Chapter 3 Mass Relationships in Chemistry Stoichiometry Mass







- Slides: 11

Chapter 3 Mass Relationships in Chemistry; Stoichiometry

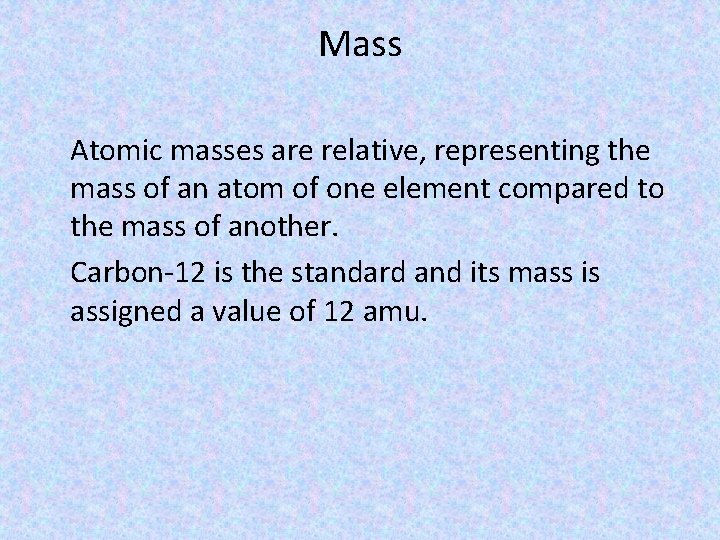
Mass Atomic masses are relative, representing the mass of an atom of one element compared to the mass of another. Carbon-12 is the standard and its mass is assigned a value of 12 amu.

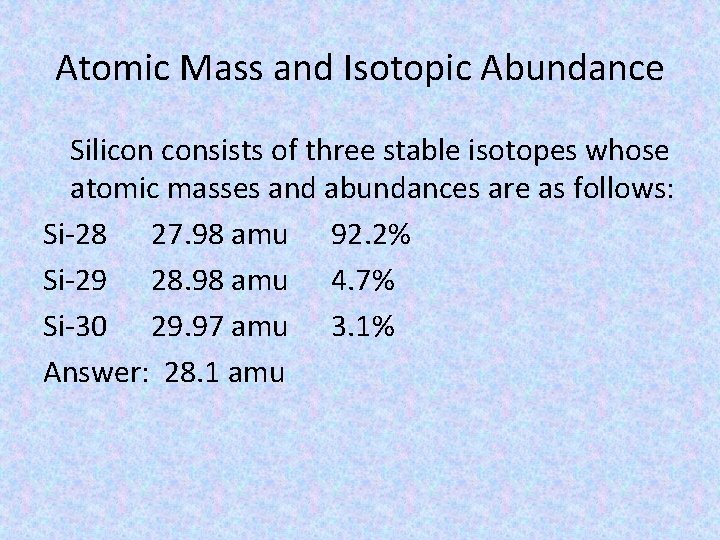
Atomic Mass and Isotopic Abundance Silicon consists of three stable isotopes whose atomic masses and abundances are as follows: Si-28 27. 98 amu 92. 2% Si-29 28. 98 amu 4. 7% Si-30 29. 97 amu 3. 1% Answer: 28. 1 amu

% Composition Percent composition = total mass of element/total mass of compound X 100

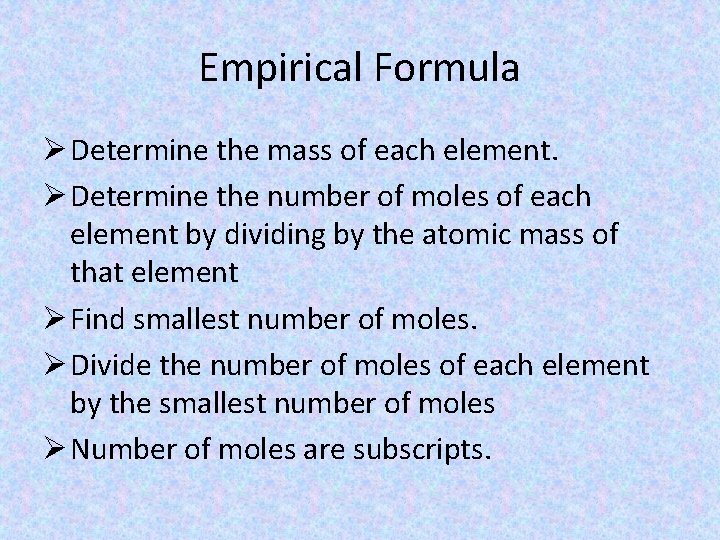
Empirical Formula Ø Determine the mass of each element. Ø Determine the number of moles of each element by dividing by the atomic mass of that element Ø Find smallest number of moles. Ø Divide the number of moles of each element by the smallest number of moles Ø Number of moles are subscripts.

Examples A sample of compound is made up of 78. 20 g of potassium and 32. 06 g sulfur. What is the empirical formula? 78. 20 g K x 1 mol K/39. 10 g K = 2. 000 mol K 32. 06 g S x 1 mol S/32. 06 g S = 1. 000 mol S Divide each by 1. 000 K 2 S 1 Formula: K 2 S

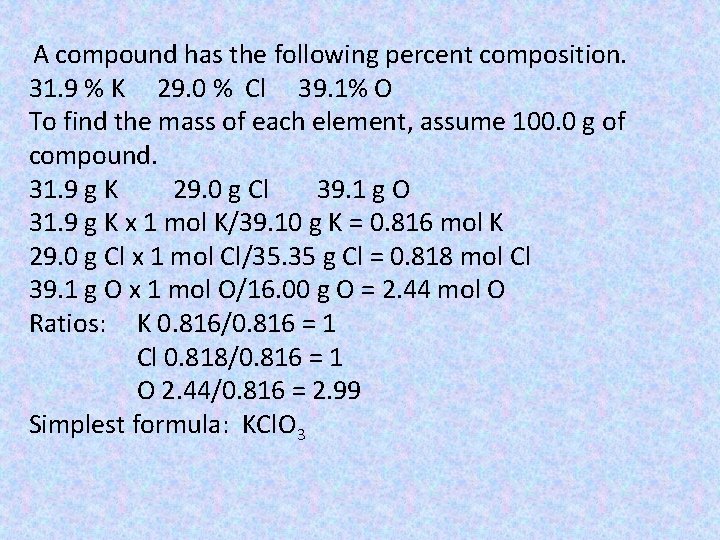
A compound has the following percent composition. 31. 9 % K 29. 0 % Cl 39. 1% O To find the mass of each element, assume 100. 0 g of compound. 31. 9 g K 29. 0 g Cl 39. 1 g O 31. 9 g K x 1 mol K/39. 10 g K = 0. 816 mol K 29. 0 g Cl x 1 mol Cl/35. 35 g Cl = 0. 818 mol Cl 39. 1 g O x 1 mol O/16. 00 g O = 2. 44 mol O Ratios: K 0. 816/0. 816 = 1 Cl 0. 818/0. 816 = 1 O 2. 44/0. 816 = 2. 99 Simplest formula: KCl. O 3

Rounding to determine integer ratios To get an integer, it is ok to round down by at most 0. 1. Ø 2. 09 can be rounded down to 2 To get an integer, it is ok to round up by at most 0. 1. Ø 2. 93 can be rounded up to 3 ØIf that is not possible, multiply each ratio by small whole numbers until multiplication by one small number yields ratios that are all integers. Ø 1. 50 x 2 = 3 1. 33 x 3 = 4

Molecular Formulas Multiple: molar mass/simplest formula mass


 Stoichiometry example
Stoichiometry example Stoichiometry worksheet #2 (mole-mass mass-mole problems)
Stoichiometry worksheet #2 (mole-mass mass-mole problems) Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Modern chemistry chapter 9 review answers
Modern chemistry chapter 9 review answers Chapter 11 chapter assessment stoichiometry answer key
Chapter 11 chapter assessment stoichiometry answer key Stoichiometry chapter 9 test
Stoichiometry chapter 9 test Thermite reaction formula
Thermite reaction formula Stoichiometry defintion
Stoichiometry defintion General chemistry 1 stoichiometry
General chemistry 1 stoichiometry Ap chemistry stoichiometry
Ap chemistry stoichiometry Molar mass of chocolate chips
Molar mass of chocolate chips Charles law indirect or direct
Charles law indirect or direct