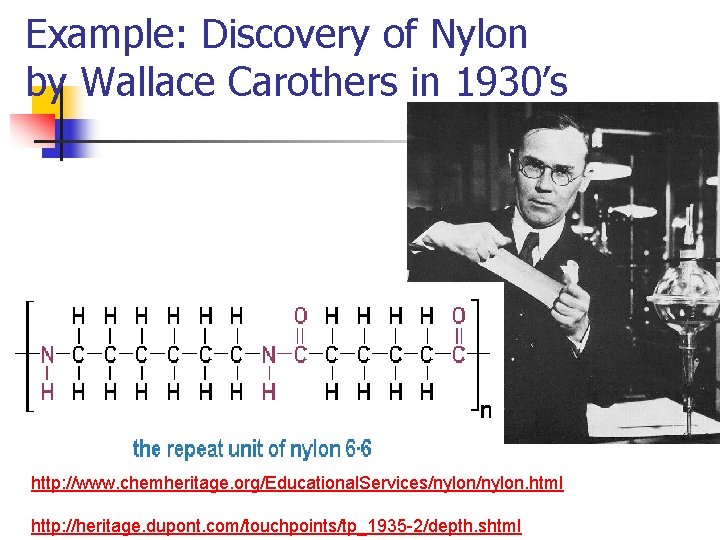
Example: Discovery of Nylon by Wallace Carothers in 1930’s http: //www. chemheritage. org/Educational. Services/nylon. html http: //heritage. dupont. com/touchpoints/tp_1935 -2/depth. shtml

Microscopic- Macroscopic n Micro –(small) n n Microscopic- objects can be seen with a microscope. Macro-(from afar) n Macroscopic- objects are seen without a microscope.

Part II – A Brief History and the Scientific Method

Aristotle (Greece, 4 th Century BC) Philosopher who believed that: n There are 4 elements: earth, water, air, fire. n Matter is perpetually divisible.

Democritus (Greece, 4 th Century BC) First atomic theory n Atom (indivisible). n

Alchemists (~300 BC-1650 AD) China, India, Arabia, Europe, Egypt • Aiming to: §Change common metals to gold. §Develop medicines. • Developed lab equipment. • Mystical.

Galileo Galilei (Italy 1564 AD) Father of the scientific method (along with the Englishman Francis Bacon 1500’s). n

Antoine Lavoisier (France 1743 -1794) Regarded as the Father of Chemistry. n Designed equipment. n Used observations and measurements. n Discovered nitrogen. n

Antoine Lavoisier (cont’d) n Discovered the Law of Conservation of Mass: n In a chemical reaction mass is conserved.

Antoine Lavoisier (cont’d) n n Explained burning as reaction with oxygen. Old theory: release of “phlogiston”.

Question: n Does an iron nail gain mass or lose mass when it rusts (a form of burning)?

John Dalton n (England 1766 -1844) Atomic theory

Amedeo Avogadro (Italy, 1776 -1856) n n Avogadro’s Number 6. 02 x 1023 One mole of any substance contains 6. 02 x 1023 particles.

Dmitri Mendeléev (Russia, 1834 -1907) n First Periodic Table of elements.

The Scientific Method n Steps followed during scientific investigations.

Scientific Method n n Observation- recognition of a problem. Hypothesis- a proposed explanation of an observation n n an educated guess must be testable. Experiment- a procedure used to test a hypothesis (measurement, data collection, manipulated and responding variables) Theory Law

Theory n n A well tested explanation for a broad set of observations. May use models. May allow predictions. Theories may change to explain new observations.

Law n n A statement that summarizes results of observations, but does not explain them. Changes or is abandoned when contradicted by new experiments.

Note: n The order of the steps can vary and additional steps may be added.

“No number of experiments can prove me right; a single experiment can prove me wrong. ” Albert Einstein

Part III Math and Chemistry n Math- the language of Science

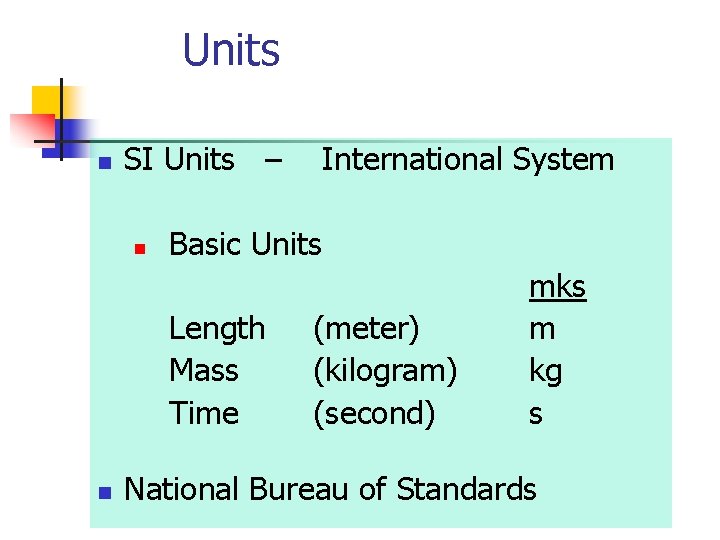
Units n SI Units – n Basic Units Length Mass Time n International System (meter) (kilogram) (second) mks m kg s National Bureau of Standards

Solving Word Problems n n n Analyze n List knowns and unknowns. n Devise a plan. n Write the math equation to be used. Calculate n If needed, rearrange the equation to solve for the unknown. n Substitute the knowns with units in the equation and express the answer with units. Evaluate n Is the answer reasonable?
 They are mrs garcia and mrs castro
They are mrs garcia and mrs castro They are mrs garcia and mrs castro
They are mrs garcia and mrs castro Mrs. darling was ___________ of mrs. s.
Mrs. darling was ___________ of mrs. s. Metodo de coyle
Metodo de coyle Coyle method
Coyle method Coyle and castello method
Coyle and castello method Caitlin coyle
Caitlin coyle Do coyle clil
Do coyle clil Philippe goret
Philippe goret Coyle
Coyle Coyle health and wellbeing
Coyle health and wellbeing Gauss law ap physics c
Gauss law ap physics c Aaron coyle
Aaron coyle University of nottingham
University of nottingham Dr christine coyle
Dr christine coyle What part of speech is mrs
What part of speech is mrs Part whole model subtraction
Part whole model subtraction Unit ratio definition
Unit ratio definition Part part whole
Part part whole Technical object description example
Technical object description example 3 parts of the bar
3 parts of the bar The phase of the moon you see depends on ______.
The phase of the moon you see depends on ______. 미니탭 gage r&r 해석
미니탭 gage r&r 해석 Ib chemistry functional groups
Ib chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Epic poem the odyssey
Epic poem the odyssey The odyssey and epic poetry an introduction part 1
The odyssey and epic poetry an introduction part 1 What rules must an epic follow?
What rules must an epic follow? What are the parts of the globe theatre
What are the parts of the globe theatre Good introduction paragraph
Good introduction paragraph The odyssey and epic poetry: an introduction, part 1
The odyssey and epic poetry: an introduction, part 1 The odyssey
The odyssey Homers epic poem
Homers epic poem The odyssey and epic poetry: an introduction, part 1
The odyssey and epic poetry: an introduction, part 1 Hamlet, part 1: an introduction to elizabethan theater
Hamlet, part 1: an introduction to elizabethan theater An introduction to shakespeare and romeo and juliet part 1
An introduction to shakespeare and romeo and juliet part 1 The odyssey and epic poetry an introduction part 1
The odyssey and epic poetry an introduction part 1 Mrcog registration
Mrcog registration The odyssey and epic poetry an introduction part 1
The odyssey and epic poetry an introduction part 1 The odyssey and epic poetry an introduction part 1
The odyssey and epic poetry an introduction part 1