Formation of Ammonia Proportional Relationships Stoichiometry mass relationships




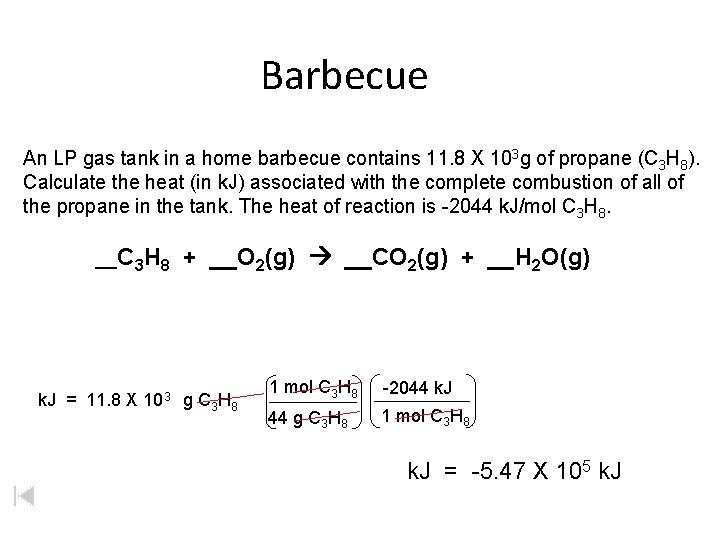

- Slides: 37

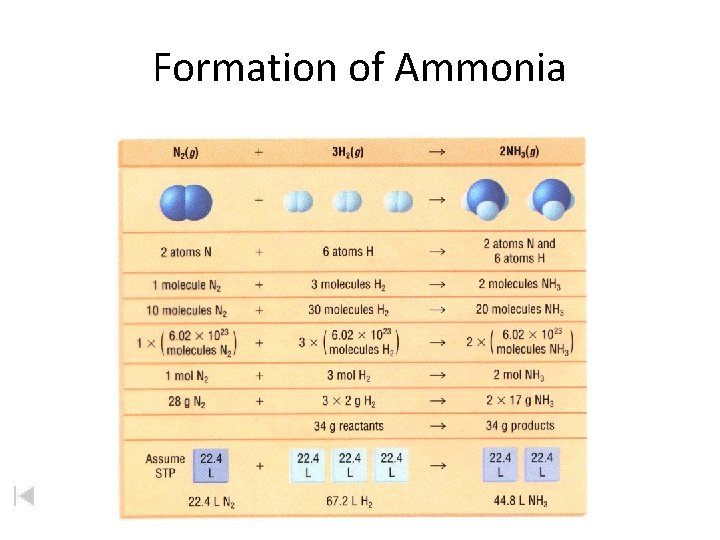
Formation of Ammonia

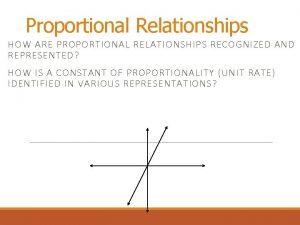
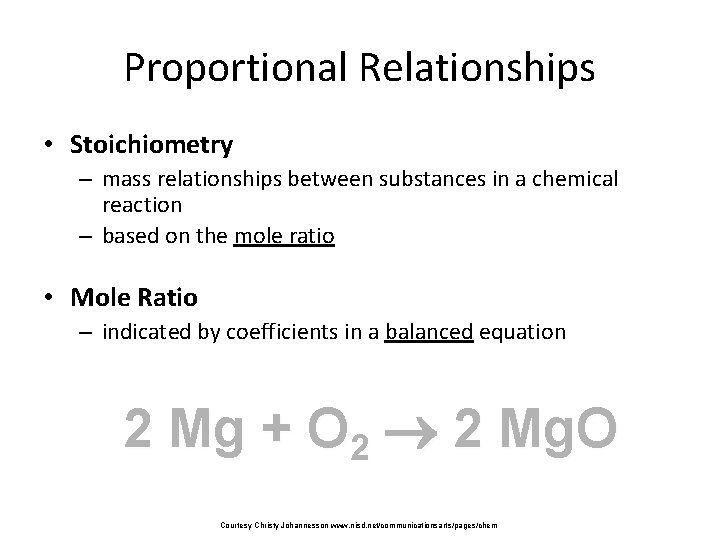
Proportional Relationships • Stoichiometry – mass relationships between substances in a chemical reaction – based on the mole ratio • Mole Ratio – indicated by coefficients in a balanced equation 2 Mg + O 2 2 Mg. O Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

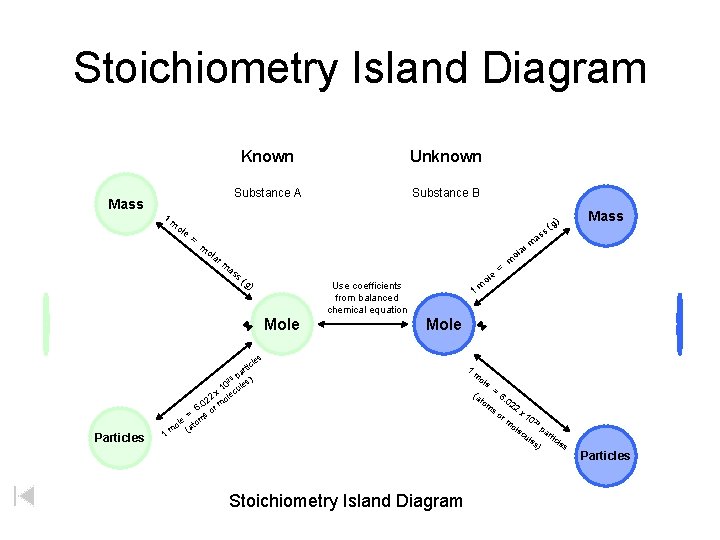
Stoichiometry Island Diagram Mass 1 Known Unknown Substance A Substance B ole = m as a ol s( g) Use coefficients from balanced chemical equation Mole e ol m = m 1 Mole les 1 Mass a rm m ola r Particles ) (g ss m c rti pa ) 23 0 les x 1 lecu 2 o 02 6. or m = s m ole (ato m Stoichiometry Island Diagram 1 m ole (a to m = 6. 02 so 2 x 1 0 23 ole pa cu r les ticle s ) rm Particles

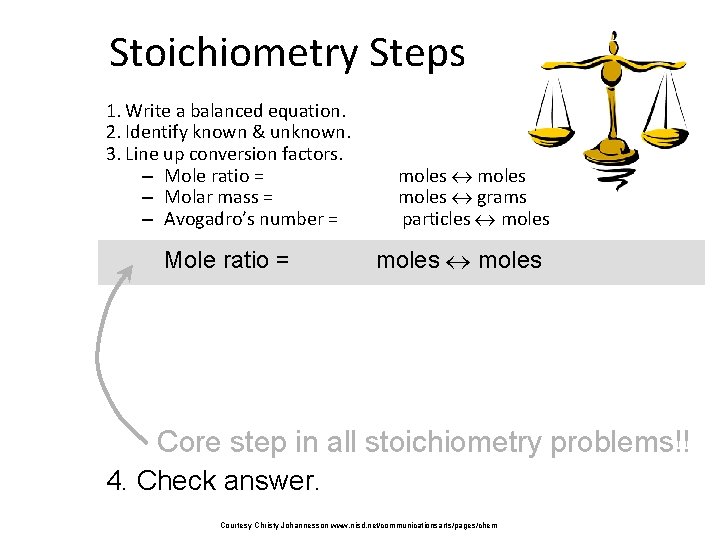
Stoichiometry Steps 1. Write a balanced equation. 2. Identify known & unknown. 3. Line up conversion factors. – Mole ratio = – Molar mass = – Avogadro’s number = – Mole ratio = moles grams particles moles Core step in all stoichiometry problems!! 4. Check answer. Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

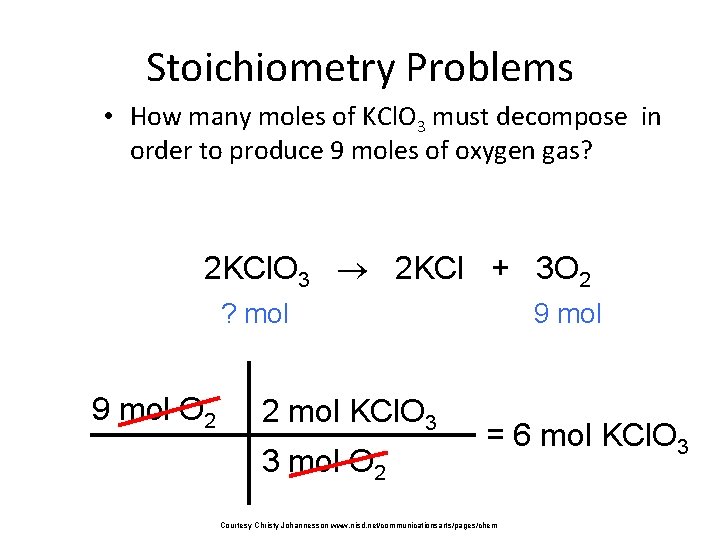
Stoichiometry Problems • How many moles of KCl. O 3 must decompose in order to produce 9 moles of oxygen gas? 2 KCl. O 3 2 KCl + 3 O 2 ? mol 9 mol O 2 2 mol KCl. O 3 3 mol O 2 9 mol = 6 mol KCl. O 3 Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

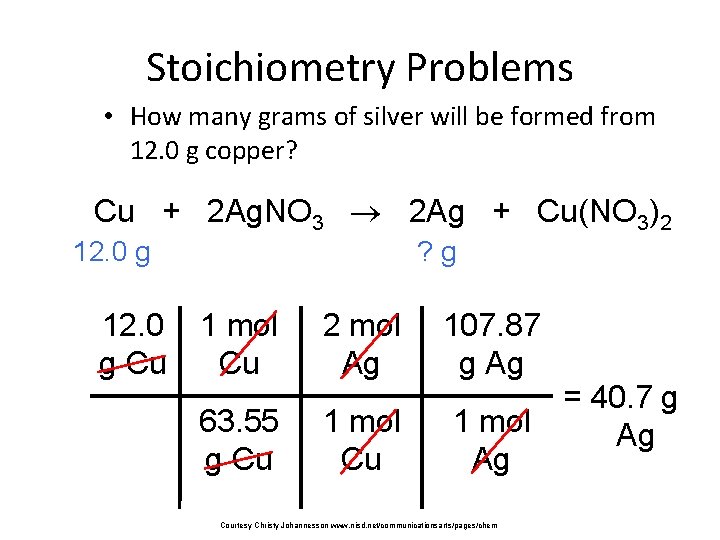
Stoichiometry Problems • How many grams of silver will be formed from 12. 0 g copper? Cu + 2 Ag. NO 3 2 Ag + Cu(NO 3)2 12. 0 g Cu ? g 1 mol Cu 2 mol Ag 107. 87 g Ag 63. 55 g Cu 1 mol Ag Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem = 40. 7 g Ag

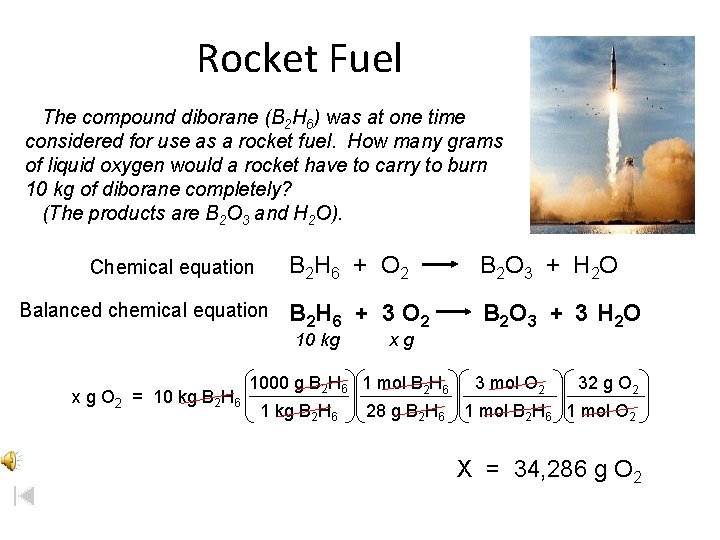
Rocket Fuel The compound diborane (B 2 H 6) was at one time considered for use as a rocket fuel. How many grams of liquid oxygen would a rocket have to carry to burn 10 kg of diborane completely? (The products are B 2 O 3 and H 2 O). Chemical equation Balanced chemical equation B 2 H 6 + O 2 B 2 O 3 + H 2 O B 2 H 6 + 3 O 2 B 2 O 3 + 3 H 2 O 10 kg x g O 2 = 10 kg B 2 H 6 xg 1000 g B 2 H 6 1 mol B 2 H 6 1 kg B 2 H 6 28 g B 2 H 6 3 mol O 2 32 g O 2 1 mol B 2 H 6 1 mol O 2 X = 34, 286 g O 2

Limiting Reactants • Limiting Reactant – used up in a reaction – determines the amount of product • Excess Reactant – added to ensure that the other reactant is completely used up – cheaper & easier to recycle Courtesy Christy Johannesson www. nisd. net/communicationsarts/pages/chem

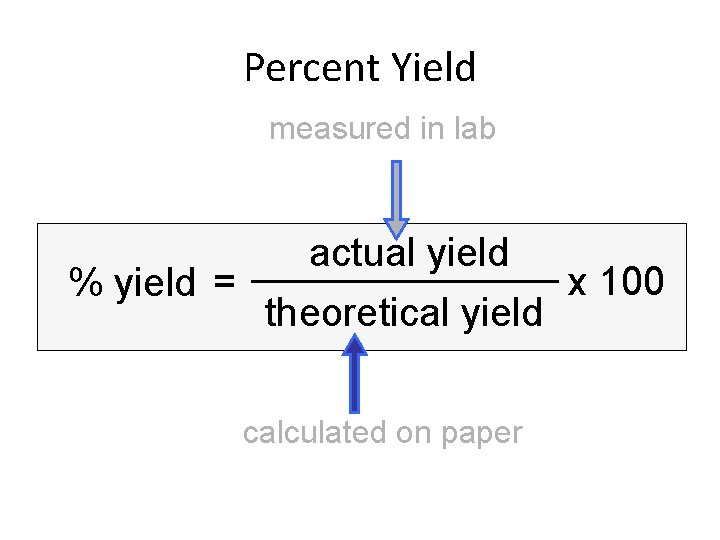
Percent Yield measured in lab % yield = actual yield theoretical yield calculated on paper x 100

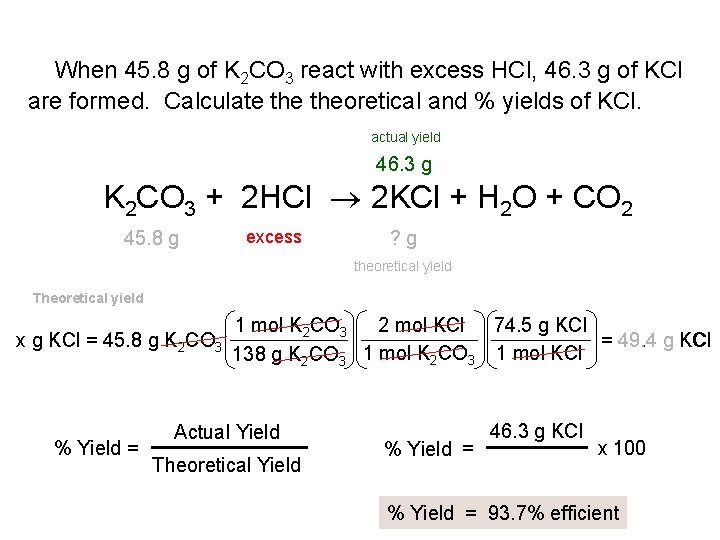
When 45. 8 g of K 2 CO 3 react with excess HCl, 46. 3 g of KCl are formed. Calculate theoretical and % yields of KCl. actual yield 46. 3 g K 2 CO 3 + 2 HCl 2 KCl + H 2 O + CO 2 45. 8 g excess ? g theoretical yield Theoretical yield x g KCl = 45. 8 g K 2 CO 3 % Yield = 1 mol K 2 CO 3 2 mol KCl 74. 5 g KCl = 49. 4 g KCl 1 mol K CO 1 mol KCl 138 g K 2 CO 3 2 3 Actual Yield Theoretical Yield % Yield = 46. 3 g KCl x 100 % Yield = 93. 7% efficient

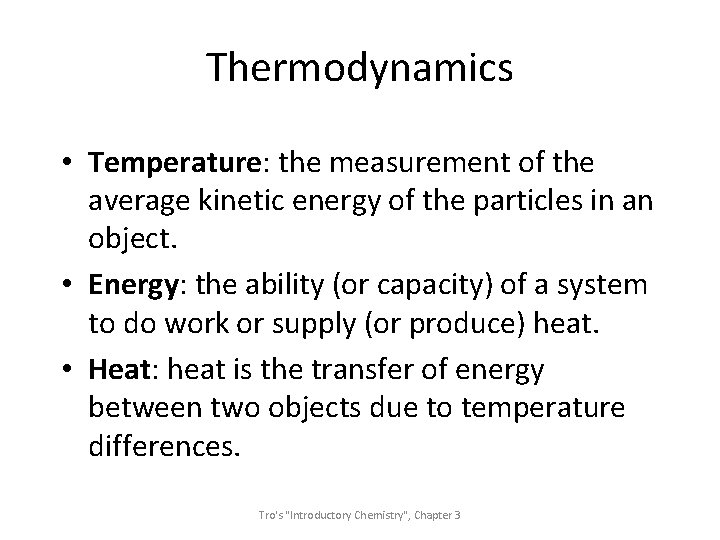
Thermodynamics • Temperature: the measurement of the average kinetic energy of the particles in an object. • Energy: the ability (or capacity) of a system to do work or supply (or produce) heat. • Heat: heat is the transfer of energy between two objects due to temperature differences. Tro's "Introductory Chemistry", Chapter 3

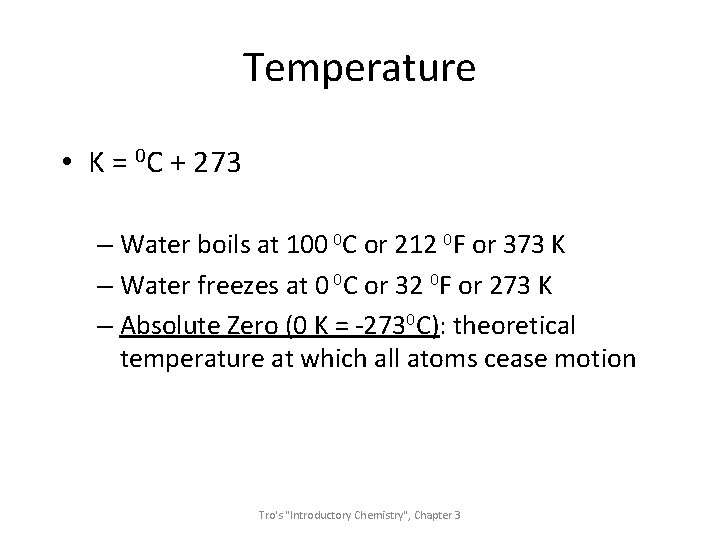
Temperature • K = 0 C + 273 – Water boils at 100 0 C or 212 0 F or 373 K – Water freezes at 0 0 C or 32 0 F or 273 K – Absolute Zero (0 K = -2730 C): theoretical temperature at which all atoms cease motion Tro's "Introductory Chemistry", Chapter 3

Law of Conservation of Energy • “Energy can neither be created nor destroyed. ” • The total amount of energy in the universe is constant. There is no process that can increase or decrease that amount. • However, we can transfer energy from one place (a system) in the universe to another (surroundings), and we can change its form. • Energy is stored in chemical bonds of matter.

Units of Energy • Calorie (cal) is the amount of energy needed to raise one gram of water by 1 °C. – kcal = energy needed to raise 1000 g of water 1 °C. – food calories = kcals. Energy Conversion Factors 1 calorie (cal) 1 Calorie (Cal) = = 4. 184 joules (J) 1000 calories (cal)

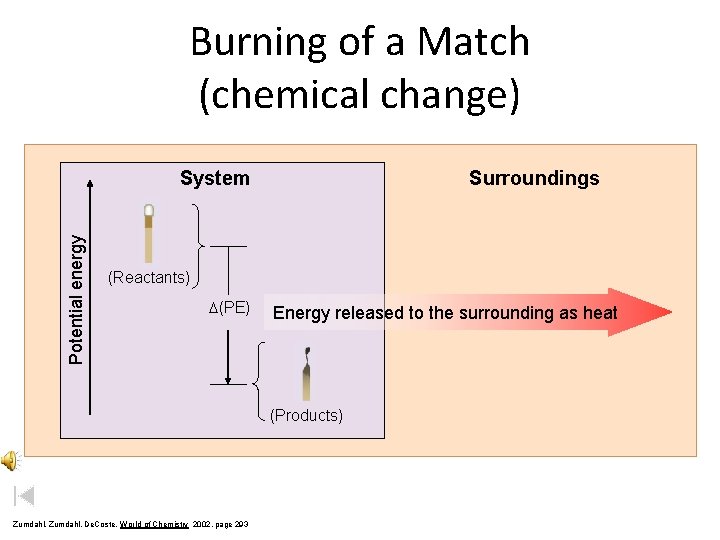
Burning of a Match (chemical change) Potential energy System Surroundings (Reactants) D(PE) Energy released to the surrounding as heat (Products) Zumdahl, De. Coste, World of Chemistry 2002, page 293

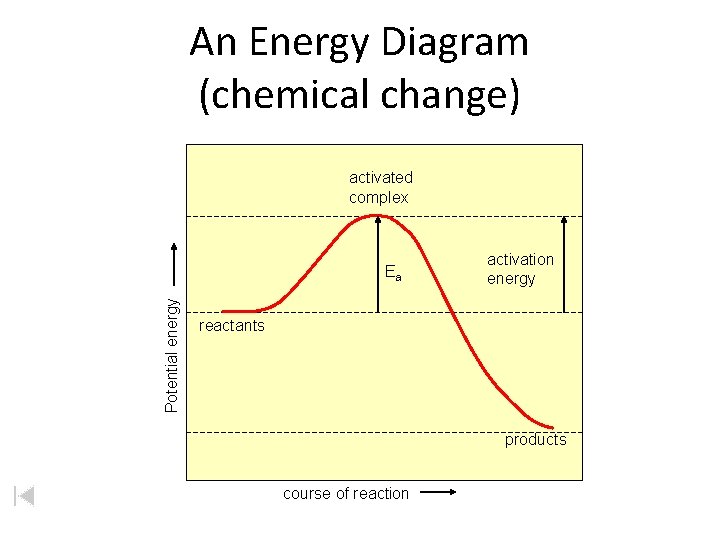
An Energy Diagram (chemical change) activated complex Potential energy Ea activation energy reactants products course of reaction

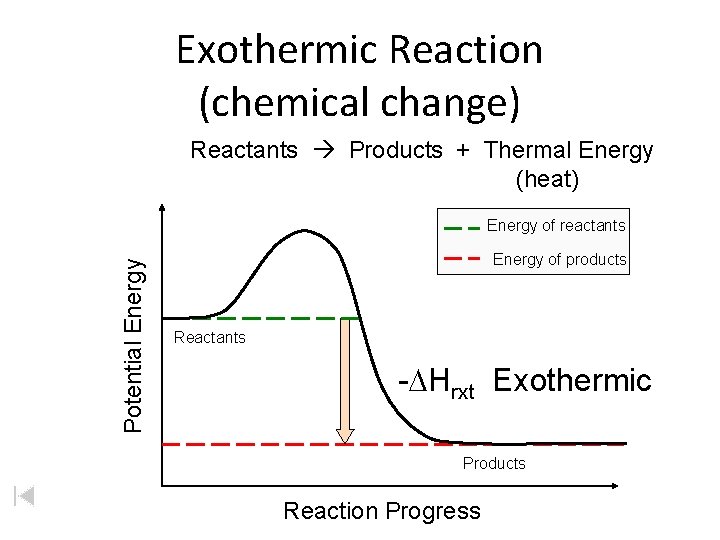
Exothermic Reaction (chemical change) Reactants Products + Thermal Energy (heat) Potential Energy of reactants Energy of products Reactants -DHrxt Exothermic Products Reaction Progress

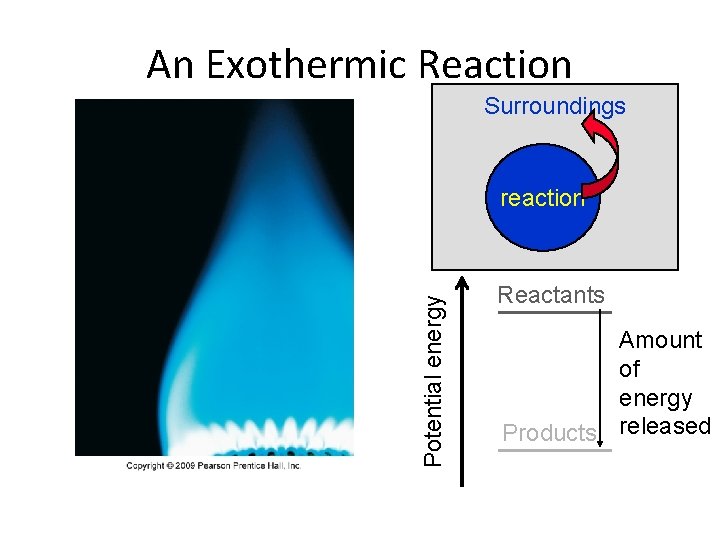
An Exothermic Reaction Surroundings Potential energy reaction Reactants Amount of energy Products released

The Zeppelin LZ 129 Hindenburg catching fire on May 6, 1937 at Lakehurst Naval Air Station in New Jersey.

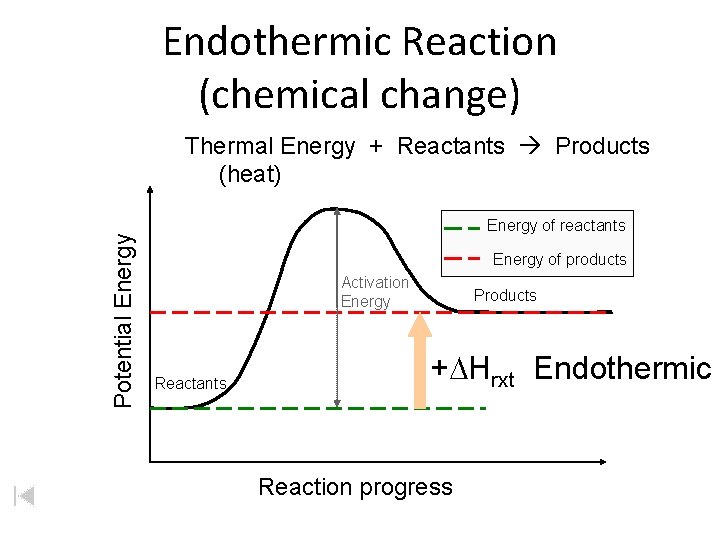
Endothermic Reaction (chemical change) Potential Energy Thermal Energy + Reactants Products (heat) Energy of reactants Energy of products Activation Energy Reactants Products +DHrxt Endothermic Reaction progress

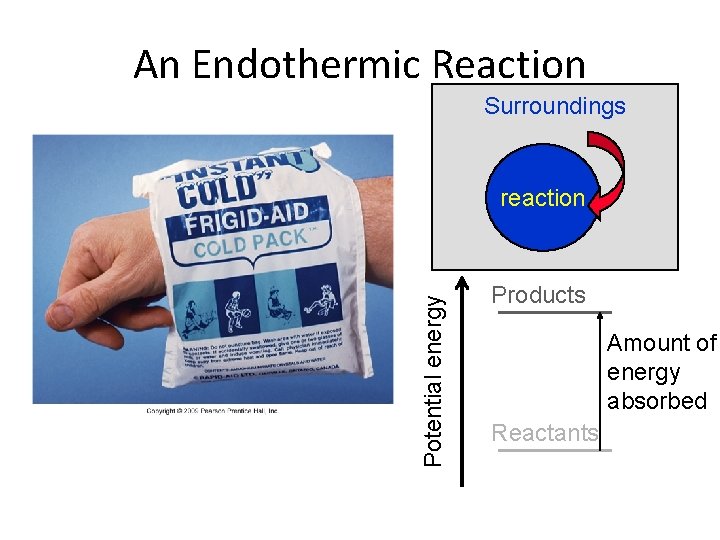
An Endothermic Reaction Surroundings Potential energy reaction Products Amount of energy absorbed Reactants

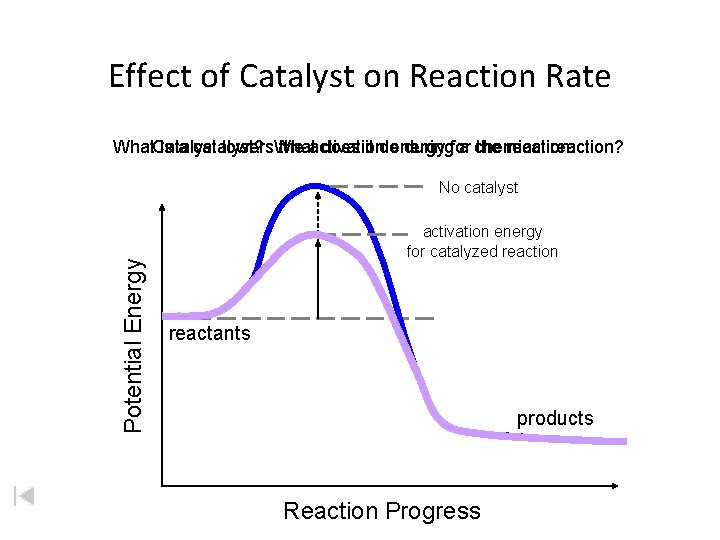
Effect of Catalyst on Reaction Rate What. Catalyst is a catalyst? does it do duringfor a chemical reaction? lowers. What the activation energy the reaction. Potential Energy No catalyst activation energy for catalyzed reaction reactants products Reaction Progress
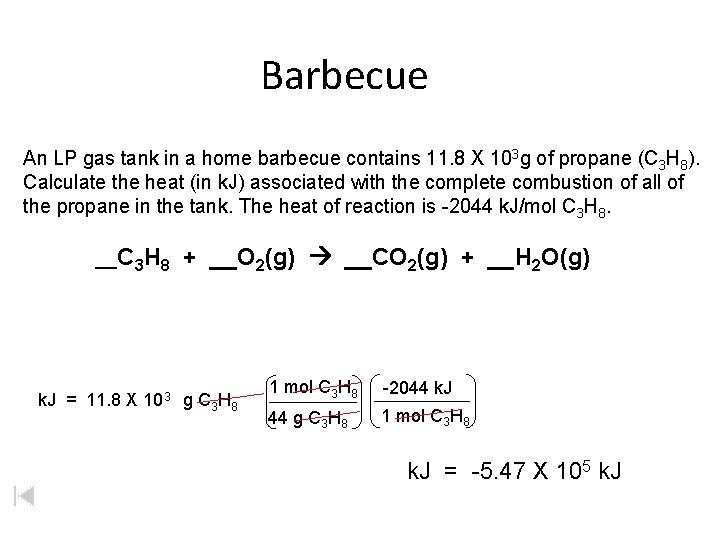
Barbecue An LP gas tank in a home barbecue contains 11. 8 X 103 g of propane (C 3 H 8). Calculate the heat (in k. J) associated with the complete combustion of all of the propane in the tank. The heat of reaction is -2044 k. J/mol C 3 H 8. __C 3 H 8 + __O 2(g) __CO 2(g) + __H 2 O(g) k. J = 11. 8 X 103 g C 3 H 8 1 mol C 3 H 8 -2044 k. J 44 g C 3 H 8 1 mol C 3 H 8 k. J = -5. 47 X 105 k. J

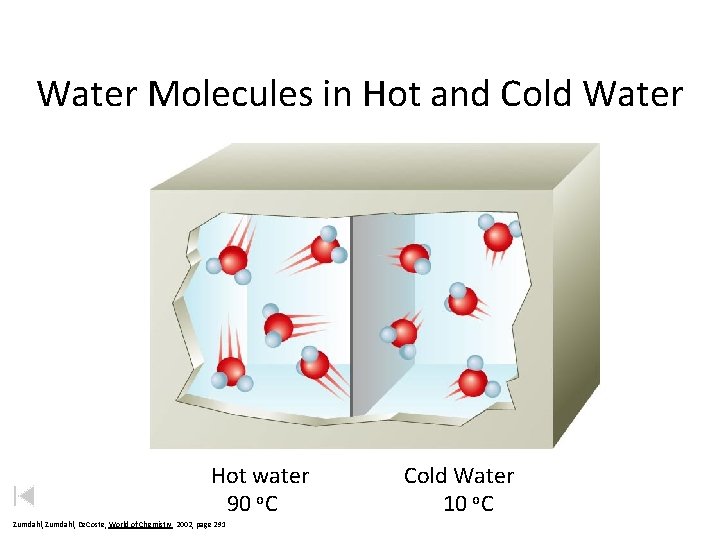
Water Molecules in Hot and Cold Water Hot water 90 o. C Zumdahl, De. Coste, World of Chemistry 2002, page 291 Cold Water 10 o. C

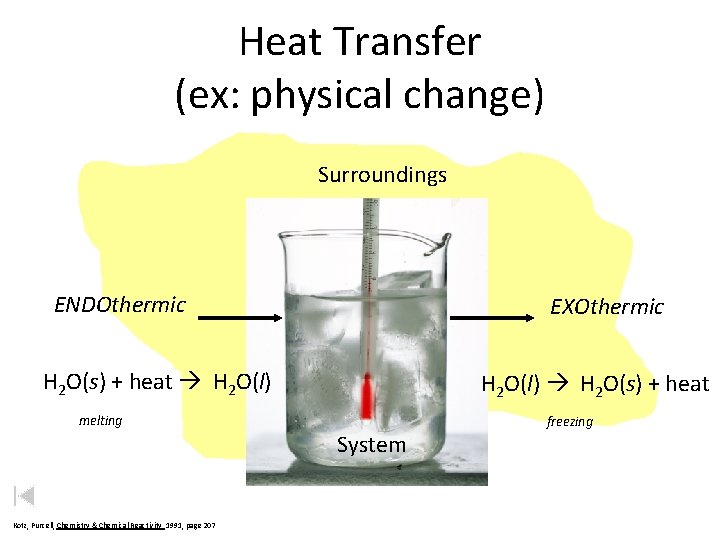
Heat Transfer (ex: physical change) Surroundings ENDOthermic System H 2 O(s) + heat H 2 O(l) H 2 O(s) + heat melting System Kotz, Purcell, Chemistry & Chemical Reactivity 1991, page 207 EXOthermic freezing

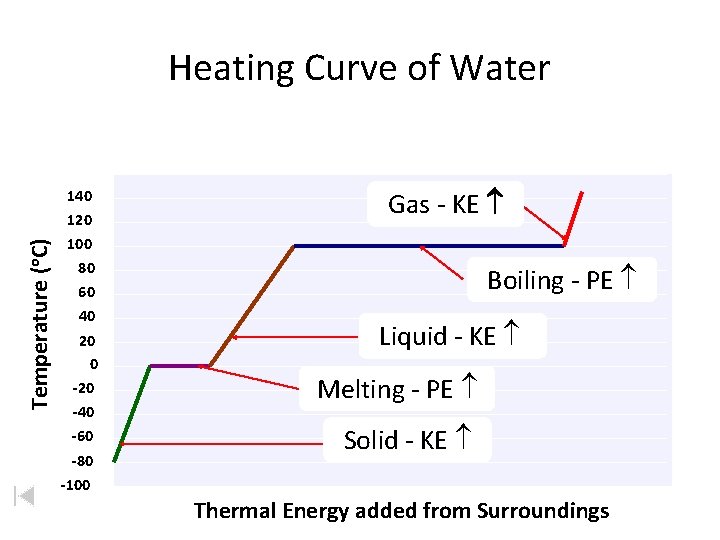
Temperature (o. C) Heating Curve of Water 140 120 100 80 60 40 20 0 -20 -40 -60 -80 -100 Gas - KE Boiling - PE Liquid - KE Melting - PE Solid - KE Thermal Energy added from Surroundings

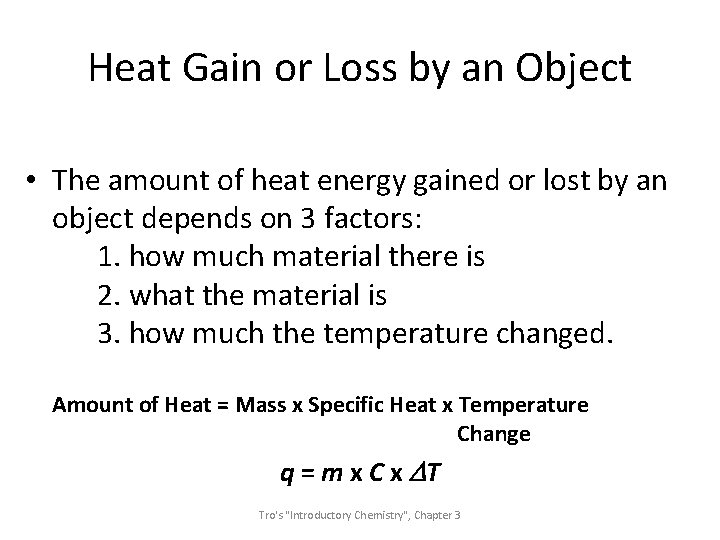
Heat Gain or Loss by an Object • The amount of heat energy gained or lost by an object depends on 3 factors: 1. how much material there is 2. what the material is 3. how much the temperature changed. Amount of Heat = Mass x Specific Heat x Temperature Change q = m x C x DT Tro's "Introductory Chemistry", Chapter 3

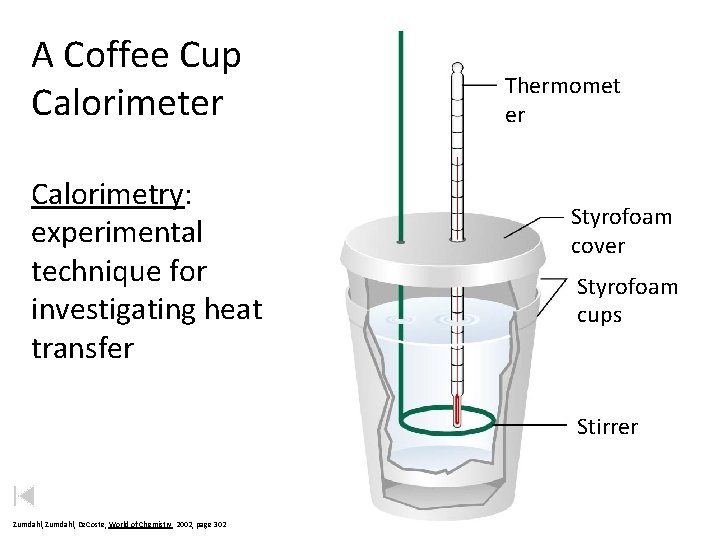
A Coffee Cup Calorimeter Calorimetry: experimental technique for investigating heat transfer Thermomet er Styrofoam cover Styrofoam cups Stirrer Zumdahl, De. Coste, World of Chemistry 2002, page 302

Heat Capacity • Heat capacity is the amount of heat a substance must absorb to raise its temperature by 1 °C. – cal/°C or J/°C. – Metals have low heat capacities; insulators have high heat capacities. • Specific heat = heat capacity of 1 gram of the substance. – cal/g°C or J/g°C. – Water’s specific heat = 4. 184 J/g°C for liquid. • Or 1. 000 cal/g°C. • It is less for ice and steam. 30

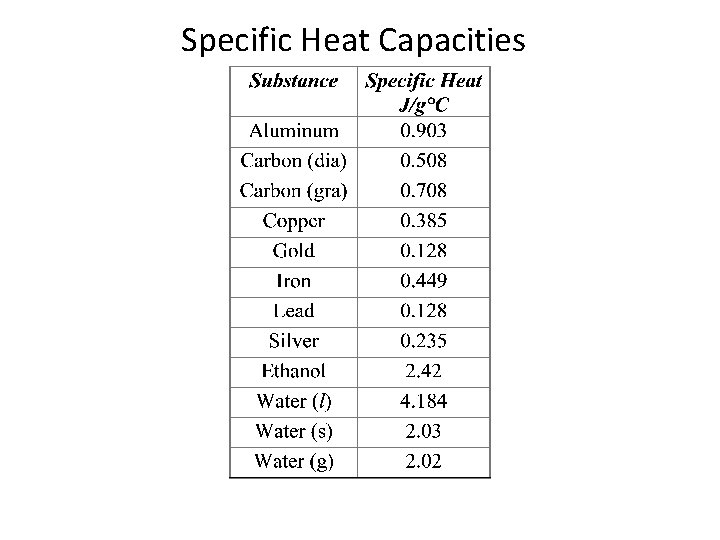
Specific Heat Capacities

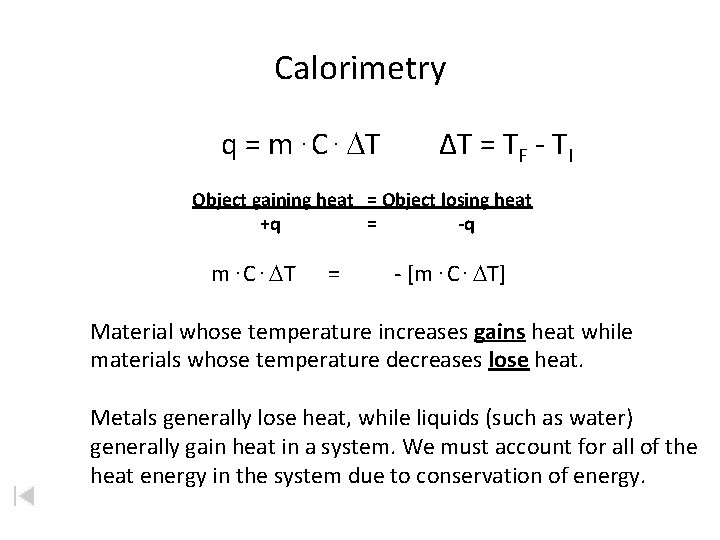
Calorimetry q = m. C. DT ΔT = TF - TI Object gaining heat = Object losing heat +q = -q m. C. DT = - [m. C. DT] Material whose temperature increases gains heat while materials whose temperature decreases lose heat. Metals generally lose heat, while liquids (such as water) generally gain heat in a system. We must account for all of the heat energy in the system due to conservation of energy.

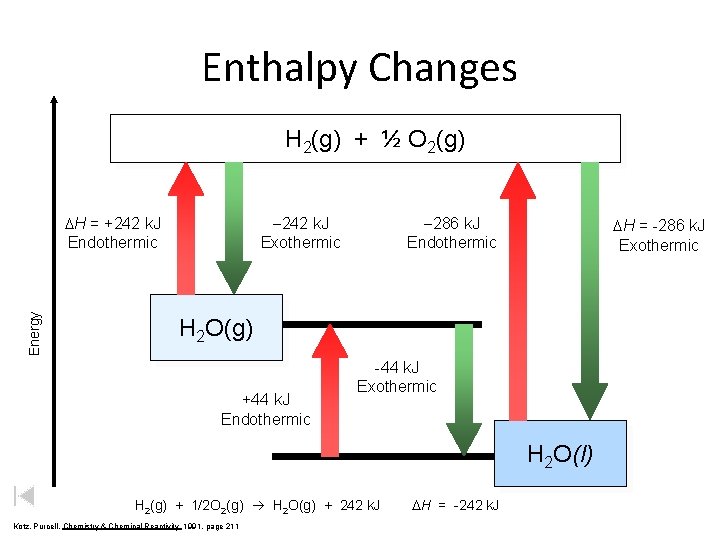
Enthalpy Changes H 2(g) + ½ O 2(g) Energy DH = +242 k. J Endothermic -242 k. J Exothermic -286 k. J Endothermic DH = -286 k. J Exothermic H 2 O(g) +44 k. J Endothermic -44 k. J Exothermic H 2 O(l) H 2(g) + 1/2 O 2(g) H 2 O(g) + 242 k. J Kotz, Purcell, Chemistry & Chemical Reactivity 1991, page 211 DH = -242 k. J

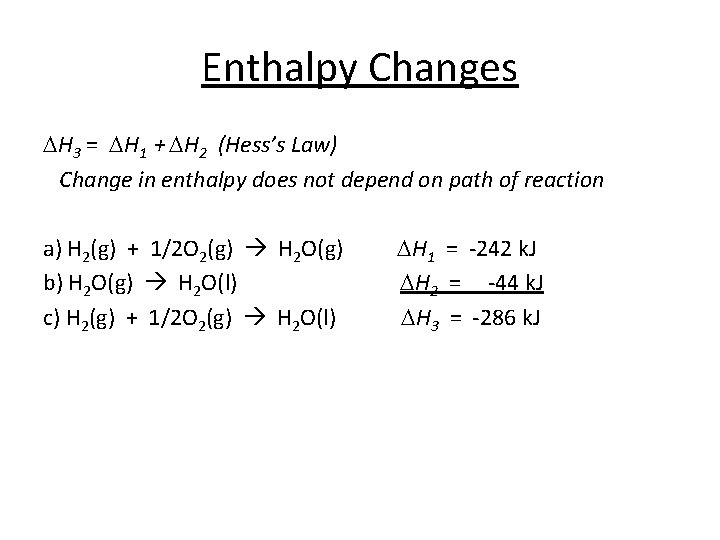
Enthalpy Changes DH 3 = DH 1 + DH 2 (Hess’s Law) Change in enthalpy does not depend on path of reaction a) H 2(g) + 1/2 O 2(g) H 2 O(g) b) H 2 O(g) H 2 O(l) c) H 2(g) + 1/2 O 2(g) H 2 O(l) DH 1 = -242 k. J DH 2 = -44 k. J DH 3 = -286 k. J

Hess’s Law a) N 2(g) + O 2(g) 2 NO(g) b) 2 NO(g) + O 2(g) 2 NO 2(g) c) N 2(g) + 2 O 2(g) 2 NO 2(g) DH 1 = +180 k. J DH 2 = -112 k. J DH 3 = +68 k. J

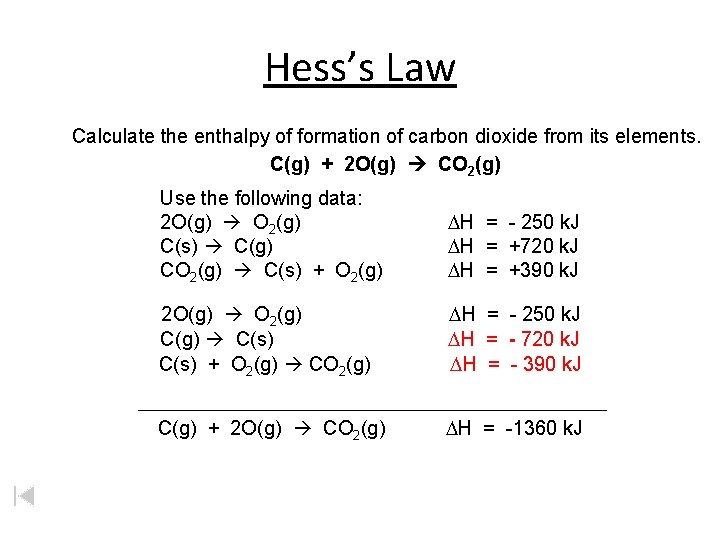
Hess’s Law Calculate the enthalpy of formation of carbon dioxide from its elements. C(g) + 2 O(g) CO 2(g) Use the following data: 2 O(g) O 2(g) C(s) C(g) CO 2(g) C(s) + O 2(g) DH = - 250 k. J DH = +720 k. J DH = +390 k. J 2 O(g) O 2(g) C(g) C(s) + O 2(g) CO 2(g) DH = - 250 k. J DH = - 720 k. J DH = - 390 k. J C(g) + 2 O(g) CO 2(g) DH = -1360 k. J

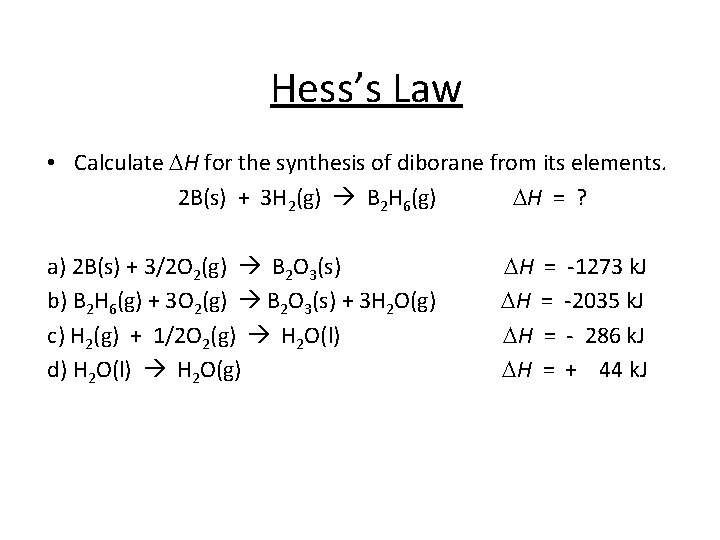
Hess’s Law • Calculate DH for the synthesis of diborane from its elements. 2 B(s) + 3 H 2(g) B 2 H 6(g) DH = ? a) 2 B(s) + 3/2 O 2(g) B 2 O 3(s) b) B 2 H 6(g) + 3 O 2(g) B 2 O 3(s) + 3 H 2 O(g) c) H 2(g) + 1/2 O 2(g) H 2 O(l) d) H 2 O(l) H 2 O(g) DH DH = = -1273 k. J -2035 k. J - 286 k. J + 44 k. J

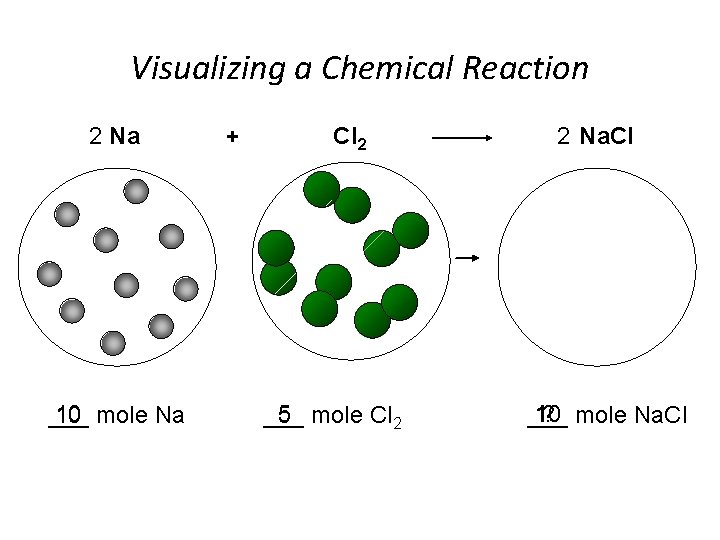
Visualizing a Chemical Reaction 2 Na 10 mole Na ___ + Cl 2 5 mole Cl 2 ___ 2 Na. Cl 10 ? mole Na. Cl ___
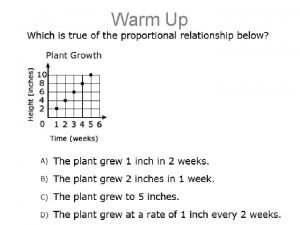
 Proportional vs nonproportional
Proportional vs nonproportional Stoichiometry mass to mass formula
Stoichiometry mass to mass formula Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Non proportional table
Non proportional table Inveresly proportional
Inveresly proportional Proportional vs non proportional graphs worksheet
Proportional vs non proportional graphs worksheet Directly vs indirectly proportional
Directly vs indirectly proportional What is not a proportional relationship
What is not a proportional relationship To find the height h of a dinosaur in a museum
To find the height h of a dinosaur in a museum Stoichiometry island diagram
Stoichiometry island diagram Graph proportional
Graph proportional 7.5 using proportional relationships
7.5 using proportional relationships 7-5 using proportional relationships
7-5 using proportional relationships Interpreting proportional graphs worksheet
Interpreting proportional graphs worksheet What makes two quantities proportional
What makes two quantities proportional Jeopardy proportional relationships
Jeopardy proportional relationships Representing proportional relationships lesson 3-1
Representing proportional relationships lesson 3-1 Identify proportional relationships
Identify proportional relationships Proportional and nonproportional situations
Proportional and nonproportional situations Proportional graph
Proportional graph Proportional and nonproportional relationships answer key
Proportional and nonproportional relationships answer key Whats a proportional relationship
Whats a proportional relationship Graphing proportional relationships quiz
Graphing proportional relationships quiz Lesson 4-4 proportional and nonproportional situations
Lesson 4-4 proportional and nonproportional situations Lesson 2 proportional relationships
Lesson 2 proportional relationships Math antics proportional relationships
Math antics proportional relationships Connections web for proportional relationships
Connections web for proportional relationships Using proportional relationships
Using proportional relationships Molar mass of chocolate chips
Molar mass of chocolate chips Formation initiale vs formation continue
Formation initiale vs formation continue Transamination and oxidative deamination
Transamination and oxidative deamination Fsfffs
Fsfffs Lone pairs and bond pairs
Lone pairs and bond pairs Gdd
Gdd Non aqueous solvents examples
Non aqueous solvents examples Haber-bosch process flow diagram
Haber-bosch process flow diagram Ammonia awareness safety program
Ammonia awareness safety program Preparation of buffer solution lab
Preparation of buffer solution lab