Chapter 3 2 Names and Formulas of Ionic





















- Slides: 24

Chapter 3. 2 Names and Formulas of Ionic Compounds Remember ions are just atoms with charges = different # of electrons

Ionic Compounds l Each ionic compound has a name and a chemical formula that tells you what types of ions it contains. l All ionic compounds are composed of metal ions which are ALWAYS positive (+) and non-metal ions which are ALWAYS negative (-) l With ionic compounds, the positive ion always goes first THEN the negative ion Just be positive first!

NAMING Ionic Compounds… FIRST, count the capital letters. • If there are two capitals, there are only two elements. Go to Step A. • If there are more than two capital letters, go to Step B (we will get to this later).

ONLY TWO CAPITAL LETTERS Ca. O, Li 2 S Step A 1. Name the metal first (just write it from the periodic table) 2. Find the non-metal (from the periodic table but Change the non-metal ending to –ide

Example, Na. Cl: l There are two capital letters. Go to Step A. l The first part of the name, “sodium”, describes the metal ion. l The second part of the name, “chlorine”, describes the non-metal ion. l The negative, non metal ion gets an “-ide” ending. ¡(In this case, chlorine gets changed to chloride. ) = sodium chloride

Example, Mg. Br 2: l There are two capital letters. Go to Step A. l Metal = magnesium (remember, positives always go first) l Non-metal = bromine (negatives always go second) l Change the non-metal ending to –ide; bromine becomes bromide l Mg. Br 2 = magnesium bromide

Example: l Li 3 N l Lithium l Nitrogen = Nitride l Lithium Nitride

Try… l Al. Br 3 l Na 2 O l Mg 3 P 2 l Textbook page 86 #1 a-o l Combining Capacities Worksheet

How to Write Formulas for Ionic Compounds do combining capacity worksheet first l Find the symbol for the metal, include the charge (always positive) l Find the symbol for the non-metal, include the charge (always negative) l Metal first, non-metal second l Remember charge = combining capacity l Use the “Hand Hold” method or “Drop and Swap”

Example… l Calcium Nitride ¡Ca 2+ ¡N 3¡Hand hold method: l. Ca 3 N 2 l. So… 3 Calcium are needed to bind with 2 Nitrogen

Example… l Lithium Bromide ¡Li+ ¡Br¡Drop and Swap or hand hold ¡Li. Br ¡(don’t show subscripts if they equal 1)

Try… l Sodium Chloride l Magnesium Fluoride l Aluminum Oxide l Calcium Nitride l Potassium Sulphide l Textbook page 87 #1 a-f, #2 (e. s. l. ) l Worksheet on Naming Compounds and Writing Formulas

Compounds Containing Multivalent Metals l Multivalent = multiple combining capacities ¡Multiple ion charges ¡Ex/ Iron l. Can be Fe 3+ or Fe 2+ l Same rules and steps apply for writing formulas

Example: l Gold (I) Oxide ¡Au+ ¡O 2 - l Drop and Swap ¡Au 2 O

Naming for multivalents ¡Must specify (with roman numerals) which ion we’re using l. Fe 3+ = Iron (III) and Fe 2+ = Iron (II) 1. Find the metal 2. Check if it has more than one charge ¡If it does, write down the possible ions 3. Note the charge for the negative ion 4. Balance out the positives and negatives to determine the ion charge of the metal 5. Write the roman numeral 6. Write the non-metal with changed ending

Example: l Mn. I 2 1. Manganese 2. Can be Mn 2+ or Mn 4+ 3. The charge for iodine is -1. 4. There are two iodines in the formula l 2 x -1 = -2 5. The Mn ion must be +2 to balance out the -2 charge, therefore write (II). 6. Iodine = iodide Answer = Manganese (II) iodide

Try… l Chromium (II) fluoride l Tin (IV) sulphide l Fe 2 Se 3 l Bi. Cl 5 l Textbook pg. 89 #1 e. s. l. l Textbook pg. 90 #1 e. s. l. l Multivalents Worksheet

3 or more capital letters If you have 3 or more capital letter you have a PAI (polyatomic ion) PAI = a molecule (more than one atom) that has a charge (makes it an ion) and is tightly bonded together PAI’s never get broken up. They have their own names for the whole molecule and are on the back of your periodic table

MORE THAN TWO CAPITALS Na. NO 3, K 2 CO 3, NH 4 NO 3 Step B 1. Name the metal first l Note: Ammonium (NH 4+) is a PAI that is positively charged and acts like a metal. If it is in the compound, write its name first, just like a metal. 2. LOOK UP THE PAI and write its name directly from the sheet. Don’t change the ending!

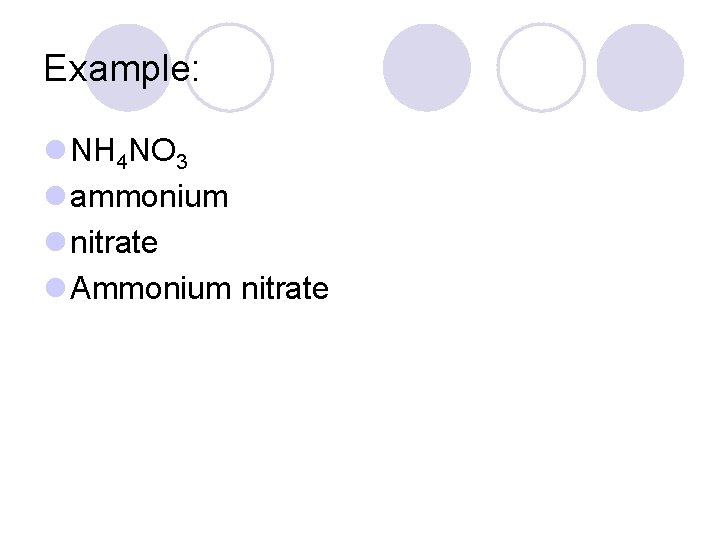
Example: l NH 4 NO 3 l ammonium l nitrate l Ammonium nitrate

Writing Formulas with Polyatomic Ions l Find the metal ion and include charge l Find the polyatomic ion (PAI chart on the backside of the periodic table handout) and include charge l Drop and Swap ¡Note: If a subscript is added after the PAI (that did not belong to the PAI before) then brackets must be put around the PAI group

Example l Iron (III) hydroxide ¡Fe 3+ ¡OH 1 - l Drop and Swap or Hand Hold ¡Fe(OH)3 (keep polyatomic ion in brackets)

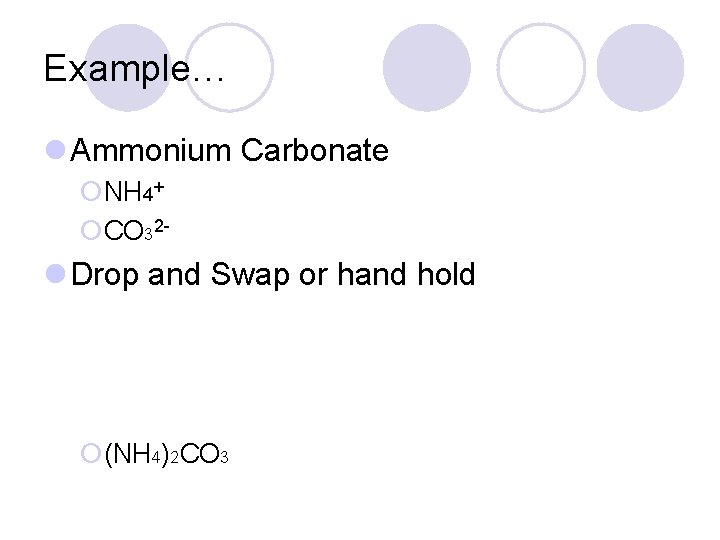
Example… l Ammonium Carbonate ¡NH 4+ ¡CO 32 - l Drop and Swap or hand hold ¡(NH 4)2 CO 3

Try… l Calcium carbonate l Magnesium perchlorate l Barium hydroxide l Lithium hydrogen carbonate l Sodium hydroxide l Pg. 91 #1 e. s. l. , 2 e. s. l. l PAI Worksheet