Formula for Compounds ending in ide Compounds whos









- Slides: 18

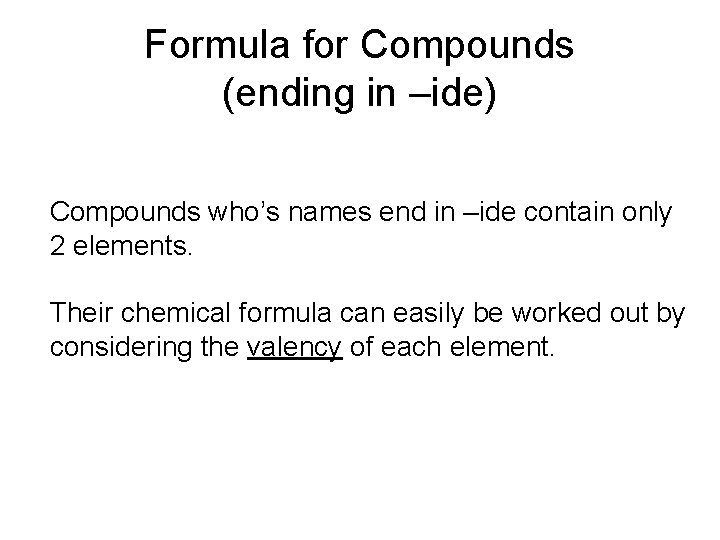
Formula for Compounds (ending in –ide) Compounds who’s names end in –ide contain only 2 elements. Their chemical formula can easily be worked out by considering the valency of each element.

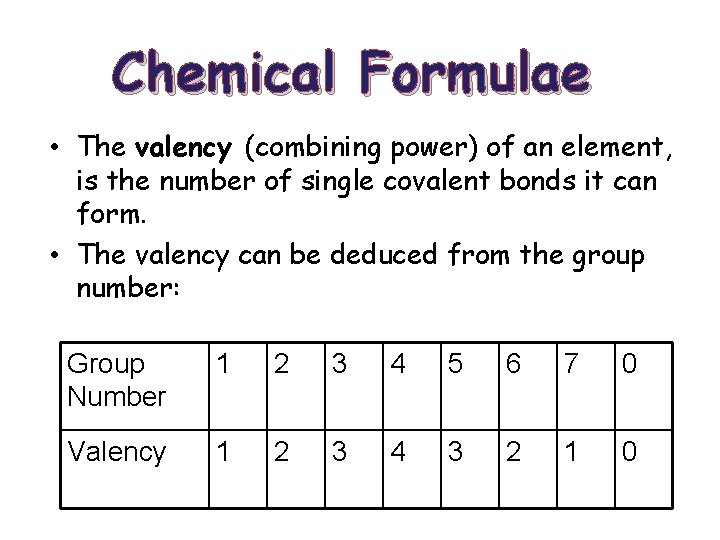
Chemical Formulae • The valency (combining power) of an element, is the number of single covalent bonds it can form. • The valency can be deduced from the group number: Group Number 1 2 3 4 5 6 7 0 Valency 1 2 3 4 3 2 1 0

Writing Formula - Steps 1. Write the symbols 2. Underneath, write the valency 3. Swap them over and write numbers as a subscript. 4. Cancel out any common factor 5. Leave out ‘ 1’ if present.

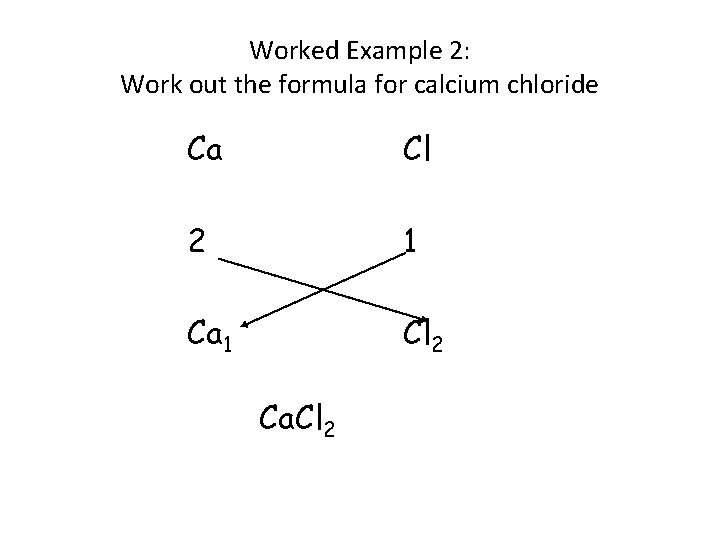
Worked Example 2: Work out the formula for calcium chloride Ca Cl 2 1 Ca 1 Cl 2 Ca. Cl 2

Further Examples: Pg 28 Q 9 1. Boron fluoride 6. Magnesium phosphide 2. Carbon sulphide 7. Hydrogen sulphide 3. Hydrogen 8. Potassium oxide fluoride 4. Sodium sulphide 9. Carbon chloride 5. Aluminium oxide 10. Calcium hydride 1. BF 3 6. Mg 3 P 2 2. CS 2 7. H 2 S 3. HF 8. K 2 O 4. Na 2 S 9. CCl 4 5. Al 2 O 3 10. Ca. H 2

Lesson Starter Write the formula for the following: 1) Lithium sulphide 2) Calcium nitride 3) nitrogen oxide 4) Magnesium hydride 5) Sodium iodide 6) phosphorous chloride 7) Aluminium sulphide 8) Carbon hydride 9) Magnesium oxide 10) Hydrogen oxide 1)Li 2 S 2)Ca 3 N 2 3)N 2 O 3 4)Mg. H 2 5)Na. I 6)PCl 3 7)Al 2 S 3 8)CH 4 9)Mg. O 10) H 2 O

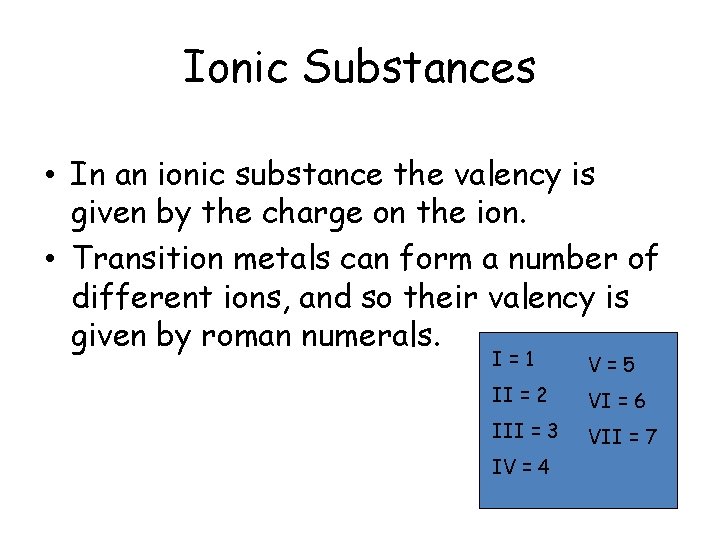
Ionic Substances • In an ionic substance the valency is given by the charge on the ion. • Transition metals can form a number of different ions, and so their valency is given by roman numerals. I=1 V=5 II = 2 VI = 6 III = 3 VII = 7 IV = 4

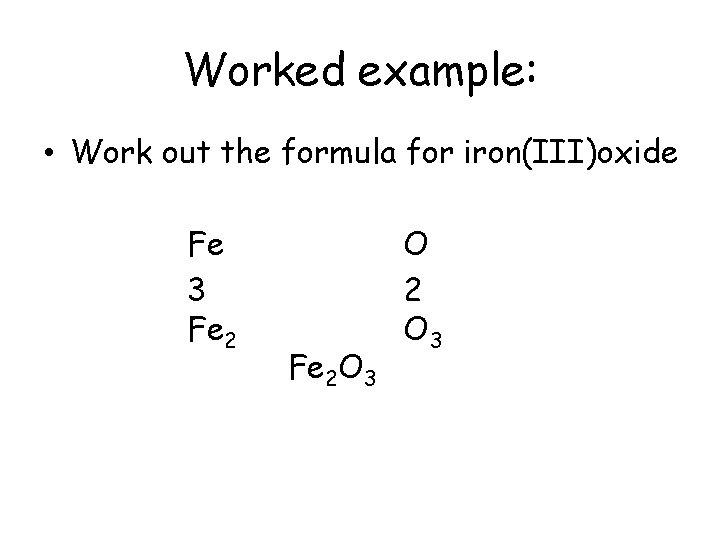
Worked example: • Work out the formula for iron(III)oxide Fe 3 Fe 2 O 3 O 2 O 3

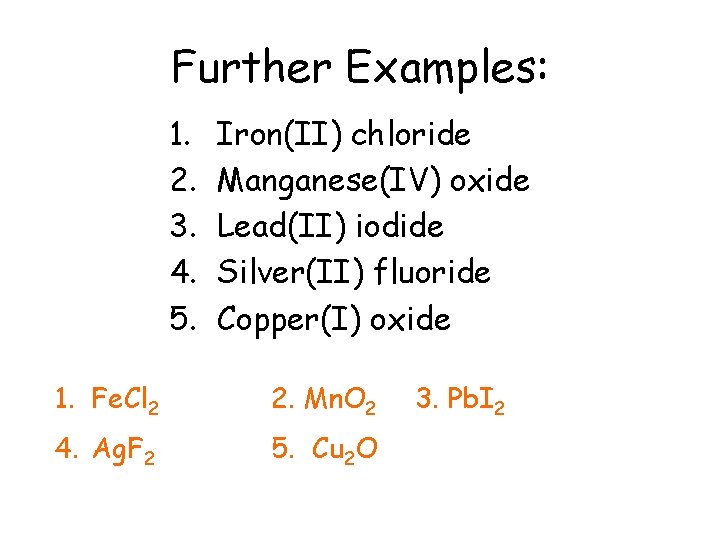
Further Examples: 1. 2. 3. 4. 5. Iron(II) chloride Manganese(IV) oxide Lead(II) iodide Silver(II) fluoride Copper(I) oxide 1. Fe. Cl 2 2. Mn. O 2 4. Ag. F 2 5. Cu 2 O 3. Pb. I 2

Group Ions • Ions containing 2 or more different types of atom are known as group ions. • The formula for these can be found on page 8 of the data booklet. • N. B. The charge is on the WHOLE GROUP of atoms • The valency is equal to the number of charges. • Always place the formula for the group ion in brackets.

Worked Example 1: Calcium Nitrate Symbol Valency Swap

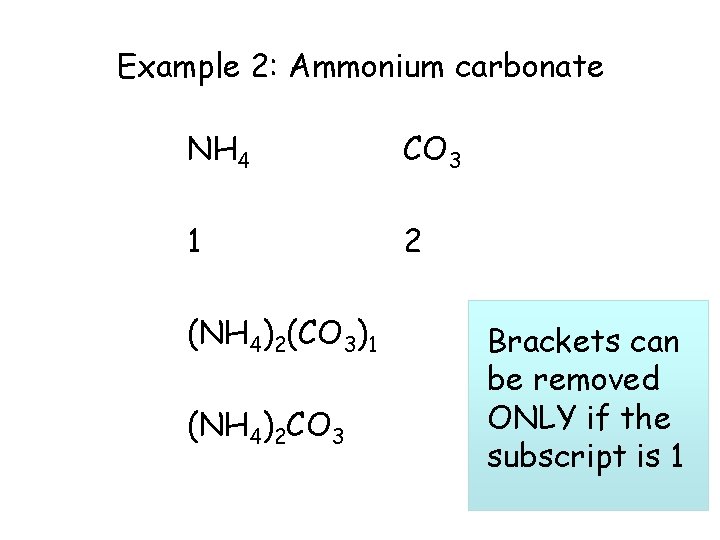
Example 2: Ammonium carbonate NH 4 CO 3 1 2 (NH 4)2(CO 3)1 (NH 4)2 CO 3 Brackets can be removed ONLY if the subscript is 1

Further Examples: 1. 2. 3. 4. 5. Magnesium hydroxide Ammonium phosphate Potassium chromate Aluminium sulphate Calcium hydrogensulphite 1. Mg(OH)2 2. (NH 4)3 PO 4 4. Al 2(SO 4)3 5. Ca(HSO 3)2 3. K 2 Cr. O 4

Group ion Practice: Yellow Book Page 29 Q 11 Int 2 Textbook Page 61 Q 5&6 Mixed Questions: Yellow Book Page 29 Q 12 Int 2 Textbook Page 64 Q 11

Formula from Prefixes • In some cases the name of the compound tells us its chemical formula. This information is given in the form of a prefix. • If only one prefix is given it can be assumed that only one atom of the other element is present. • N. B. You do not need to ‘cross over’ if a prefix is given.

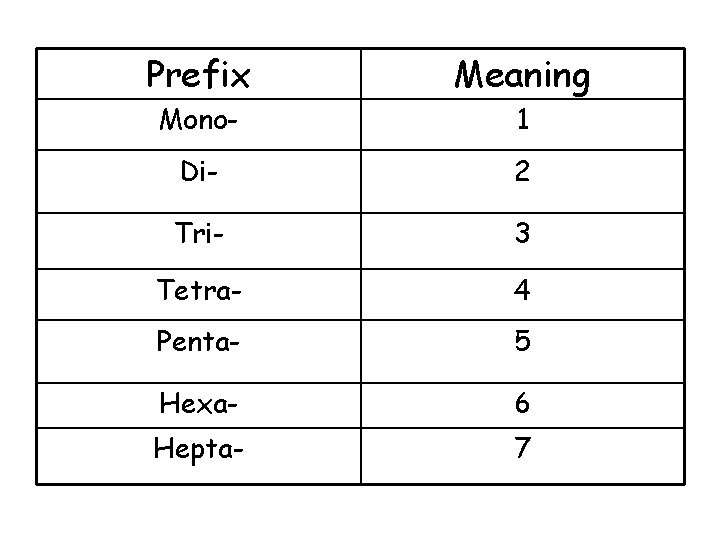
Prefix Meaning Mono- 1 Di- 2 Tri- 3 Tetra- 4 Penta- 5 Hexa- 6 Hepta- 7

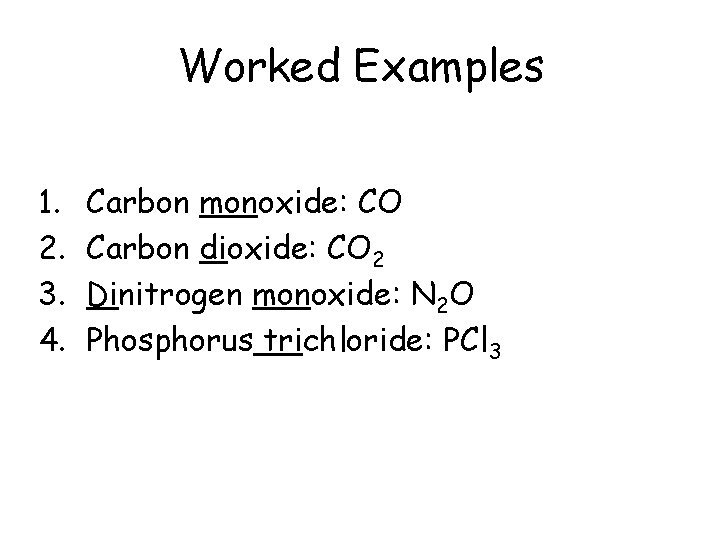
Worked Examples 1. 2. 3. 4. Carbon monoxide: CO Carbon dioxide: CO 2 Dinitrogen monoxide: N 2 O Phosphorus trichloride: PCl 3

Further Examples 1. 2. 3. 4. 5. Diphosphorus trioxide Silicon tetrachloride Divanadium pentoxide Tetraarsenic hexaoxide Uranium hexafluoride 1. P 2 O 3 2. Si. Cl 4 3. V 2 O 5 4. As 4 O 6 5. UF 6